Impact of Oxygen Delivery on the Development of Acute Kidney Injury in Patients Undergoing Valve Heart Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Patients
2.3. Study Variables and Definitions
2.4. Anesthesia and CPB Management
Intraoperative Care and Anesthesia
2.5. Statistical Analysis
3. Results
Multivariable Model for DO2i and CSA-AKI
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zakkar, M.; Bruno, V.D.; Guida, G.; Angelini, G.D.; Chivasso, P.; Suleiman, M.S.; Bryan, A.J.; Ascione, R. Postoperative acute kidney injury defined by RIFLE criteria predicts early health outcome and long-term survival in patients undergoing redo coronary artery bypass graft surgery. J. Thorac. Cardiovasc. Surg. 2016, 152, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lannemyr, L.; Bragadottir, G.; Krumbholz, V.; Redfors, B.; Sellgren, J.; Ricksten, S.E. Effects of Cardiopulmonary Bypass on Renal Perfusion, Filtration, and Oxygenation in Patients Undergoing Cardiac Surgery. Anesthesiology 2017, 126, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.G.; Ince, C.; Joles, J.A.; Smith, D.W.; May, C.N.; O’Connor, P.M.; Gardiner, B.S. Haemodynamic influences on kidney oxygenation: Clinical implications of integrative physiology. Clin. Exp. Pharmacol. Physiol. 2013, 40, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Loubon, C.; Fernandez-Molina, M.; Carrascal-Hinojal, Y.; Fulquet-Carreras, E. Cardiac surgery-associated acute kidney injury. Ann. Card. Anaesth. 2016, 19, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Newland, R.F.; Baker, R.A.; Woodman, R.J.; Barnes, M.B.; Willcox, T.W.; Australian and New Zealand Collaborative Perfusion Registry. Predictive Capacity of Oxygen Delivery During Cardiopulmonary Bypass on Acute Kidney Injury. Ann. Thorac. Surg. 2019, 108, 1807–1814. [Google Scholar] [CrossRef]
- Magruder, J.T.; Dungan, S.P.; Grimm, J.C.; Harness, H.L.; Wierschke, C.; Castillejo, S.; Barodka, V.; Katz, N.; Shah, A.S.; Whitman, G.J. Nadir Oxygen Delivery on Bypass and Hypotension Increase Acute Kidney Injury Risk after Cardiac Operations. Ann. Thorac. Surg. 2015, 100, 1697–1703. [Google Scholar] [CrossRef]
- Patel, H.; Parikh, N.; Shah, R.; Patel, R.; Thosani, R.; Shah, P.; Prajapat, L. Effect of Goal-directed Hemodynamic Therapy in Postcardiac Surgery Patients. Indian J. Crit. Care Med. 2020, 24, 321–326. [Google Scholar] [CrossRef]
- Ranucci, M.; Johnson, I.; Willcox, T.; Baker, R.A.; Boer, C.; Baumann, A.; Justison, G.A.; de Somer, F.; Exton, P.; Agarwal, S.; et al. Goal-directed perfusion to reduce acute kidney injury: A randomized trial. J. Thorac. Cardiovasc. Surg. 2018, 156, 1918–1927.e2. [Google Scholar] [CrossRef] [Green Version]
- Ranucci, M. Perioperative renal failure: Hypoperfusion during cardiopulmonary bypass? Semin. Cardiothorac. Vasc. Anesth. 2007, 11, 265–268. [Google Scholar] [CrossRef]
- Ranucci, M.; Romitti, F.; Isgro, G.; Cotza, M.; Brozzi, S.; Boncilli, A.; Ditta, A. Oxygen delivery during cardiopulmonary bypass and acute renal failure after coronary operations. Ann. Thorac. Surg. 2005, 80, 2213–2220. [Google Scholar] [CrossRef]
- Lankadeva, Y.R.; Cochrane, A.D.; Marino, B.; Iguchi, N.; Hood, S.G.; Bellomo, R.; May, C.N.; Evans, R.G. Strategies that improve renal medullary oxygenation during experimental cardiopulmonary bypass may mitigate postoperative acute kidney injury. Kidney Int. 2019, 95, 1338–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brezis, M.; Rosen, S. Hypoxia of the renal medulla—Its implications for disease. N. Engl. J. Med. 1995, 332, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.G.; Lankadeva, Y.R.; Cochrane, A.D.; Marino, B.; Iguchi, N.; Zhu, M.Z.L.; Hood, S.G.; Smith, J.A.; Bellomo, R.; Gardiner, B.S.; et al. Renal haemodynamics and oxygenation during and after cardiac surgery and cardiopulmonary bypass. Acta Physiol. 2018, 222, e12995. [Google Scholar] [CrossRef] [PubMed]
- de Somer, F.; Mulholland, J.W.; Bryan, M.R.; Aloisio, T.; Van Nooten, G.J.; Ranucci, M. O2 delivery and CO2 production during cardiopulmonary bypass as determinants of acute kidney injury: Time for a goal-directed perfusion management? Crit. Care 2011, 15, R192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkins, N.J.; Schisterman, E.F. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am. J. Epidemiol. 2006, 163, 670–675. [Google Scholar] [CrossRef] [Green Version]
- Abadeer, A.I.; Kurlansky, P.; Chiuzan, C.; Truby, L.; Radhakrishnan, J.; Garan, R.; Topkara, V.; Yuzefpolskaya, M.; Colombo, P.; Takeda, K.; et al. Importance of stratifying acute kidney injury in cardiogenic shock resuscitated with mechanical circulatory support therapy. J. Thorac. Cardiovasc. Surg. 2017, 154, 856–864.e4. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.K.; Kim, W.J.; Chin, J.H.; Lee, E.H.; Don Hahm, K.; Yeon Sim, J.; Cheol Choi, I. Intraoperative renal regional oxygen desaturation can be a predictor for acute kidney injury after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2014, 28, 564–571. [Google Scholar] [CrossRef]
- Baker, R.A. Variation in Measurement and Reporting of Goal Directed Perfusion Parameters. J. Extra-Corpor. Technol. 2017, 49, P2–P7. [Google Scholar]
- Gaffney, A.M.; Sladen, R.N. Acute kidney injury in cardiac surgery. Curr. Opin. Anaesthesiol. 2015, 28, 50–59. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Loubon, C.; Fernandez-Molina, M.; Fierro, I.; Jorge-Monjas, P.; Carrascal, Y.; Gomez-Herreras, J.I.; Tamayo, E. Postoperative kidney oxygen saturation as a novel marker for acute kidney injury after adult cardiac surgery. J. Thorac. Cardiovasc. Surg. 2019, 157, 2340–2351.e3. [Google Scholar] [CrossRef]
- Ortega-Loubon, C.; Fernandez-Molina, M.; Paneda-Delgado, L.; Jorge-Monjas, P.; Carrascal, Y. Predictors of Postoperative Acute Kidney Injury after Coronary Artery Bypass Graft Surgery. Braz. J. Cardiovasc. Surg. 2018, 33, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Jorge-Monjas, P.; Bustamante-Munguira, J.; Lorenzo, M.; Heredia-Rodriguez, M.; Fierro, I.; Gomez-Sanchez, E.; Hernandez, A.; Alvarez, F.J.; Bermejo-Martin, J.F.; Gomez-Pesquera, E.; et al. Predicting cardiac surgery-associated acute kidney injury: The CRATE score. J. Crit. Care 2016, 31, 130–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newland, R.F.; Baker, R.A. Low Oxygen Delivery as a Predictor of Acute Kidney Injury during Cardiopulmonary Bypass. J. Extra-Corpor. Technol. 2017, 49, 224–230. [Google Scholar] [PubMed]
- von Heymann, C.; Sander, M.; Foer, A.; Heinemann, A.; Spiess, B.; Braun, J.; Kramer, M.; Grosse, J.; Dohmen, P.; Dushe, S.; et al. The impact of an hematocrit of 20% during normothermic cardiopulmonary bypass for elective low risk coronary artery bypass graft surgery on oxygen delivery and clinical outcome—A randomized controlled study [ISRCTN35655335]. Crit. Care 2006, 10, R58. [Google Scholar] [CrossRef] [Green Version]
- Oshita, T.; Hiraoka, A.; Nakajima, K.; Muraki, R.; Arimichi, M.; Chikazawa, G.; Yoshitaka, H.; Sakaguchi, T. A Better Predictor of Acute Kidney Injury after Cardiac Surgery: The Largest Area under the Curve below the Oxygen Delivery Threshold during Cardiopulmonary Bypass. J. Am. Heart Assoc. 2020, 9, e015566. [Google Scholar] [CrossRef]
- Reagor, J.A.; Clingan, S.; Gao, Z.; Morales, D.L.S.; Tweddell, J.S.; Bryant, R.; Young, W.; Cavanaugh, J.; Cooper, D.S. Higher Flow on Cardiopulmonary Bypass in Pediatrics Is Associated with a Lower Incidence of Acute Kidney Injury. Semin. Thorac. Cardiovasc. Surg. 2020, 32, 1015–1020. [Google Scholar] [CrossRef]
- Mukaida, H.; Matsushita, S.; Kuwaki, K.; Inotani, T.; Minami, Y.; Saigusa, A.; Amano, A. Time-dose response of oxygen delivery during cardiopulmonary bypass predicts acute kidney injury. J. Thorac. Cardiovasc. Surg. 2019, 158, 492–499. [Google Scholar] [CrossRef]
- Jefayri, M.K.; Grace, P.A.; Mathie, R.T. Attenuation of reperfusion injury by renal ischaemic preconditioning: The role of nitric oxide. BJU Int. 2000, 85, 1007–1013. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, S.R.; Kandler, K.; Nielsen, R.V.; Cornelius Jakobsen, P.; Knudsen, N.N.; Ranucci, M.; Christian Nilsson, J.; Ravn, H.B. Duration of critically low oxygen delivery is associated with acute kidney injury after cardiac surgery. Acta Anaesthesiol. Scand. 2019, 63, 1290–1297. [Google Scholar] [CrossRef]
- Hendrix, R.H.J.; Ganushchak, Y.M.; Weerwind, P.W. Oxygen delivery, oxygen consumption and decreased kidney function after cardiopulmonary bypass. PLoS ONE 2019, 14, e0225541. [Google Scholar] [CrossRef]
- Ranucci, M.; Pavesi, M.; Mazza, E.; Bertucci, C.; Frigiola, A.; Menicanti, L.; Ditta, A.; Boncilli, A.; Conti, D. Risk factors for renal dysfunction after coronary surgery: The role of cardiopulmonary bypass technique. Perfusion 1994, 9, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Azau, A.; Markowicz, P.; Corbeau, J.J.; Cottineau, C.; Moreau, X.; Baufreton, C.; Beydon, L. Increasing mean arterial pressure during cardiac surgery does not reduce the rate of postoperative acute kidney injury. Perfusion 2014, 29, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Urzua, J.; Troncoso, S.; Bugedo, G.; Canessa, R.; Muñoz, H.; Lema, G.; Valdivieso, A.; Irarrazaval, M.; Moran, S.; Meneses, G. Renal function and cardiopulmonary bypass: Effect of perfusion pressure. J. Cardiothorac. Vasc. Anesth. 1992, 6, 299–303. [Google Scholar] [CrossRef]
- Sirvinskas, E.; Andrejaitiene, J.; Raliene, L.; Nasvytis, L.; Karbonskiene, A.; Pilvinis, V.; Sakalauskas, J. Cardiopulmonary bypass management and acute renal failure: Risk factors and prognosis. Perfusion 2008, 23, 323–327. [Google Scholar] [CrossRef]
- Sirvinskas, E.; Benetis, R.; Raliene, L.; Andrejaitiene, J. The influence of mean arterial blood pressure during cardiopulmonary bypass on postoperative renal dysfunction in elderly patients. Perfusion 2012, 27, 193–198. [Google Scholar] [CrossRef]
- Kanji, H.D.; Schulze, C.J.; Hervas-Malo, M.; Wang, P.; Ross, D.B.; Zibdawi, M.; Bagshaw, S.M. Difference between pre-operative and cardiopulmonary bypass mean arterial pressure is independently associated with early cardiac surgery-associated acute kidney injury. J. Cardiothorac. Surg. 2010, 5, 71. [Google Scholar] [CrossRef] [Green Version]
- Habib, R.H.; Zacharias, A.; Schwann, T.A.; Riordan, C.J.; Engoren, M.; Durham, S.J.; Shah, A. Role of hemodilutional anemia and transfusion during cardiopulmonary bypass in renal injury after coronary revascularization: Implications on operative outcome. Crit. Care Med. 2005, 33, 1749–1756. [Google Scholar] [CrossRef]
- Karkouti, K. Transfusion and risk of acute kidney injury in cardiac surgery. Br. J. Anaesth. 2012, 109 (Suppl. 1), i29–i38. [Google Scholar] [CrossRef] [Green Version]
- Almac, E.; Ince, C. The impact of storage on red cell function in blood transfusion. Best Pract. Res. Clin. Anaesthesiol. 2007, 21, 195–208. [Google Scholar] [CrossRef]
- Tinmouth, A.; Fergusson, D.; Yee, I.C.; Hebert, P.C.; Investigators, A.; the Canadian Critical Care Trials Group. Clinical consequences of red cell storage in the critically ill. Transfusion 2006, 46, 2014–2027. [Google Scholar] [CrossRef]
| Characteristics | Total (n 782) | CSA-AKI (231) | CSA-AKI Free (551) | p Value |
|---|---|---|---|---|
| Preoperative data | ||||
| Population characteristics | ||||
| Age, y, median (IQR) | 70 (63–77) | 74 (67–78) | 69 (62–76) | <0.001 |
| Female | 381 (48.7) | 112 (48.5) | 269 (48.8) | 0.932 |
| BMI, kg/m2, median (IQR) | 27.0 (24.4–29.8) | 27.1 (24.6–29.3) | 27.0 (24.3–30.0) | 0.923 |
| EuroSCORE II, median (IQR) | 1.7 (1.2–2.1) | 1.8 (1.4–2.2) | 1.6 (1.2–2.0) | <0.001 |
| Smoker | 163 (20.8) | 52 (22.5) | 111 (20.1) | 0.043 |
| Current Smoker | 63 (8.1) | 10 (4.3) | 53 (9.6) | 0.043 |
| Hypertension | 689 (88.1) | 216 (93.5) | 473 (85.8) | 0.003 |
| Diabetes mellitus | 154 (19.7) | 54 (23.4) | 100 (18.1) | 0.141 |
| Dyslipidemia | 574 (73.4) | 176 (76.2) | 398 (72.2) | 0.253 |
| COPD | 43 (5.5) | 12 (5.2) | 31 (5.6) | 0.533 |
| Peripheral Vascular Disease | 17 (2.2) | 8 (3.5) | 9 (1.6) | 0.109 |
| Prior Cardiac Surgery | 49 (6.3) | 19 (8.2) | 30 (5.4) | 0.339 |
| Prior Stroke | 36 (4.6) | 17 (7.4) | 19 (3.4) | 0.106 |
| AF | 257 (33.0) | 75 (32.3) | 182 (33.0) | 0.948 |
| NYHA 3 | 230 (29.4) | 73 (31.6) | 157 (28.5) | 0.371 |
| NYHA 4 | 8 (1.0) | 4 (1.7) | 4 (0.7) | |
| Preoperative SCr, median (IQR) | 0.8 (0.7–0.9) | 1.21 (0.7–1.7) | 0.95 (0.7–1.2) | <0.001 |
| LVEF | 62 (58–65) | 62 (58–65) | 62 (59–65) | 0.740 |
| Intraoperative data | ||||
| CBP time, min, median (IQR) | 97 (80–123) | 102 (83–131) | 95 (80–117) | <0.001 |
| Aortic Cross-Clamp time, min, median (IQR) | 72 (57–93) | 75 (60–99) | 70 (56–91) | <0.001 |
| Surgical procedure | ||||
| Aortic Surgery | 374 (47.7) | 105 (45.4) | 269 (48.8) | 0.007 |
| Mitral Surgery | 170 (21.7) | 48 (20.8) | 122 (22.1) | 0.007 |
| Mitral + Aortic Surgery | 115 (14.7) | 38 (16.5) | 77 (14.0) | 0.007 |
| Mitral + Tricuspid Surgery | 87 (11.1) | 24 (10.4) | 63 (11.4) | 0.007 |
| Mitral + Aortic + Tricuspid Surgery | 24 (3.1) | 11 (4.8) | 13 (2.4) | 0.007 |
| Tricuspid Surgery | 12 (1.5) | 5 (2.2) | 7 (1.3) | 0.007 |
| Red blood cell Transfusion | 94 (12.0) | 42 (18.2) | 52 (9.5) | <0.001 |
| Lactate, mg/dL | 25 (19–32) | 26 (21–33) | 24 (19–32) | <0.001 |
| DO2 indexed start CPB (mL min−1 m−2) | 315.6 (283.0–350.2) | 296.4 (330.6–271.3) | 322.2 (290.3–357.3) | <0.001 |
| DO2 indexed end CPB (mL min−1 m−2) | 316.8 (284.8–354.9) | 298.4 (272.1–330.8) | 323.3 (293.0–351.7) | <0.001 |
| Univariate Analysis | ||
|---|---|---|
| Variables | OR (95% CI) | p Value |
| Age, y | 1.06 (1.03–1.07) | <0.001 |
| CBP time, min | 1.01 (1.00–1.02) | <0.001 |
| Aortic Cross-Clamp time, min | 1.01 (1.01–1.02) | 0.001 |
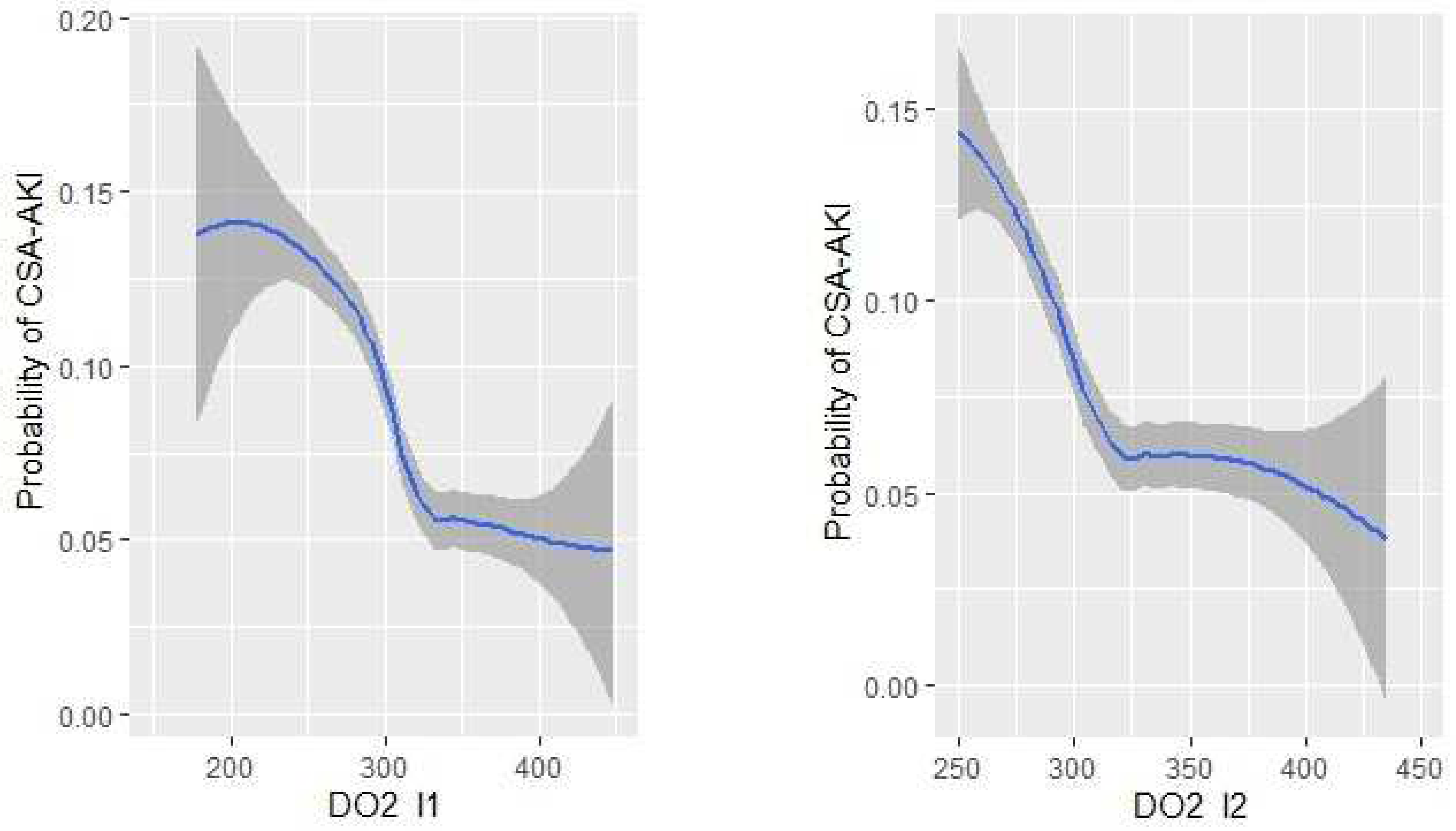
| DO2 indexed during CBP (mL/min/m2) | 0.99 (0.98–0.99) | <0.001 |
| DO2 indexed end CBP (mL/min/m2) | 0.99 (0.99–0.99) | 0.002 |
| Multivariable Analysis | ||
|---|---|---|
| Variables | OR (95% CI) | p Value |
| Age, y | 1.07 (1.03–1.11) | <0.001 |
| CBP time, min | 1.01 (1.01–1.02) | <0.001 |
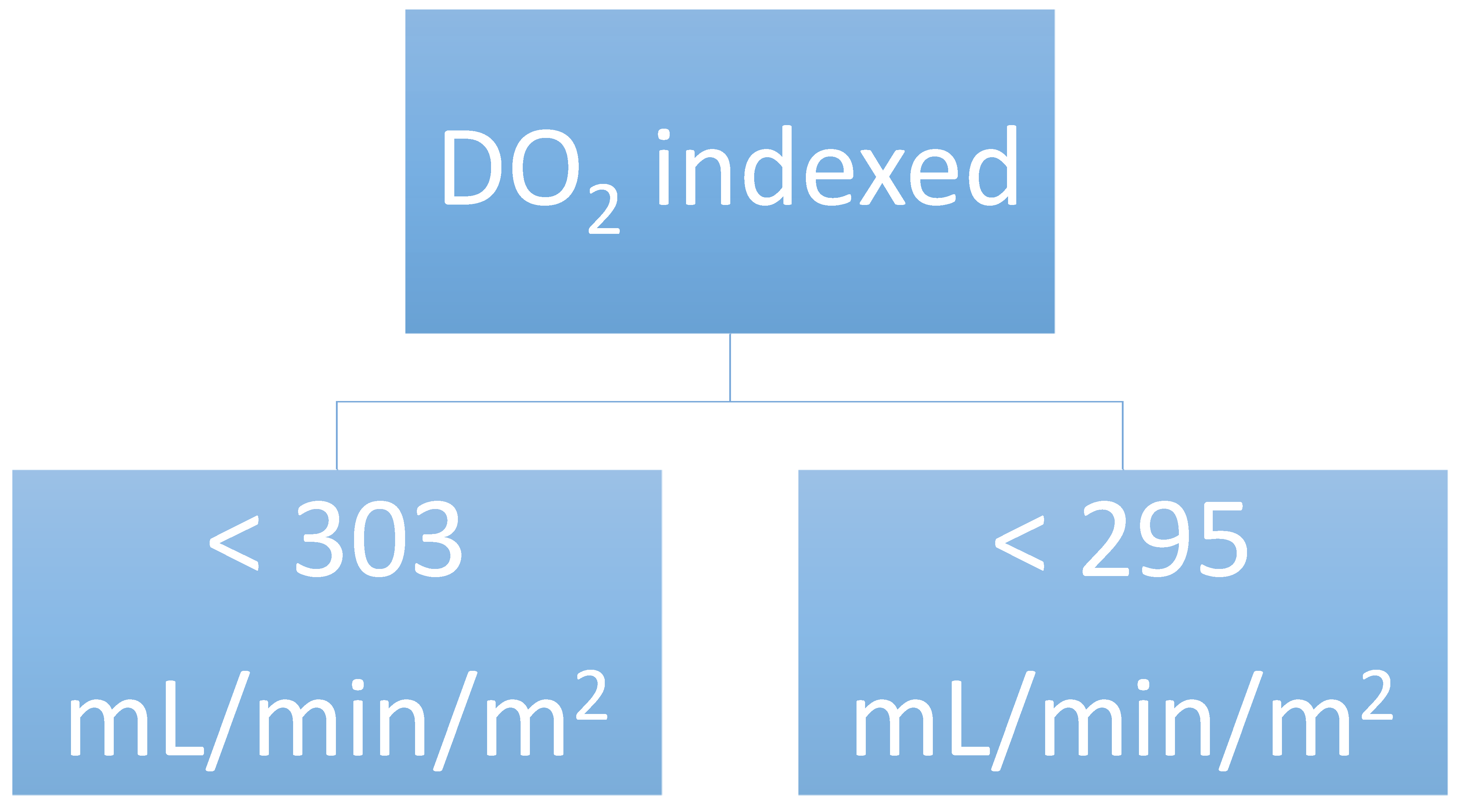
| DO2 indexed during CPB < 303 mL min−1 m−2 | 1.90 (1.12–3.24) | 0.018 |
| DO2 indexed end CPB < 295 mL min−1 m−2 | 1.94 (1.15–3.29) | 0.014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrasco-Serrano, E.; Jorge-Monjas, P.; Muñoz-Moreno, M.F.; Gómez-Sánchez, E.; Priede-Vimbela, J.M.; Bardají-Carrillo, M.; Cubero-Gallego, H.; Tamayo, E.; Ortega-Loubon, C. Impact of Oxygen Delivery on the Development of Acute Kidney Injury in Patients Undergoing Valve Heart Surgery. J. Clin. Med. 2022, 11, 3046. https://doi.org/10.3390/jcm11113046
Carrasco-Serrano E, Jorge-Monjas P, Muñoz-Moreno MF, Gómez-Sánchez E, Priede-Vimbela JM, Bardají-Carrillo M, Cubero-Gallego H, Tamayo E, Ortega-Loubon C. Impact of Oxygen Delivery on the Development of Acute Kidney Injury in Patients Undergoing Valve Heart Surgery. Journal of Clinical Medicine. 2022; 11(11):3046. https://doi.org/10.3390/jcm11113046
Chicago/Turabian StyleCarrasco-Serrano, Elena, Pablo Jorge-Monjas, María Fé Muñoz-Moreno, Esther Gómez-Sánchez, Juan Manuel Priede-Vimbela, Miguel Bardají-Carrillo, Héctor Cubero-Gallego, Eduardo Tamayo, and Christian Ortega-Loubon. 2022. "Impact of Oxygen Delivery on the Development of Acute Kidney Injury in Patients Undergoing Valve Heart Surgery" Journal of Clinical Medicine 11, no. 11: 3046. https://doi.org/10.3390/jcm11113046
APA StyleCarrasco-Serrano, E., Jorge-Monjas, P., Muñoz-Moreno, M. F., Gómez-Sánchez, E., Priede-Vimbela, J. M., Bardají-Carrillo, M., Cubero-Gallego, H., Tamayo, E., & Ortega-Loubon, C. (2022). Impact of Oxygen Delivery on the Development of Acute Kidney Injury in Patients Undergoing Valve Heart Surgery. Journal of Clinical Medicine, 11(11), 3046. https://doi.org/10.3390/jcm11113046







