Remote Assessment of Quality of Life and Functional Exercise Capacity in a Cohort of COVID-19 Patients One Year after Hospitalization (TELECOVID)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
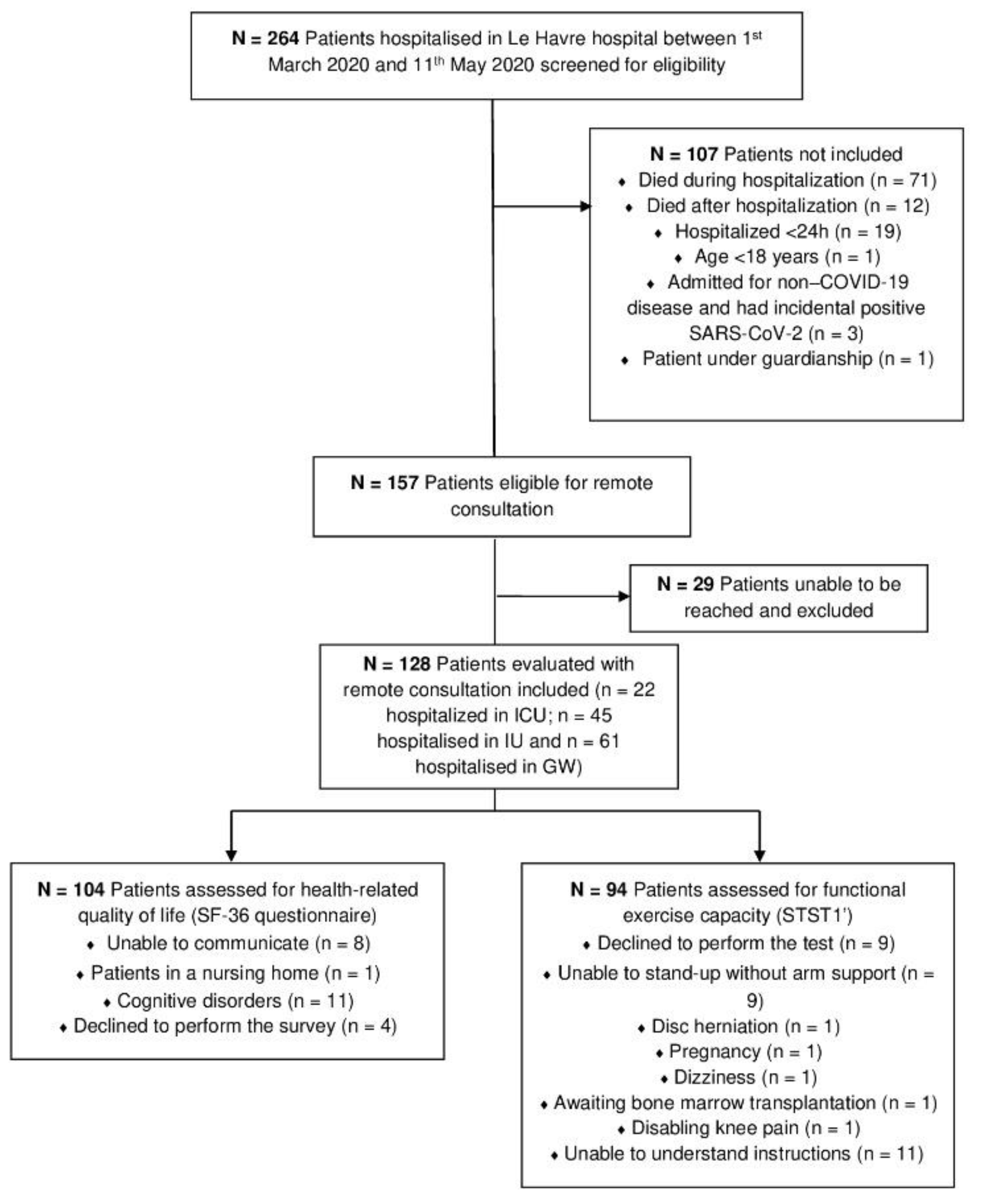
2.2. Participants
2.3. Data Extraction
2.4. Remote Assessment
2.5. Measurements and Outcomes
2.6. Statistical Analyses
3. Results
3.1. Study Population
3.2. Patients’ Characteristics
3.3. Remote Assessment
3.4. Results of the Logistic Regressions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, H.; Soe, M.M.; Konnor, R.; Dantes, R.; Haass, K.; Dudeck, M.A.; Gross, C.; Leaptrot, D.; Sapiano, M.R.P.; Allen-Bridson, K.; et al. Hospital capacities and shortages of healthcare resources among US hospitals during the coronavirus disease 2019 (COVID-19) pandemic, National Healthcare Safety Network (NHSN), March 27–July 14, 2020. Infect. Control Hosp. Epidemiol. 2021, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Salje, H.; Kiem, C.T.; Lefrancq, N.; Courtejoie, N.; Bosetti, P.; Paireau, J.; Andronico, A.; Hozé, N.; Hozé, J.; Dubost, C.L.; et al. Estimating the burden of SARS-CoV-2 in France. Science 2020, 369, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Pesenti, A.; Cecconi, M. Critical Care Utilization for the COVID-19 Outbreak in Lombardy, Italy: Early Experience and Forecast During an Emergency Response. JAMA 2020, 323, 1545–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, H.; Athar, S.; Harhara, T.; Elhag, S.A.; MElnour, S.; Sukkar, H.H.; Kamour, A.M. Post-infectious and post-acute sequelae of critically ill adults with COVID-19. PLoS ONE 2021, 16, e0252763. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.T.; Hamilton, F.W.; Milne, A.; Morley, A.J.; Viner, J.; Attwood, M.; Noel, A.; Gunning, S.; Hatrick, J.; Hamilton, S.; et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: Results from a prospective UK cohort. Thorax 2021, 76, 399–401. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Vaes, A.W.; Goërtz, Y.M.J.; Van Herck, M.; Machado, F.V.C.; Meys, R.; Delbressine, J.M.; Houben-Wilke, S.; Gaffron, S.; Maier, D.; Burtin, C.; et al. Recovery from COVID-19: A sprint or marathon? 6-month follow-up data from online long COVID-19 support group members. ERJ Open Res. 2021, 7, 00141–2021. [Google Scholar] [CrossRef]
- Romero-Duarte, Á.; Rivera-Izquierdo, M.; de Alba, I.G.-F.; Pérez-Contreras, M.; Fernández-Martínez, N.F.; Ruiz-Montero, R.; Serrano-Ortiz, A.; González-Serna, R.O.; Salcedo-Leal, I.; Jiménez-Mejías, E.; et al. Sequelae, persistent symptomatology and outcomes after COVID-19 hospitalization: The ANCOHVID multicentre 6-month follow-up study. BMC Med. 2021, 19, 129. [Google Scholar] [CrossRef]
- The Writing Committee for the COMEBAC Study Group; Morin, L.; Savale, L.; Pham, T.; Colle, R.; Figueiredo, S.; Harrois, A.; Harrois, M.; Lecoq, A.-L.; Meyrignac, O.; et al. Four-Month Clinical Status of a Cohort of Patients After Hospitalization for COVID-19. JAMA 2021, 325, 1525. [Google Scholar] [CrossRef] [PubMed]
- Monaghesh, E.; Hajizadeh, A. The role of telehealth during COVID-19 outbreak: A systematic review based on current evidence. BMC Public Health 2020, 20, 1193. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, E.M.; Jiménez, F.N.; Chen, K.; Davoodi, N.M.; Li, M.; Strauss, D.H.; Zou, M.; Guthrie, K.; Merchant, R.C. Telehealth was Beneficial during COVID-19 for Older Americans: A Qualitative Study with Physicians. J. Am. Geriatr. Soc. 2021, 69, 3034–3043. [Google Scholar] [CrossRef]
- Casariego-Vales, E.; Blanco-López, R.; Rosón-Calvo, B.; Suárez-Gil, R.; Santos-Guerra, F.; Dobao-Feijoo, M.; Ares-Rico, R.; Bal-Alvaredo, M.; On Behalf of The Telea-COVID Lugo Comanagement Team. Efficacy of Telemedicine and Telemonitoring in At-Home Monitoring of Patients with COVID-19. JCM 2021, 10, 2893. [Google Scholar] [CrossRef] [PubMed]
- Cox, N.S.; Scrivener, K.; Holland, A.E.; Jolliffe, L.; Wighton, A.; Nelson, S.; McCredie, L.; Lannin, N.A. A Brief Intervention to Support Implementation of Telerehabilitation by Community Rehabilitation Services During COVID-19: A Feasibility Study. Arch. Phys. Med. Rehabil. 2021, 102, 789–795. [Google Scholar] [CrossRef]
- Martin, I.; Braem, F.; Baudet, L.; Poncin, W.; Fizaine, S.; Aboubakar, F.; Froidure, A.; Pilette, C.; Liistro, G.; De Greef, J.; et al. Follow-up of functional exercise capacity in patients with COVID-19: It is improved by telerehabilitation. Respir. Med. 2021, 183, 106438. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef] [Green Version]
- Katz, S. Assessing Self-maintenance: Activities of Daily Living, Mobility, and Instrumental Activities of Daily Living. J. Am. Geriatr. Soc. 1983, 31, 721–727. [Google Scholar] [CrossRef]
- Rockwood, K. A global clinical measure of fitness and frailty in elderly people. Can. Med. Assoc. J. 2005, 173, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Darvall, J.N.; Bellomo, R.; Paul, E.; Bailey, M.; Young, P.J.; Reid, A.; Rockwood, K.; Pilcher, D. Routine Frailty Screening in Critical Illness. Chest 2021, 160, 1292–1303. [Google Scholar] [CrossRef]
- Ware, J.E.; Sherbourne, C.D. The MOS 36-ltem Short-Form Health Survey (SF-36): I. Conceptual Framework and Item Selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Leplège, A.; Ecosse, E.; Verdier, A.; Perneger, T.V. The French SF-36 Health Survey. J. Clin. Epidemiol. 1998, 51, 1013–1023. [Google Scholar] [CrossRef]
- Mishra, G.D.; Hockey, R.; Dobson, A.J. A comparison of SF-36 summary measures of physical and mental health for women across the life course. Qual. Life Res. 2014, 23, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Ozalevli, S.; Ozden, A.; Itil, O.; Akkoclu, A. Comparison of the Sit-to-Stand Test with 6min walk test in patients with chronic obstructive pulmonary disease. Respir. Med. 2007, 101, 286–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strassmann, A.; Steurer-Stey, C.; Lana, K.D.; Zoller, M.; Turk, A.J.; Suter, P.; Puhan, M.A. Population-based reference values for the 1-min sit-to-stand test. Int. J. Public Health 2013, 58, 949–953. [Google Scholar] [CrossRef] [Green Version]
- Gelman, A. Scaling regression inputs by dividing by two standard deviations. Stat. Med. 2008, 27, 2865–2873. [Google Scholar] [CrossRef]
- Smondack, P.; Gravier, F.-É.; Prieur, G.; Repel, A.; Muir, J.-F.; Cuvelier, A.; Combret, Y.; Medrinal, C.; Bonnevie, T. Kinésithérapie et COVID-19: De la réanimation à la réhabilitation à domicile. Synthèse des recommandations internationales. Rev. Des Mal. Respir. 2020, 37, 811–822. [Google Scholar] [CrossRef]
- Paneroni, M.; Vitacca, M.; Bernocchi, P.; Bertacchini, L.; Scalvini, S. Feasibility of tele-rehabilitation in survivors of COVID-19 pneumonia. Pulmonology 2021. [Google Scholar] [CrossRef]
- Thomas, P.; Baldwin, C.; Bissett, B.; Boden, I.; Gosselink, R.; Granger, C.L.; Hodgson, C.; Jones, A.Y.M.; Kho, M.E.; Moses, R.; et al. Physiotherapy management for COVID-19 in the acute hospital setting: Clinical practice recommendations. J. Physiother. 2020, 66, 73–82. [Google Scholar] [CrossRef]
- Labenz, C.; Kremer, W.M.; Schattenberg, J.M.; Wörns, M.-A.; Toenges, G.; Weinmann, A.; Galle1, P.R.; Sprinzl, M.F. Clinical Frailty Scale for risk stratification in patients with SARS-CoV-2 infection. J. Investig. Med. 2020, 68, 1199–1202. [Google Scholar] [CrossRef]
- Andrés-Esteban, E.M.; Quintana-Diaz, M.; Ramírez-Cervantes, K.L.; Benayas-Peña, I.; Silva-Obregón, A.; Magallón-Botaya, R.; Santolalla-Arnedo, I.; Santolalla-Arnedo, R.; Santolalla-Arnedo, V. Outcomes of hospitalized patients with COVID-19 according to level of frailty. PeerJ 2021, 9, e11260. [Google Scholar] [CrossRef] [PubMed]
- Heesakkers, H.; van der Hoeven, J.G.; Corsten, S.; Janssen, I.; Ewalds, E.; Simons, K.S.; Westerhof, B.; Westerhof, T.C.D.; Jacobs, C.; van Santen, S.; et al. Clinical Outcomes among Patients with 1-Year Survival Following Intensive Care Unit Treatment for COVID-19. JAMA 2022. Available online: https://jamanetwork.com/journals/jama/fullarticle/2788504 (accessed on 28 January 2022).
- Covinsky, K.E.; Palmer, R.M.; Fortinsky, R.H.; Counsell, S.R.; Stewart, A.L.; Kresevic, D.; Burant, C.J.; Landefeld, C.S. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: Increased vulnerability with age. J. Am. Geriatr. Soc. 2003, 51, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Walle-Hansen, M.M.; Ranhoff, A.H.; Mellingsæter, M.; Wang-Hansen, M.S.; Myrstad, M. Health-related quality of life, functional decline, and long-term mortality in older patients following hospitalisation due to COVID-19. BMC Geriatr. 2021, 21, 199. [Google Scholar] [CrossRef] [PubMed]
- Paneroni, M.; Simonelli, C.; Saleri, M.; Bertacchini, L.; Venturelli, M.; Troosters, T.; Ambrosino, N.; Vitacca, M. Muscle Strength and Physical Performance in Patients Without Previous Disabilities Recovering From COVID-19 Pneumonia. Am. J. Phys. Med. Rehabil. 2021, 100, 105–109. [Google Scholar] [CrossRef]
- Medrinal, C.; Prieur, G.; Bonnevie, T.; Gravier, F.-E.; Mayard, D.; Desmalles, E.; Smondack, P.; Lamia, B.; Combret, Y.; Fossat, G. Muscle weakness, functional capacities and recovery for COVID-19 ICU survivors. BMC Anesth. 2021, 21, 64. [Google Scholar] [CrossRef]
- Vanhorebeek, I.; Latronico, N.; Van den Berghe, G. ICU-acquired weakness. Intensive Care Med. 2020, 46, 637–653. [Google Scholar] [CrossRef]
| Complete Study Sample (N = 128) | Patients Discharged from ICU (N = 22) | Patients Discharged from IU (N = 45) | Patients Discharged from GW (N = 61) | p-Value ‡ | |
|---|---|---|---|---|---|
| Sex F/M, n | 62/66 | 8/14 | 19/26 | 35/26 | 0.140 |
| Age (years), median (IQR) | 69 (20.8) | 64.5 (18) | 64 (23.5) | 72 (18.5) | 0.004 |
| BMI (kg.m2), median (IQR) | 27.6 (7.2) | 29.8 (7.2) | 27.7 (6.8) | 27.3 (7.3) | 0.057 |
| Working status | 0.046 | ||||
| Active, n (%) | 44 (34) | 8 (36) | 21 (47) | 15 (25) | |
| Retired, n (%) | 80 (63) | 14 (64) | 21 (47) | 45 (73) | |
| Unable to work, n (%) | 4 (3) | 0 (0) | 3 (6) | 1 (2) | |
| KATZ score at admission (0–6) *, median (IQR) | 6 (0) | 6 (0) | 6 (0) | 6 (2) | 0.003 |
| Clinical Frailty Scale at admission (1–9), median (IQR) | 2.5 (2) | 2 (1) | 3 (2.5) | 3 (4) | 0.017 |
| Comorbidities | |||||
| Chronic respiratory failure, n (%) | 16 (13) | 2 (9) | 7 (16) | 7 (11) | 0.713 |
| Chronic cardiac failure, n (%) | 70 (55) | 11 (50) | 21 (47) | 38 (62) | 0.248 |
| Diabetes, n (%) | 29 (23) | 9 (41) | 5 (11) | 15 (25) | 0.021 |
| Obesity, n (%) | 41 (32) | 11 (50) | 14 (31) | 16 (26) | 0.121 |
| Other, n (%) ** | 42 (32) | 7 (32) | 14 (31) | 21 (34) | 0.932 |
| Length of stay in highest-care ward admitted to (days), median (IQR) *** | 8 (7) | 11.5 (11) | 6 (5.5) | 9 (6.5) | 0.002 |
| Total hospital length of stay (days), median (IQR) | 9 (10.5) | 17.5 (9.5) | 7 (7) | 9 (6.5) | <0.001 |
| Respiratory support | |||||
| Low-flow oxygen, n (%) | 92 (72) | 21 (95) | 28 (62) | 43 (70) | 0.017 |
| High-flow oxygen, n (%) | 14 (11) | 13 (59) | 1 (2) | 0 (0) | <0.001 |
| CPAP/NIV, n (%) | 4 (3) | 4 (18) | 0 (0) | 0 (0) | <0.001 |
| Invasive MV, n (%) | 14 (11) | 14 (64) | 0 (0) | 0 (0) | <0.001 |
| Referral to the hospital rehabilitation unit, n (%) | 34 (27) | 5 (23) | 9 (20) | 20 (33) | 0.306 |
| Length of stay in rehabilitation unit (days), median (IQR) | 12 (7.5) | 10 (11.5) | 14 (11) | 12 (8) | 0.677 |
| KATZ score at discharge (0–6), median (IQR) | 6 (2) | 6 (2) | 6 (1) | 6 (4.5) | 0.074 |
| Clinical Frailty Scale at discharge (1–9), median (IQR) | 4 (3) | 4 (2) | 4 (2) | 5 (4) | 0.246 |
| Discharged home, n (%) | 117 (91) | 21 (95) | 43 (96) | 53 (87) | 0.220 |
| Home-based rehabilitation, n (%) | 15 (12) | 9 (41) | 2 (4) | 4 (7) | <0.001 |
| Number of rehabilitation sessions, median (IQR) | 20 (70) | 10 (22) | 70 (100) | 57.5 (122) | 0.140 |
| Discharged to other rehabilitation centers, n (%) | 11 (9) | 2 (9) | 2 (4) | 7 (11) | 0.441 |
| Length of stay in rehabilitation center (days), median (IQR) | 81 (110) | 72 (95) | 90 (260) | 28 (110) | 0.346 |
| Complete Study Sample (N = 128) | Patients Discharged from ICU (N = 22) | Patients Discharged from IU (N = 45) | Patients Discharged from GW (N = 61) | p-Value ‡ | |
|---|---|---|---|---|---|
| KATZ score at one year (0–6) *, median (IQR) | 6 (0) | 6 (0) | 6 (0) | 6 (5) | <0.001 |
| Clinical Frailty Scale at one year (0–6), median (IQR) | 3 (3) | 2 (1) | 3 (2) | 4 (5) | 0.006 |
| Number of STST1′ repetitions §, median (IQR) | 23 (22) | 29 (20) | 23 (18) | 20 (29) | 0.090 |
| Number of STST1′ repetitions § (%predicted value), median (IQR) | 67 (58) | 84 (35) | 61 (50) | 61 (83) | 0.089 |
| End STST1′ dyspnea § (Borg 0–10), median (IQR) | 3 (2) | 3 (2) | 4 (2) | 3 (2) | 0.211 |
| SF-36 sub scores ¶ | |||||
| Physical functioning (%), median (IQR) | 65 (35) | 68 (50) | 70 (38) | 65 (54) | 0.903 |
| Role limitations due to physical health (%), median (IQR) | 75 (75) | 62.5 (100) | 75 (75) | 63 (100) | 0.177 |
| Pain (%), median (IQR) | 78 (55) | 70 (53) | 80 (65) | 78 (55) | 0.837 |
| General Health (%), median (IQR) | 60 (35) | 65 (23) | 55 (36) | 50 (38) | 0.268 |
| Emotional well-being (%), median (IQR) | 72 (28) | 80 (17) | 74 (25) | 66 (32) | 0.081 |
| Role limitations due to emotional problems (%), median (IQR) | 100 (77) | 100 (100) | 100 (77) | 100 (77) | 0.879 |
| Vitality (%), median (IQR) | 50 (29) | 50 (23) | 50 (33) | 50 (24) | 0.975 |
| Social functioning (%), median (IQR) | 100 (38) | 100 (41) | 88 (50) | 100 (25) | 0.378 |
| Health change (%), median (IQR) | 50 (25) | 50 (31) | 50 (25) | 25 (25) | 0.061 |
| SF-36 Physical health component score(%), median (IQR)¶ | 65 (38) | 68 (46) | 68 (37) | 63 (49) | 0.789 |
| SF-36 Mental health component score (%), median (IQR)¶ | 77 (37) | 79 (41) | 69 (33) | 77 (39) | 0.884 |
| Returned to work at one year/active patients, n (%) | 34/49 (69) | 5/9 (56) | 18/24 (75) | 11/16 (69) | 0.557 |
| Poor Physical HRQOL (PCS < 50% Predicted Value) | Poor Mental HRQOL (MCS < 50% Predicted Value) | Poor Functional Exercise Capacity (STST1′ < 50% Predicted Value) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | Std OR (95%CI) | p-Value | OR (95%CI) | Std OR (95%CI) | p-Value | OR (95%CI) | Std OR (95%CI) | p-Value | |
| Age | 1.04 (1–1.07) | 1.74 (1.07–3) | 0.03 | 1.02 (0.99–1.06) | 1.40 (0.87–2.36) | 0.18 | 1.02 (0.99–1.06) | 1.42 (0.9–2.27) | 0.14 |
| Sex | 1.87 (0.8–4.56) | 1.87 (0.8–4.56) | 0.16 | 1.47 (0.61–3.62) | 1.47 (0.61–3.62) | 0.39 | 1.44 (0.62–3.36) | 1.44 (0.62–3.36) | 0.40 |
| BMI | 0.96 (0.89–1.03) | 0.77 (0.48–1.19) | 0.26 | 0.96 (0.88–1.03) | 0.77 (0.47–1.2) | 0.27 | 0.97 (0.91–1.04) | 0.85 (0.56–1.29) | 0.45 |
| KATZ at admission | 0.33 (0.02–0.85) | 0.14 (0–0.75) | 0.14 | 0.81 (0.44–1.51) | 0.70 (0.23–2.07) | 0.46 | - | ||
| CFS at admission | 1.95 (1.34–3.01) | 3.42 (1.71–7.6) | 0.001 | 1.32 (0.93–1.89) | 1.66 (0.87–3.21) | 0.12 | 1.93 (1.31–3.08) | 3.36 (1.64–7.92) | 0.002 |
| Chronic Cardiac Insufficiency | 2.69 (1.13–6.73) | 2.69 (1.13–6.73) | 0.03 | 1.41 (0.59–3.42) | 1.41 (0.59–3.42) | 0.44 | 2.04 (0.88–4.86) | 2.04 (0.88–4.86) | 0.10 |
| Chronic Respiratory Insufficiency | 1.21 (0.35–3.77) | 1.21 (0.35–3.77) | 0.75 | 2.03 (0.62–6.29) | 2.03 (0.62–6.29) | 0.22 | 2.18 (0.61–10.26) | 2.18 (0.61–10.26) | 0.26 |
| Diabetes | 2.20 (0.83–5.77) | 2.20 (0.83–5.77) | 0.11 | 2.10 (0.77–5.58) | 2.10 (0.77–5.58) | 0.14 | 1.65 (0.59–5.07) | 1.65 (0.59–5.07) | 0.35 |
| Obesity | 0.74 (0.29–1.8) | 0.74 (0.29–1.8) | 0.52 | 0.57 (0.2–1.46) | 0.57 (0.2–1.46) | 0.26 | 0.96 (0.39–2.41) | 0.96 (0.39–2.41) | 0.93 |
| Service | 0.97 (0.55–1.67) | 0.97 (0.55–1.67) | 0.90 | 0.91 (0.51–1.6) | 0.91 (0.51–1.6) | 0.76 | 0.71 (0.41–1.24) | 0.71 (0.41–1.24) | 0.23 |
| LOS in initial ward | 1.08 (1.01–1.16) | 2.37 (1.17–5.18) | 0.02 | 1.11 (1.04–1.2) | 3.21 (1.53–7.58) | 0.004 | 1.03 (0.96–1.11) | 1.36 (0.66–3.14) | 0.43 |
| Total hospital LOS | 1.04 (0.99–1.1) | 1.59 (0.86–2.99) | 0.14 | 1.06 (1.01–1.13) | 2.10 (1.12–4.15) | 0.03 | 0.99 (0.94–1.05) | 0.91 (0.49–1.75) | 0.78 |
| Referral to the hospital rehabilitation unit | 7.76 (2.8–23.45) | 7.76 (2.8–23.45) | <0.001 | 3.28 (1.2–9.05) | 3.28 (1.2–9.05) | 0.02 | 3.14 (1.04–11.73) | 3.14 (1.04–11.73) | 0.06 |
| Rehabilitation unit LOS | 1.16 (1.07–1.28) | 3.66 (1.81–8.62) | 0.001 | 1.11 (1.04–1.21) | 2.52 (1.35–5.16) | 0.01 | 1.15 (1.03–1.34) | 3.30 (1.34–12.83) | 0.03 |
| Oxygen requirement | 1.01 (0.4–2.75) | 1.01 (0.4–2.75) | 0.98 | 2.60 (0.88–9.6) | 2.60 (0.88–9.6) | 0.11 | 0.25 (0.07–0.75) | 0.25 (0.07–0.75) | 0.02 |
| High-flow oxygen requirement | 1.37 (0.39–4.36) | 1.37 (0.39–4.36) | 0.60 | 1.62 (0.46–5.2) | 1.62 (0.46–5.2) | 0.43 | 0.37 (0.1–1.26) | 0.37 (0.1–1.26) | 0.11 |
| Invasive MV | 0.93 (0.24–3.07) | 0.93 (0.24–3.07) | 0.91 | 1.62 (0.46–5.2) | 1.62 (0.46–5.2) | 0.43 | 0.45 (0.12–1.61) | 0.45 (0.12–1.61) | 0.21 |
| KATZ at discharge | 0.62 (0.43–0.85) | 0.38 (0.18–0.73) | 0.01 | 0.60 (0.41–0.83) | 0,35 (0.17–0.68) | 0.003 | 0.56 (0.29–0.84) | 0.30 (0.08–0.71) | 0.02 |
| CFS at discharge | 1.81 (1.29–2.64) | 2.76 (1.55–5.25) | 0.001 | 2.20 (1.51–3.36) | 3.84 (2.03–7.97) | <0.001 | 1.41 (1.03–1.97) | 1.79 (1.06–3.19) | 0.04 |
| Discharged Home | 0.19 (0.03–1.03) | 0.19 (0.03–1.03) | 0.06 | 0.34 (0.06–1.95) | 0.34 (0.06–1.95) | 0.21 | 0.83 (0.11–4.51) | 0.83 (0.11–4.51) | 0.84 |
| Home-based rehabilitation | 2.68 (0.77–9.34) | 2.68 (0.77–9.34) | 0.11 | 3.18 (0.91–11.17) | 3.18 (0.91–11.17) | 0.06 | 0.99 (0.23–5.07) | 0.99 (0.23–5.07) | 0.99 |
| Number of rehabilitation sessions | 1.01 (1–1.04) | 1,39 (0.91–2.57) | 0.17 | 1.02 (1–1.04) | 1.44 (0.94–2.71) | 0.14 | 1.05 (1–1.36) | 3,08 (0.92–1114) | 0.48 |
| Discharged to other Rehabilitation Center | 8.52 (1.83–60.8) | 8.52 (1.83–60.8) | 0.01 | 3.00 (0.66–13.59) | 3.00 (0.66–13.59) | 0.14 | 0.99 (0.23–5.07) | 0.99 (0.23–5.07) | 0.99 |
| Rehabilitation Center stay length | 1.02 (1–1.05) | 1.97 (1.04–5.33) | 0.07 | 1.02 (1–1.05) | 1.99 (1.06–5.09) | 0.05 | 1.00 (0.98–1.03) | 1.14 (0.57–3.05) | 0.73 |
| Poor Physical HRQOL (PCS < 50% Predicted Value) | Poor Mental HRQOL (MCS < 50% Predicted Value) | Poor Functional Exercise Capacity (STST1′ < 50% Predicted Value) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Std OR | (95% CI) | p-Value | Std OR | (95% CI) | p-Value | Std OR | (95% CI) | p-Value | |
| Age | 0.87 | (0.45–1.69) | 0.68 | - | - | ||||
| CFS at admission | 2.10 | (0.86–5.65) | 0.12 | - | 3.64 | (1.39–10.72) | 0.01 | ||
| Chronic Cardiac Insufficiency | 1.85 | (0.55–6.48) | 0.32 | - | - | ||||
| Service LOS | 0.98 | (0.36–2.58) | 0.96 | 1.66 | (0.23–1.7) | 0.26 | - | ||
| Referral to the hospital rehabilitation unit | 2.09 | (0.24–16.59) | 0.48 | 0.35 | (0.7–4.27) | 0.40 | - | ||
| Rehabilitation unit LOS | 1.48 | (0.45–6.15) | 0.53 | 2.50 | (0.68–15.65) | 0.24 | 2.33 | (0.9–10.95) | 0.17 |
| Oxygen Requirement | - | - | 0.20 | (0.05–0.68) | 0.02 | ||||
| KATZ at discharge | 0.75 | (0.25–2.11) | 0.60 | 0.92 | (0.36–2.32) | 0.86 | 0.36 | (0.07–1.21) | 0.14 |
| CFS at discharge | 1.34 | (0.49–3.82) | 0.58 | 2.81 | (1.17–7.45) | 0.03 | 0.67 | (0.26–1.66) | 0.39 |
| Discharged to other rehabilitation Center | 4.07 | (0.56–38.37) | 0.18 | - | - | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Combret, Y.; Kerné, G.; Pholoppe, F.; Tonneville, B.; Plate, L.; Marques, M.-H.; Brunel, H.; Prieur, G.; Medrinal, C. Remote Assessment of Quality of Life and Functional Exercise Capacity in a Cohort of COVID-19 Patients One Year after Hospitalization (TELECOVID). J. Clin. Med. 2022, 11, 905. https://doi.org/10.3390/jcm11040905
Combret Y, Kerné G, Pholoppe F, Tonneville B, Plate L, Marques M-H, Brunel H, Prieur G, Medrinal C. Remote Assessment of Quality of Life and Functional Exercise Capacity in a Cohort of COVID-19 Patients One Year after Hospitalization (TELECOVID). Journal of Clinical Medicine. 2022; 11(4):905. https://doi.org/10.3390/jcm11040905
Chicago/Turabian StyleCombret, Yann, Geoffrey Kerné, Flore Pholoppe, Benjamin Tonneville, Laure Plate, Marie-Hélène Marques, Helena Brunel, Guillaume Prieur, and Clément Medrinal. 2022. "Remote Assessment of Quality of Life and Functional Exercise Capacity in a Cohort of COVID-19 Patients One Year after Hospitalization (TELECOVID)" Journal of Clinical Medicine 11, no. 4: 905. https://doi.org/10.3390/jcm11040905
APA StyleCombret, Y., Kerné, G., Pholoppe, F., Tonneville, B., Plate, L., Marques, M.-H., Brunel, H., Prieur, G., & Medrinal, C. (2022). Remote Assessment of Quality of Life and Functional Exercise Capacity in a Cohort of COVID-19 Patients One Year after Hospitalization (TELECOVID). Journal of Clinical Medicine, 11(4), 905. https://doi.org/10.3390/jcm11040905







