Association between Right Ventricular Function and Exercise Capacity in Patients with Chronic Heart Failure
Abstract
:1. Background
2. Methods
2.1. Patient Selection
2.2. Cardiopulmonary Exercise Test
2.3. Transthoracic Echocardiography
2.4. Cardiac Rehabilitation
2.5. Statistical Analysis
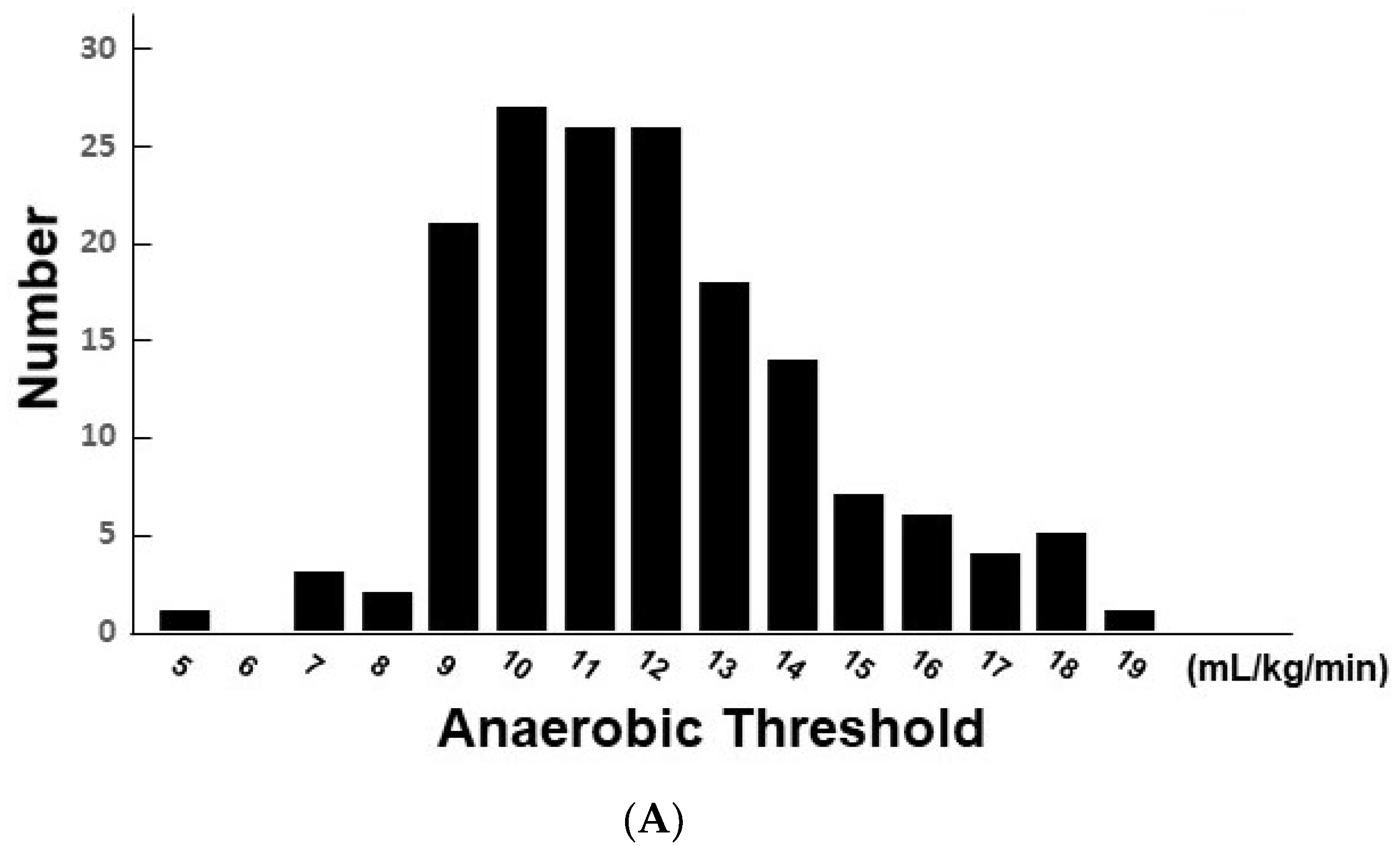
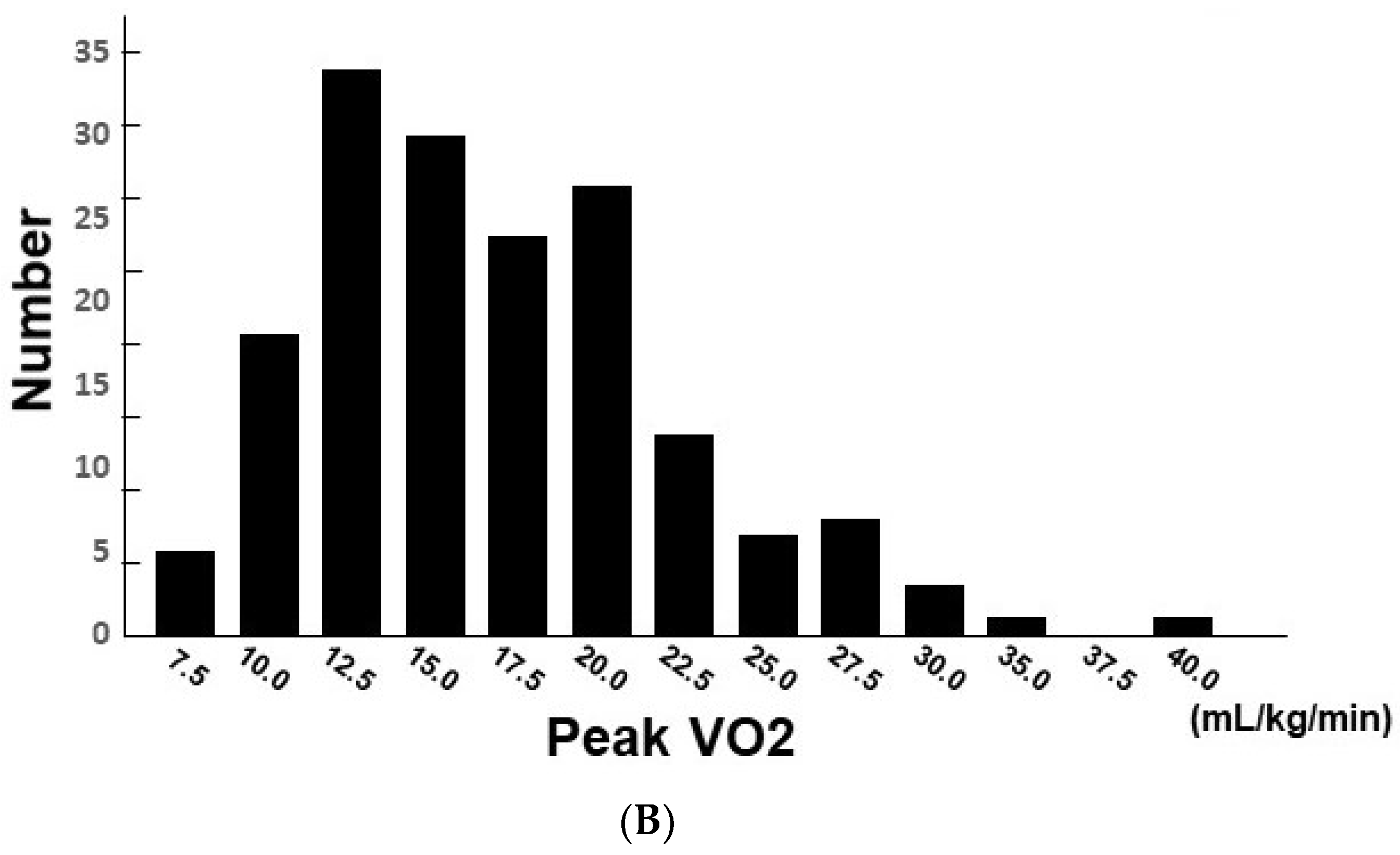
3. Results
3.1. Baseline Characteristics
3.2. Association between Clinical Parameters and AT
3.3. Association between Clinical Parameters and PVO2
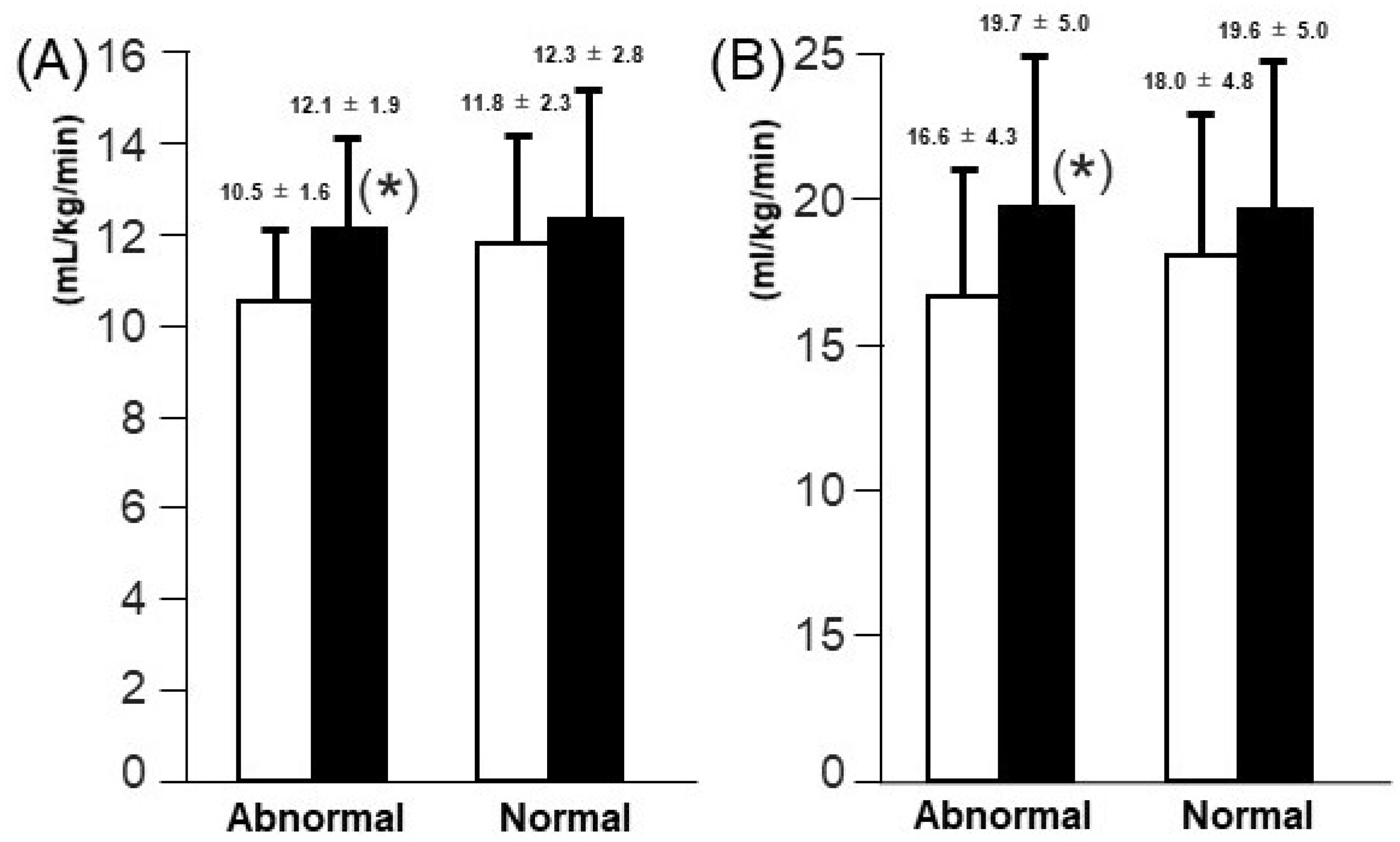
3.4. Comparison of Changes in AT and PVO2 Levels According to Initial TAPSE Levels
4. Discussion
4.1. Association between Right Ventricular Function and Exercise Capacity
4.2. Impact of Cardiac Rehabilitation for Those with Impaired Right Ventricular Function
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gardin, J.M.; Leifer, E.S.; Fleg, J.L.; Whellan, D.; Kokkinos, P.; LeBlanc, M.-H.; Wolfel, E.; Kitzman, D.W. Relationship of Doppler-echocardiographic left ventricular diastolic function to exercise performance in systolic heart failure: The HF-ACTION study. Am. Heart J. 2009, 158 (Suppl. 4), S45–S52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Di Franco, A.; Seoane, T.; Srinivasan, A.; Kampaktsis, P.N.; Geevarghese, A.; Goldburg, S.R.; Khan, S.A.; Szulc, M.; Ratcliffe, M.B.; et al. Right ventricular dysfunction impairs effort tolerance independent of left ventricular function among patients undergoing exercise stress myocardial perfusion imaging. Circ. Cardiovasc. Imaging. 2016, 9, e005115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legris, V.; Thibault, B.; Dupuis, J.; White, M.; Asgar, A.W.; Fortier, A.; Pitre, C.; Bouabdallaoui, N.; Henri, C.; O’Meara, E.; et al. Right ventricular function and its coupling to pulmonary circulation predicts exercise tolerance in systolic heart failure. ESC Heart Fail. 2021, 9, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Inoue, K.; Higashi, H.; Akazawa, Y.; Sasaki, Y.; Fujii, A.; Uetani, T.; Inaba, S.; Aono, J.; Nagai, T.; et al. Impact of right ventricular contractile reserve during low-load exercise on exercise intolerance in heart failure. ESC Heart Fail. 2020, 7, 3810–3820. [Google Scholar] [CrossRef] [PubMed]
- Gorter, T.M.; Obokata, M.; Reddy, Y.N.V.; Melenovsky, V.; A Borlaug, B. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur. Heart J. 2018, 39, 2825–2835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borlaug, B.A.; Kane, G.C.; Melenovsky, V.; Olson, T.P. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur. Heart J. 2016, 37, 3293–3302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balady, G.J.; Arena, R.; Sietsema, K.; Myers, J.; Coke, L.; Fletcher, G.F.; Forman, D.; Franklin, B.; Guazzi, M.; Gulati, M.; et al. Clinician’s guide to cardiopulmonary exercise testing in adults: A scientific statement from the American heart association. Circulation 2010, 122, 191–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. A new method for detecting anaerobic threshold by gas exchange. J. Appl. Physiol. 1986, 60, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American society of echocardiography endorsed by the European association of echocardiography, a registered branch of the European society of cardiology, and the Canadian society of echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713; quiz 86-8. [Google Scholar] [PubMed]
- JCS Joint Working Group. Guidelines for rehabilitation in patients with cardiovascular disease (JCS 2012). Circ. J. 2014, 78, 2022–2093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tajima, M.; Nakayama, A.; Uewaki, R.; Mahara, K.; Isobe, M.; Nagayama, M. Right ventricular dysfunction is associated with exercise intolerance and poor prognosis in ischemic heart disease. Heart Vessel. 2019, 34, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Bandera, F.; Generati, G.; Alfonzetti, E.; Bussadori, C.; Guazzi, M. Left atrial function dynamics during exercise in heart failure: Pathophysiological implications on the right heart and exercise ventilation inefficiency. JACC Cardiovasc. Imaging 2017, 10 Pt B, 1253–1264. [Google Scholar] [CrossRef]
- Akiyama, E.; Cinotti, R.; Čerlinskaitė, K.; Van Aelst, L.N.; Arrigo, M.; Plácido, R.; Chouihed, T.; Girerd, N.; Zannad, F.; Rossignol, P.; et al. Improved cardiac and venous pressures during hospital stay in patients with acute heart failure: An echocardiography and biomarkers study. ESC Heart Fail. 2020, 7, 996–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghio, S.; Gavazzi, A.; Campana, C.; Inserra, C.; Klersy, C.; Sebastiani, R.; Arbustini, E.; Recusani, F.; Tavazzi, L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J. Am. Coll. Cardiol. 2001, 37, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Kusunose, K.; Popovic, Z.B.; Motoki, H.; Marwick, T.H. Prognostic significance of exercise-induced right ventricular dysfunction in asymptomatic degenerative mitral regurgitation. Circ. Cardiovasc. Imaging 2013, 6, 167–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braith, R.W.; Welsch, M.A.; Feigenbaum, M.S.; Kluess, H.A.; Pepine, C.J. Neuroendocrine activation in heart failure is modified by endurance exercise training. J. Am. Coll. Cardiol. 1999, 34, 1170–1175. [Google Scholar] [CrossRef] [Green Version]
- Hambrecht, R.; Fiehn, E.; Weigl, C.; Gielen, S.; Hamann, C.; Kaiser, R.; Yu, J.; Adams, V.; Niebauer, J.; Schuler, G. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation 1998, 98, 2709–2715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haddad, F.; Hunt, S.A.; Rosenthal, D.N.; Murphy, D.J. Right ventricular function in cardiovascular disease, part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 2008, 117, 1436–1448. [Google Scholar] [CrossRef] [PubMed]
- Kind, T.; Mauritz, G.J.; Marcus, J.T.; van de Veerdonk, M.; Westerhof, N.; Vonk-Noordegraaf, A. Right ventricular ejection fraction is better reflected by transverse rather than longitudinal wall motion in pulmonary hypertension. J. Cardiovasc. Magn. Reson. 2010, 12, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Total (n = 169) | |
|---|---|
| Demographics | |
| Men | 126 (74.6%) |
| Age (years) | 70.3 ± 11.7 |
| Hypertension | 133 (78.7%) |
| Diabetes mellitus | 57 (33.7%) |
| Dyslipidemia | 128 (75.7%) |
| Chronic kidney disease | 64 (37.9%) |
| Anemia | 62 (36.7%) |
| Current smoking | 34 (20.1%) |
| New York Heart Association functional class | 2.2 ± 0.5 |
| Plasma B-type natriuretic peptide (pg/mL) | 150.5 [54.0; 312.2] |
| Etiology | |
| Ischemic heart disease | 118 (69.8%) |
| Valvular disease | 12 (7.1%) |
| Cardiomyopathy | 22 (13.0%) |
| Arrhythmia | 4 (2.4%) |
| Congenital heart disease | 2 (1.2%) |
| Others | 11 (6.5%) |
| Echocardiography | |
| Left ventricular end-diastolic diameter (mm) | 51.0 ± 8.7 |
| Left ventricular ejection fraction (%) | 52.3 ± 16.9 |
| Tricuspid annular plane systolic excursion (mm) | 18.3 ± 4.9 |
| Transmitral E velocity (m/sec) | 0.77 ± 0.24 |
| E lateral e’ ratio | 9.8 ± 3.9 |
| Anaerobic Threshold Level | Peak Level | |
|---|---|---|
| Heart rate (bpm) | 94.3 ± 17.6 | 121.2 ± 27.9 |
| Systolic blood pressure (mmHg) | 143.6 ± 26.7 | 165.0 ± 34.5 |
| VO2 (mL/kg/min) | 11.3 ± 2.5 | 18.0 ± 5.7 |
| VCO2 (mL/kg/min) | 10.7 ± 2.6 | 21.2 ± 7.3 |
| VE (L/min) | 23.2 ± 5.5 | 49.0 ± 20.1 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Beta Value | p Value | Beta Value | p Value | |
| Age | −0.9171 | 0.01 * | −0.0648 | 0.02 |
| Hypertension | 0.0928 | 0.23 * | ||
| Diabetes mellitus | 0.1881 | 0.01 * | 0.3053 | 0.20 |
| Dyslipidemia | 0.0732 | 0.06 | ||
| Chronic kidney disease | 0.2065 | <0.01 * | 0.2818 | 0.20 |
| Anemia | 0.1458 | 0.04 * | 0.0877 | 0.72 |
| Smoking | −0.0026 | 0.97 | ||
| New York Heart Association functional class | 0.0534 | <0.01 * | −10.698 | 0.03 * |
| Left ventricular end-diastolic diameter | 0.0369 | 0.89 | ||
| Left ventricular ejection fraction | 0.1015 | 0.85 | ||
| Tricuspid annular plane systolic excursion | 0.6921 | <0.01 * | 0.1224 | 0.01 * |
| Transmitral E velocity | −0.0172 | 0.03 | −0.8610 | 0.47 |
| Lateral E/e’ ratio | −0.4069 | <0.01 * | −0.0479 | 0.51 |
| Plasma B-type natriuretic peptide | −21.048 | 0.27 | ||
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Beta Value | p Value | Beta Value | p Value | |
| Age | −10.985 | <0.01 * | −0.196 | <0.01 * |
| Hypertension | 0.008 | 0.82 | ||
| Diabetes mellitus | 0.108 | <0.01 * | 0.480 | 0.25 |
| Dyslipidemia | 0.041 | 0.21 | ||
| Chronic kidney disease | 0.034 | <0.01 * | 0.719 | 0.06 |
| Anemia | 0.166 | <0.01 * | 0.656 | 0.14 |
| Current smoking | −0.057 | 0.10 | ||
| New York Heart Association functional class | −0.017 | 0.01 * | −0.390 | 0.66 |
| Left ventricular end-diastolic diameter | 0.087 | 0.49 | ||
| Left ventricular ejection fraction | 0.061 | 0.80 | ||
| Tricuspid annular plane systolic excursion | 0.319 | <0.01 * | 0.231 | <0.01 * |
| Transmitral E velocity | −0.014 | <0.01 * | −2.331 | 0.28 |
| Lateral E/e’ ratio | −0.257 | <0.01 * | −0.073 | 0.57 |
| Plasma B-type natriuretic peptide | −13.800 | 0.11 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohara, K.; Imamura, T.; Ihori, H.; Chatani, K.; Nonomura, M.; Kameyama, T.; Inoue, H. Association between Right Ventricular Function and Exercise Capacity in Patients with Chronic Heart Failure. J. Clin. Med. 2022, 11, 1066. https://doi.org/10.3390/jcm11041066
Ohara K, Imamura T, Ihori H, Chatani K, Nonomura M, Kameyama T, Inoue H. Association between Right Ventricular Function and Exercise Capacity in Patients with Chronic Heart Failure. Journal of Clinical Medicine. 2022; 11(4):1066. https://doi.org/10.3390/jcm11041066
Chicago/Turabian StyleOhara, Kazumasa, Teruhiko Imamura, Hiroyuki Ihori, Kenichi Chatani, Makoto Nonomura, Tomoki Kameyama, and Hiroshi Inoue. 2022. "Association between Right Ventricular Function and Exercise Capacity in Patients with Chronic Heart Failure" Journal of Clinical Medicine 11, no. 4: 1066. https://doi.org/10.3390/jcm11041066
APA StyleOhara, K., Imamura, T., Ihori, H., Chatani, K., Nonomura, M., Kameyama, T., & Inoue, H. (2022). Association between Right Ventricular Function and Exercise Capacity in Patients with Chronic Heart Failure. Journal of Clinical Medicine, 11(4), 1066. https://doi.org/10.3390/jcm11041066







