Influence of Guided Tissue Regeneration Techniques on the Success Rate of Healing of Surgical Endodontic Treatment: A Systematic Review and Network Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Registration
2.2. Literature Search Process
2.3. Inclusion and Exclusion Criteria
2.4. Data Extraction
2.5. Risk of Bias
2.6. Data Synthesis and Statistical Analysis
3. Results
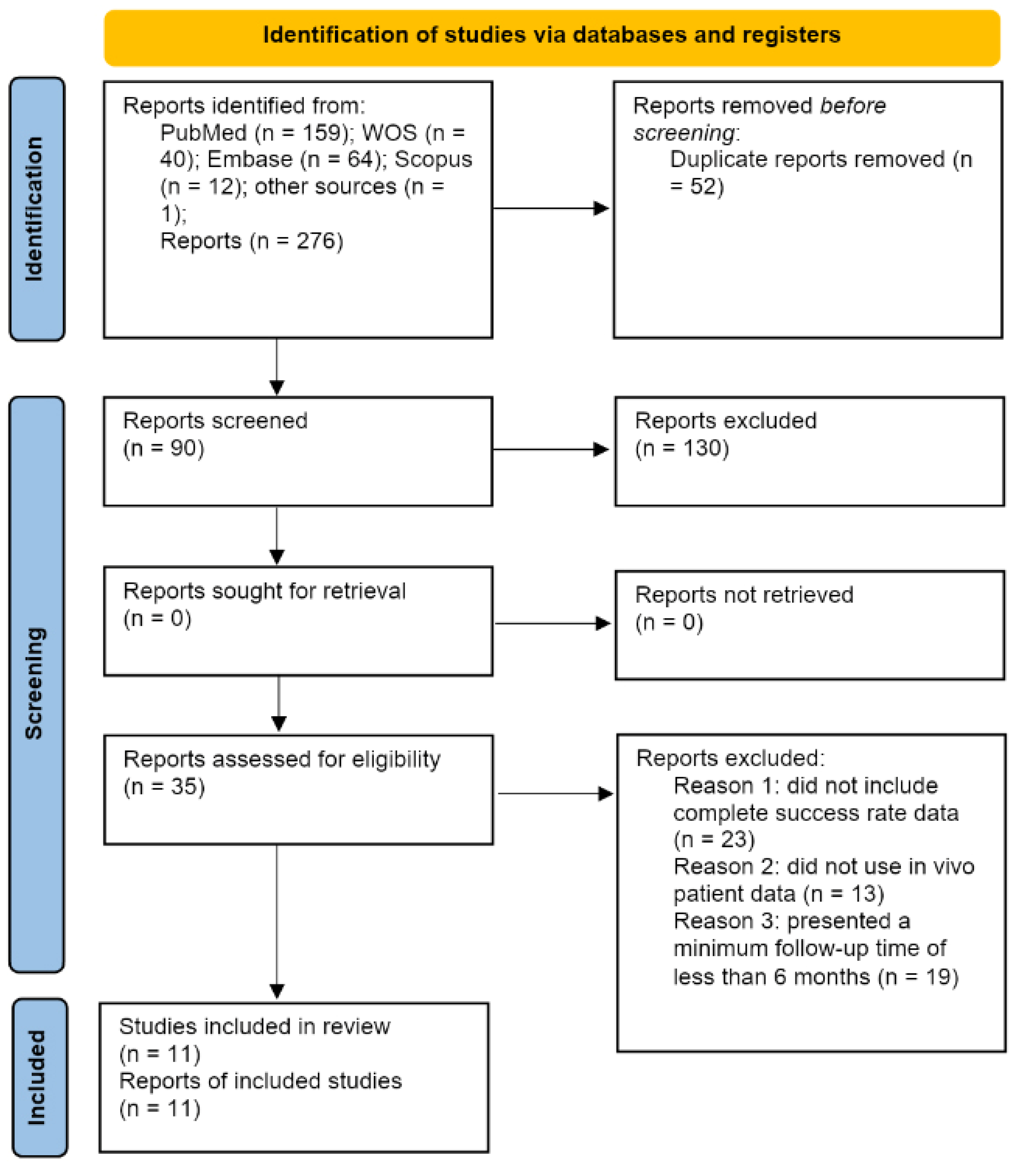
3.1. Results of the Search Process
3.2. Qualitative Analysis
3.3. Assessment of Risk of Bias
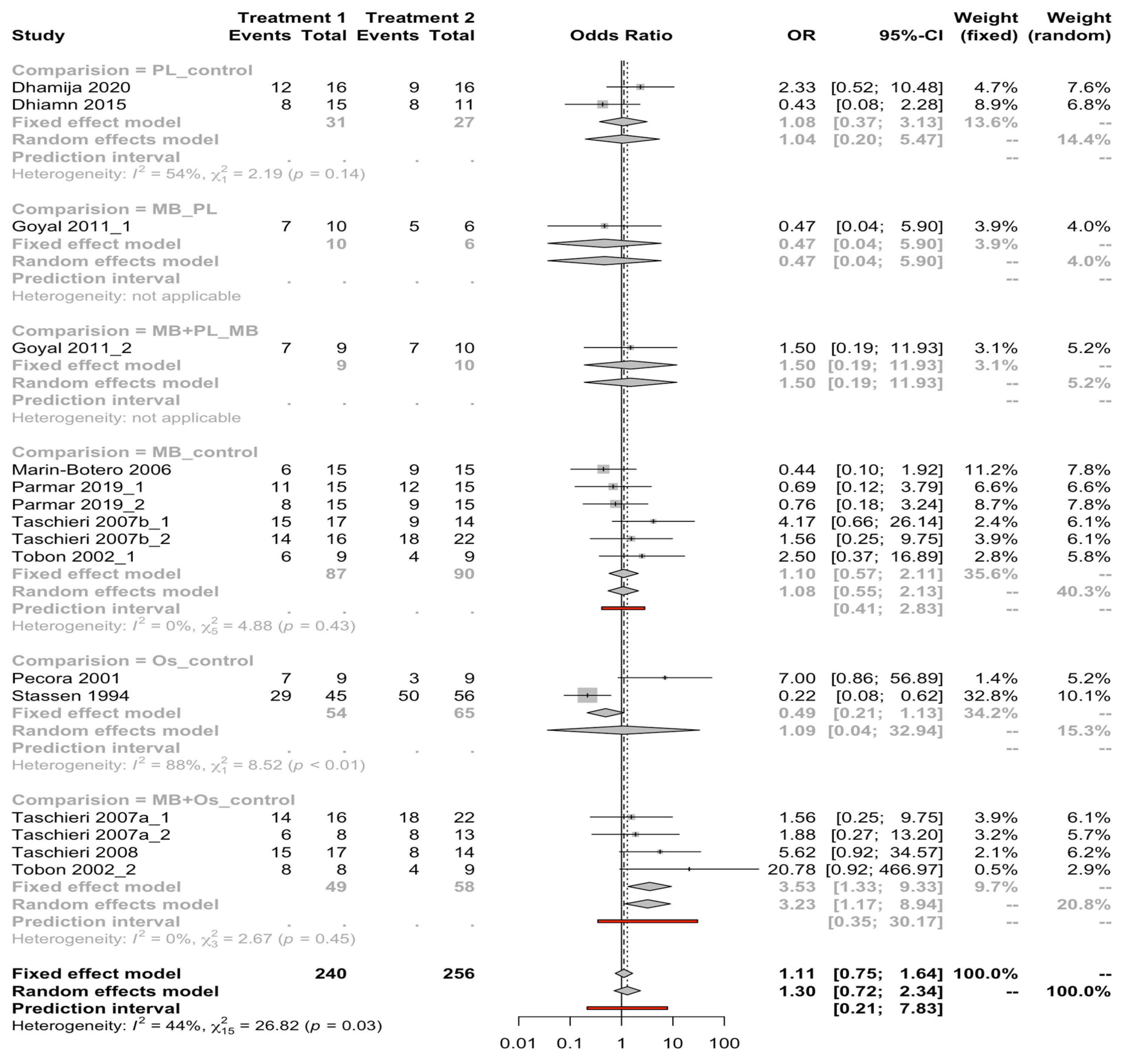
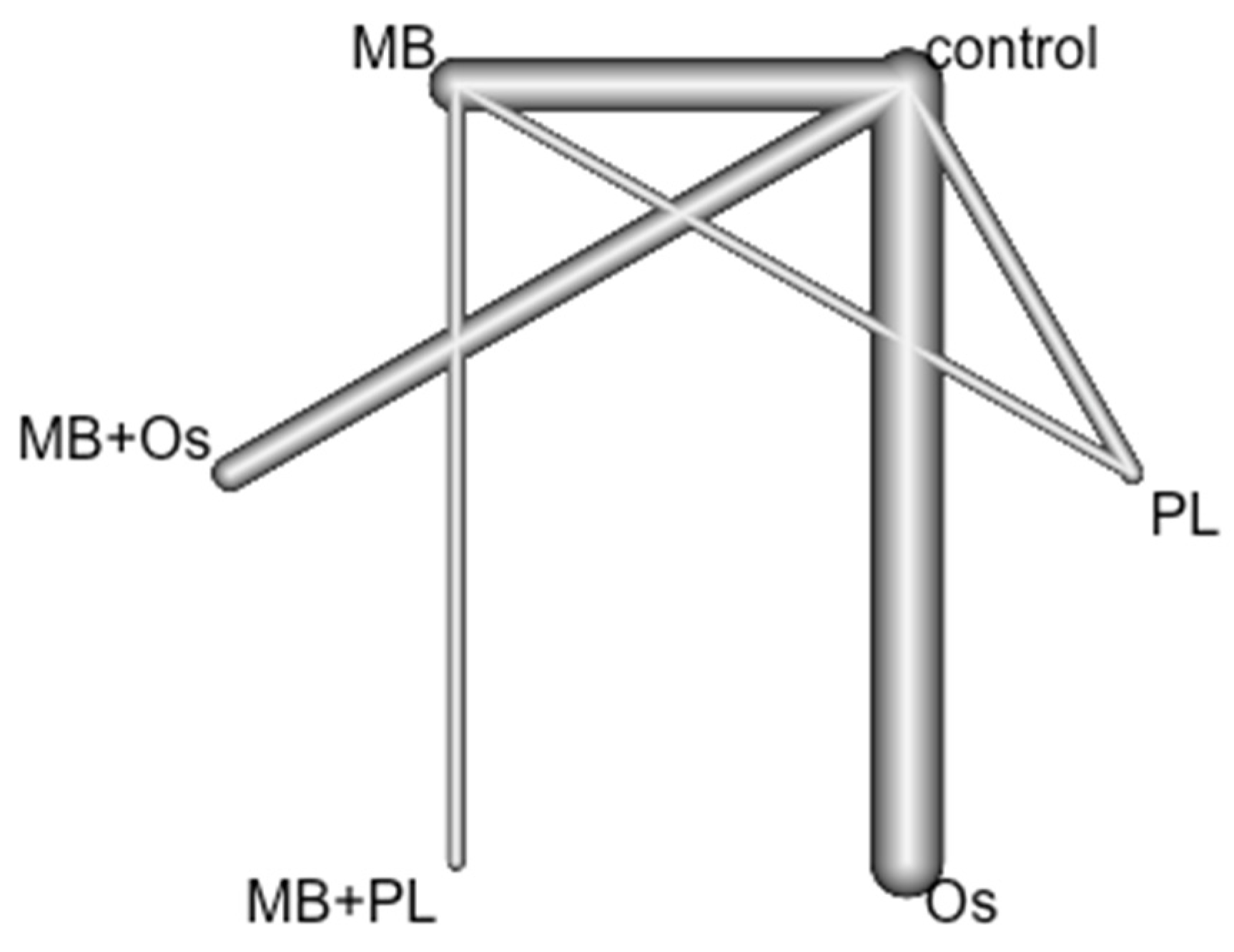
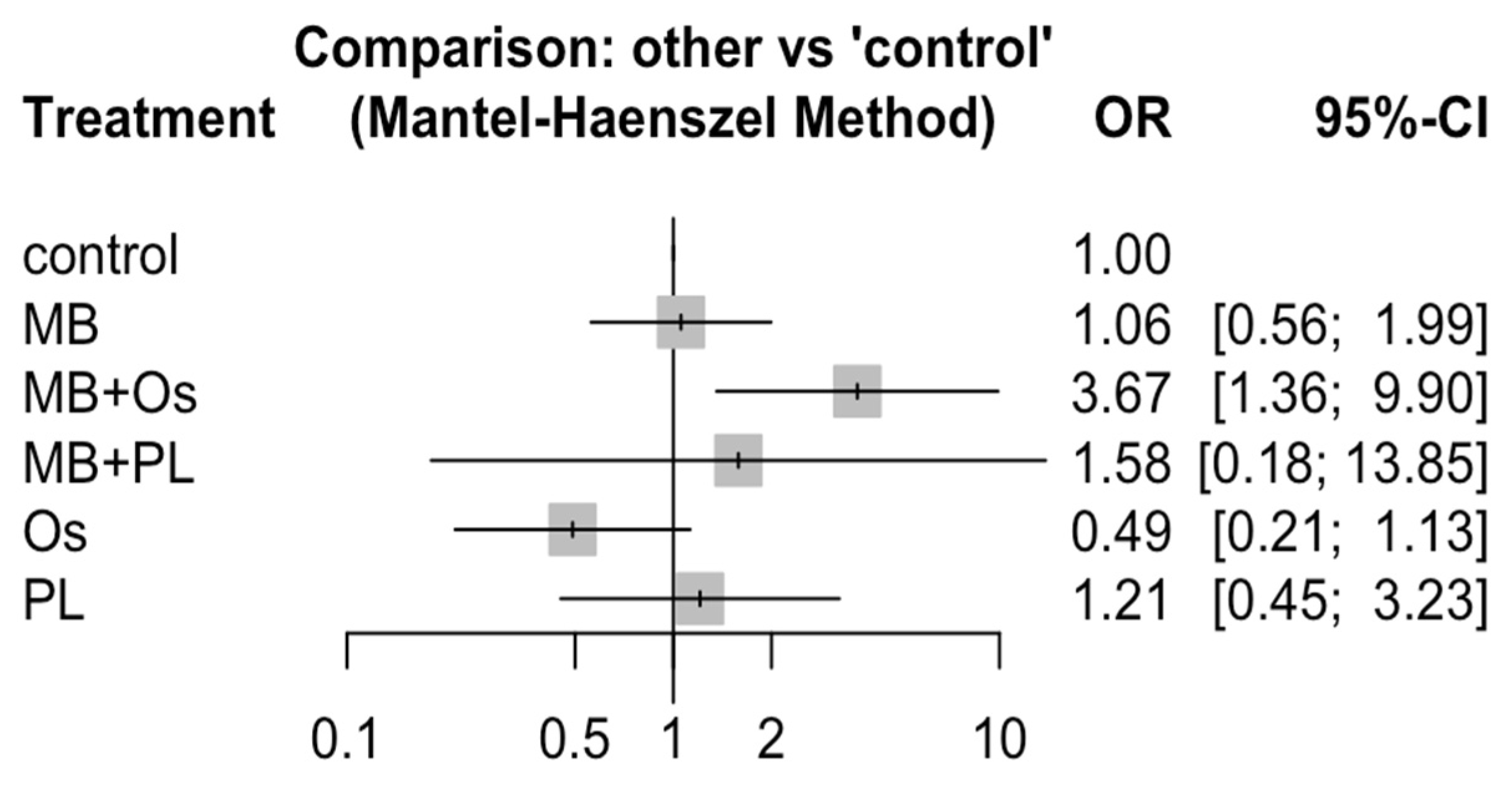
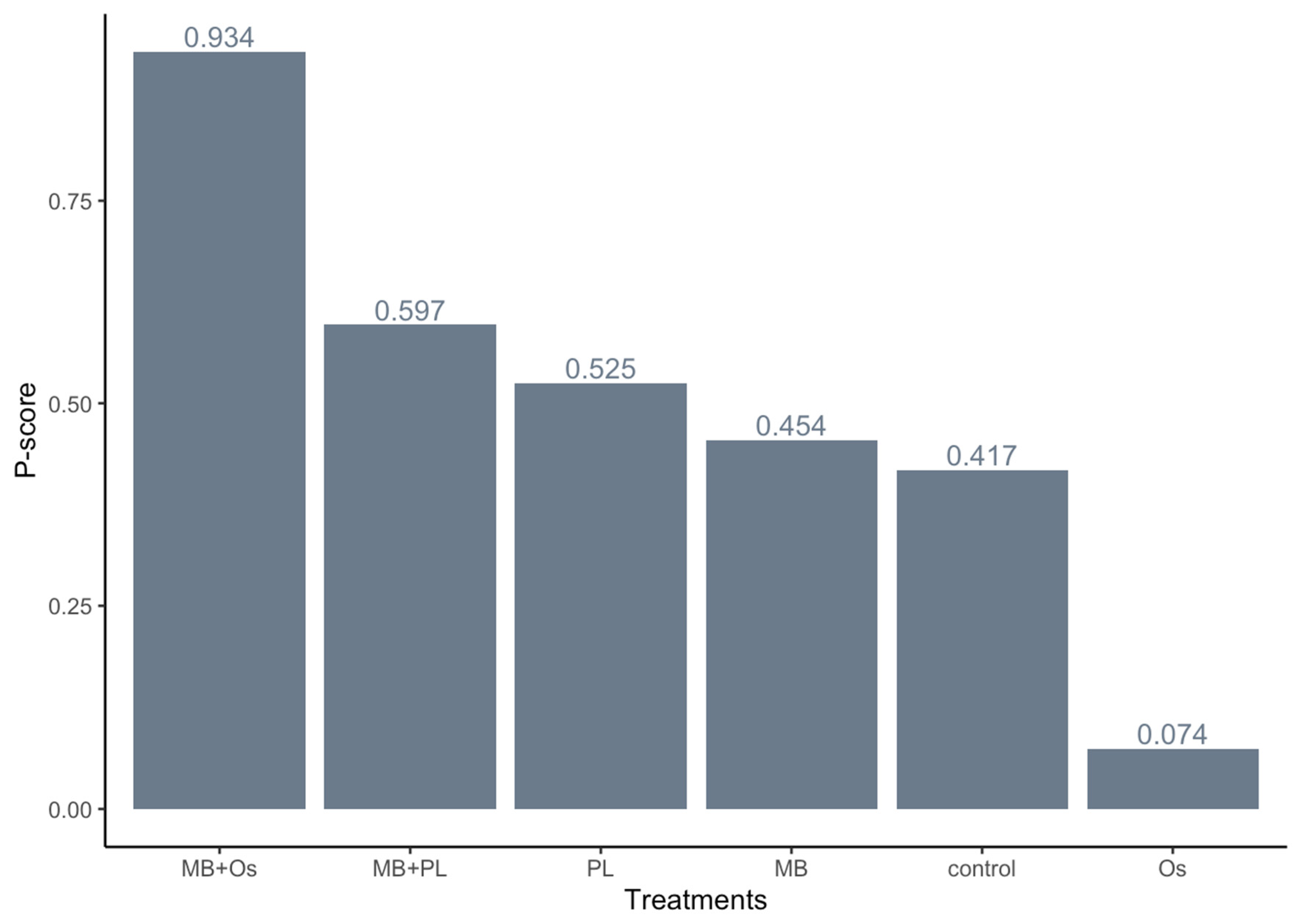
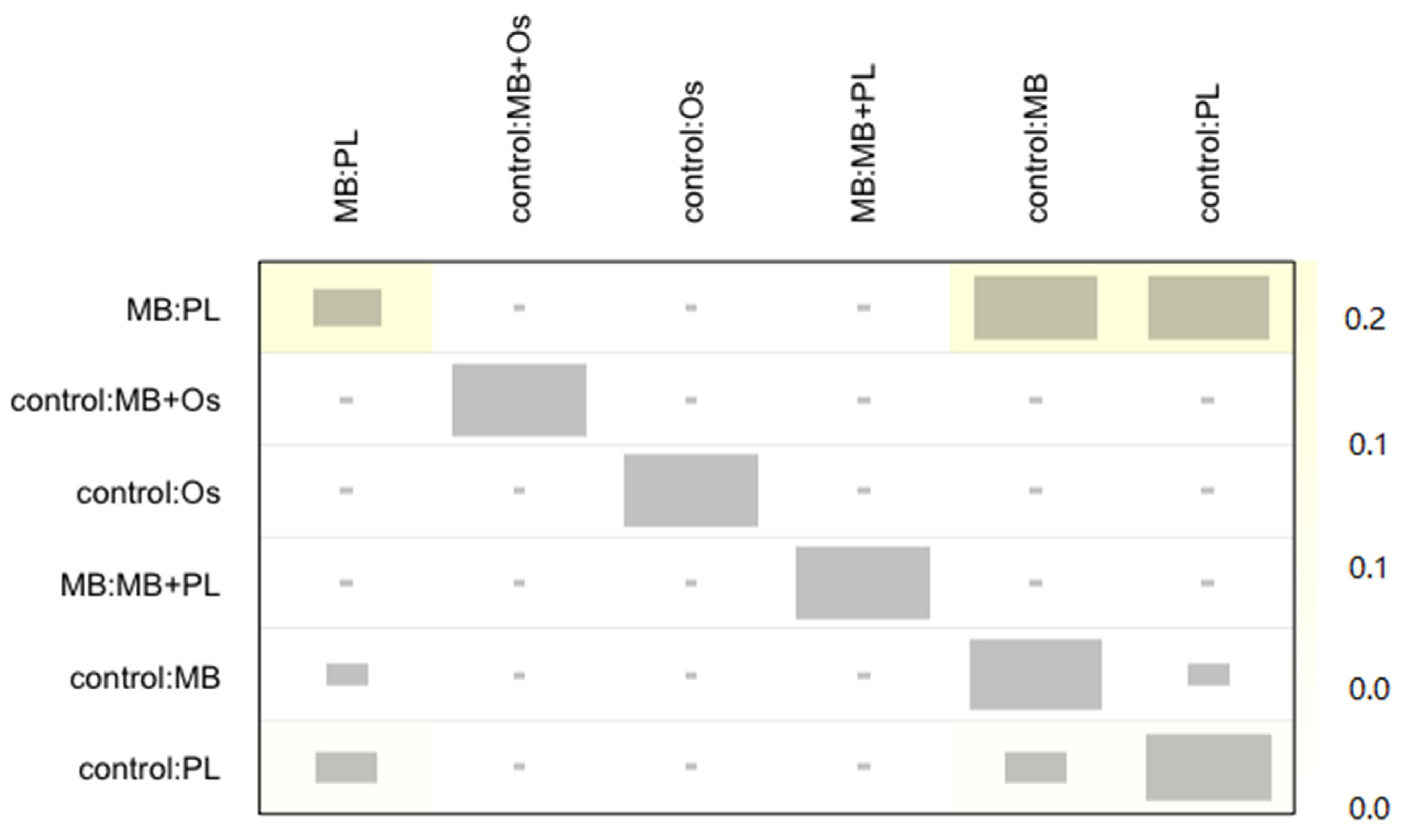
3.4. Quantitative Analysis Results
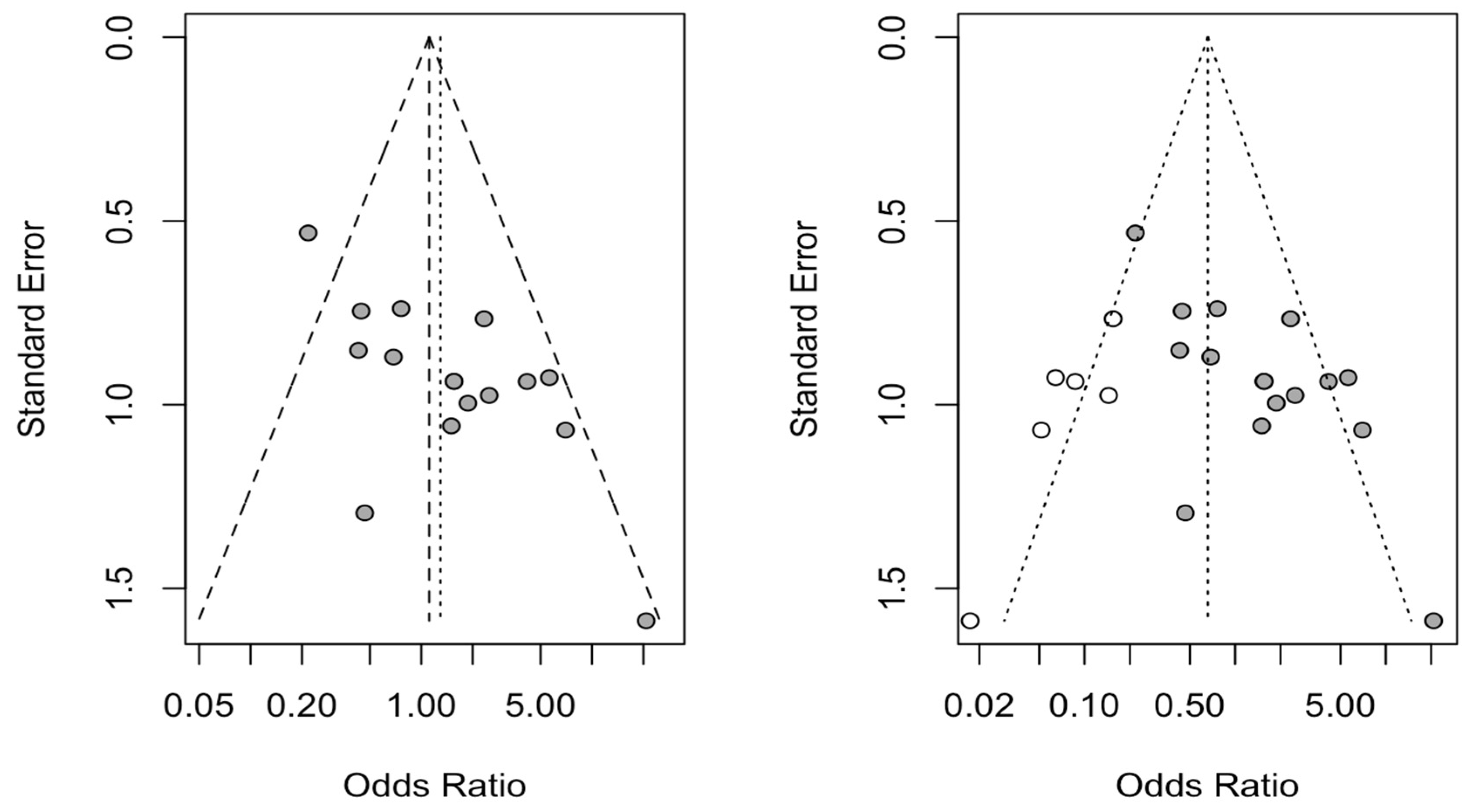
3.5. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siqueira, J.F., Jr.; Rôças, I.N. The microbiota of acute apical abscesses. J. Dent. Res. 2009, 88, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Kielbassa, A.M.; Frank, W.; Madaus, T. Radiologic assessment of quality of root canal fillings and periapical status in an Austrian subpopulation—An observational study. PLoS ONE 2017, 12, e0176724. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr. Aetiology of root canal treatment failure: Why well-treated teeth can fail. Int. Endod. J. 2001, 34, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sreedevi, P.; Varghese, N.; Varugheese, J.M. Prognosis of periapical surgery using bonegrafts: A clinical study. J. Conserv. Dent. 2011, 14, 68–72. [Google Scholar] [CrossRef]
- Chong, B.S.; Rhodes, J.S. Endodontic surgery. Br. Dent. J. 2014, 216, 281–290. [Google Scholar] [CrossRef]
- Tsesis, I.; Rosen, E.; Taschieri, S.; Telishevsky Strauss, Y.; Ceresoli, V.; Del Fabbro, M. Outcomes of surgical endodontic treatment performed by a modern technique: An updated meta-analysis of the literature. J. Endod. 2013, 39, 332–339. [Google Scholar] [CrossRef]
- Mastromihalis, N.; Goldstein, S.; Greenberg, M.; Friedman, S. Applications for guided bone regeneration in endodontic surgery. N. Y. State Dent. J. 1999, 65, 30–32. [Google Scholar]
- Kim, S.; Kratchman, S. Modern endodontic surgery concepts and practice: A review. J. Endod. 2006, 32, 601–623. [Google Scholar] [CrossRef]
- Corbella, S.; Taschieri, S.; Elkabbany, A.; Del Fabbro, M.; von Arx, T. Guided Tissue Regeneration Using a Barrier Membrane in Endodontic Surgery. Swiss Dent. J. 2016, 126, 13–25. [Google Scholar]
- Von Arx, T.; Cochran, D.L. Rationale for the application of the GTR principle using a barrier membrane in endodontic surgery: A proposal of classification and literature review. Int. J. Periodontics Restor. Dent. 2001, 21, 127–139. [Google Scholar]
- Tsesis, I.; Rosen, E.; Tamse, A.; Taschieri, S.; Del Fabbro, M. Effect of guided tissue regeneration on the outcome of surgical endodontic treatment: A systematic review and meta-analysis. J. Endod. 2011, 37, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Rouse, B.; Chaimani, A.; Li, T. Network meta-analysis: An introduction for clinicians. Intern. Emerg. Med. 2017, 12, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banzi, R.; Moja, L.; Liberati, A.; Gensini, G.F.; Gusinu, R.; Conti, A.A. Measuring the impact of evidence: The Cochrane systematic review of organised stroke care. Intern. Emerg. Med. 2009, 4, 507–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rud, J.; Andreasen, J.O.; Jensen, J.E. Radiographic criteria for the assessment of healing after endodontic surgery. Int. J. Oral Surg. 1972, 1, 195–214. [Google Scholar] [CrossRef]
- Molven, O.; Halse, A.; Grung, B. Observer strategy and the radiographic classification of healing after endodontic surgery. Int. J. Oral Maxillofac. Surg. 1987, 16, 432–439. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Dias, S.; Welton, N.J.; Caldwell, D.M.; Ades, A.E. Checking consistency in mixed treatment comparison meta-analysis. Stat. Med. 2010, 29, 932–944. [Google Scholar] [CrossRef]
- Freeman, S.C.; Fisher, D.; White, I.R.; Auperin, A.; Carpenter, J.R. Identifying inconsistency in network meta-analysis: Is the net heat plot a reliable method? Stat. Med. 2019, 38, 5547–5564. [Google Scholar] [CrossRef]
- Rücker, G.; Schwarzer, G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med. Res. Methodol. 2015, 15, 58. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.J.; Zhou, J.N.; Guo, L.H. Impact of different regenerative techniques and materials on the healing outcome of endodontic surgery: A systematic review and meta-analysis. Int. Endod. J. 2021, 54, 536–555. [Google Scholar] [CrossRef]
- Dhamija, R.; Tewari, S.; Sangwan, P.; Duhan, J.; Mittal, S. Impact of Platelet-rich Plasma in the Healing of Through-and-through Periapical Lesions Using 2-dimensional and 3-dimensional Evaluation: A Randomized Controlled Trial. J. Endod. 2020, 46, 1167–1184. [Google Scholar] [CrossRef] [PubMed]
- Stassen, L.F.; Hislop, W.S.; Still, D.M.; Moos, K.F. Use of anorganic bone in periapical defects following apical surgery--a prospective trial. Br. J. Oral Maxillofac. Surg. 1994, 32, 83–85. [Google Scholar] [CrossRef]
- Pecora, G.; De Leonardis, D.; Ibrahim, N.; Bovi, M.; Cornelini, R. The use of calcium sulphate in the surgical treatment of a ‘through and through’ periradicular lesion. Int. Endod. J. 2001, 34, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Tobón, S.I.; Arismendi, J.A.; Marín, M.L.; Mesa, A.L.; Valencia, J.A. Comparison between a conventional technique and two bone regeneration techniques in periradicular surgery. Int. Endod. J. 2002, 35, 635–641. [Google Scholar] [CrossRef]
- Marín-Botero, M.L.; Domínguez-Mejía, J.S.; Arismendi-Echavarría, J.A.; Mesa-Jaramillo, A.L.; Flórez-Moreno, G.A.; Tobón-Arroyave, S.I. Healing response of apicomarginal defects to two guided tissue regeneration techniques in periradicular surgery: A double-blind, randomized-clinical trial. Int. Endod. J. 2006, 39, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Taschieri, S.; Del Fabbro, M.; Testori, T.; Weinstein, R. Efficacy of xenogeneic bone grafting with guided tissue regeneration in the management of bone defects after surgical endodontics. J. Oral Maxillofac. Surg. 2007, 65, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Taschieri, S.; Del Fabbro, M.; Testori, T.; Saita, M.; Weinstein, R. Efficacy of guided tissue regeneration in the management of through-and-through lesions following surgical endodontics: A preliminary study. Int. J. Periodontics Restor. Dent. 2008, 28, 265–271. [Google Scholar]
- Taschieri, S.; Testori, T.; Azzola, F.; Del Fabbro, M.; Valentini, P. Régénération tissulaire guidée en chirurgie endodontique [Guided-tissue regeneration in endodontic surgery]. Rev. Stomatol. Chir. Maxillofac. 2008, 109, 213–217. [Google Scholar] [CrossRef]
- Goyal, B.; Tewari, S.; Duhan, J.; Sehgal, P.K. Comparative evaluation of platelet-rich plasma and guided tissue regeneration membrane in the healing of apicomarginal defects: A clinical study. J. Endod. 2011, 37, 773–780. [Google Scholar] [CrossRef]
- Dhiman, M.; Kumar, S.; Duhan, J.; Sangwan, P.; Tewari, S. Effect of Platelet-rich Fibrin on Healing of Apicomarginal Defects: A Randomized Controlled Trial. J. Endod. 2015, 41, 985–991. [Google Scholar] [CrossRef]
- Parmar, P.D.; Dhamija, R.; Tewari, S.; Sangwan, P.; Gupta, A.; Duhan, J.; Mittal, S. 2D and 3D radiographic outcome assessment of the effect of guided tissue regeneration using resorbable collagen membrane in the healing of through-and-through periapical lesions—A randomized controlled trial. Int. Endod. J. 2019, 52, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Salanti, G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: Many names, many benefits, many concerns for the next generation evidence synthesis tool. Res. Synth. Methods. 2012, 3, 80–97. [Google Scholar] [CrossRef]
- Garrett, K.; Kerr, M.; Hartwell, G.; O’Sullivan, S.; Mayer, P. The effect of a bioresorbable matrix barrier in endodontic surgery on the rate of periapical healing: An in vivo study. J. Endod. 2002, 28, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, J.; García, A.M.; de Vicente, J.C.; Landa, S.; López-Arranz, J.S. Bone regeneration after radicular cyst removal with and without guided bone regeneration. Int. J. Oral Maxillofac. Surg. 1998, 27, 118–120. [Google Scholar] [CrossRef]
- Taschieri, S.; Corbella, S.; Tsesis, I.; Bortolin, M.; Del Fabbro, M. Effect of guided tissue regeneration on the outcome of surgical endodontic treatment of through-and-through lesions: A retrospective study at 4-year follow-up. Oral Maxillofac. Surg. 2011, 15, 153–159. [Google Scholar] [CrossRef]
- Pantchev, A.; Nohlert, E.; Tegelberg, A. Endodontic surgery with and without inserts of bioactive glass PerioGlas—A clinical and radiographic follow-up. Oral Maxillofac. Surg. 2009, 13, 21–26. [Google Scholar] [CrossRef]
- Kattimani, V.S.; Chakravarthi, S.P.; Neelima Devi, K.N.; Sridhar, M.S.; Prasad, L.K. Comparative evaluation of bovine derived hydroxyapatite and synthetic hydroxyapatite graft in bone regeneration of human maxillary cystic defects: A clinico-radiological study. Indian J. Dent. Res. 2014, 25, 594–601. [Google Scholar] [CrossRef]
- Kattimani, V.; Lingamaneni, K.P.; Chakravarthi, P.S.; Kumar, T.S.; Siddharthan, A. Eggshell-Derived Hydroxyapatite: A New Era in Bone Regeneration. J. Craniofac. Surg. 2016, 27, 112–117. [Google Scholar] [CrossRef]
- Hamzani, Y.; Chaushu, G. Evaluation of early wound healing scales/indexes in oral surgery: A literature review. Clin. Implant. Dent. Relat. Res. 2018, 20, 1030–1035. [Google Scholar] [CrossRef]
- Haj Yahya, B.; Chaushu, G.; Hamzani, Y. Evaluation of wound healing following surgical extractions using the IPR Scale. Int. Dent. J. 2020, 71, 133–139. [Google Scholar] [CrossRef]
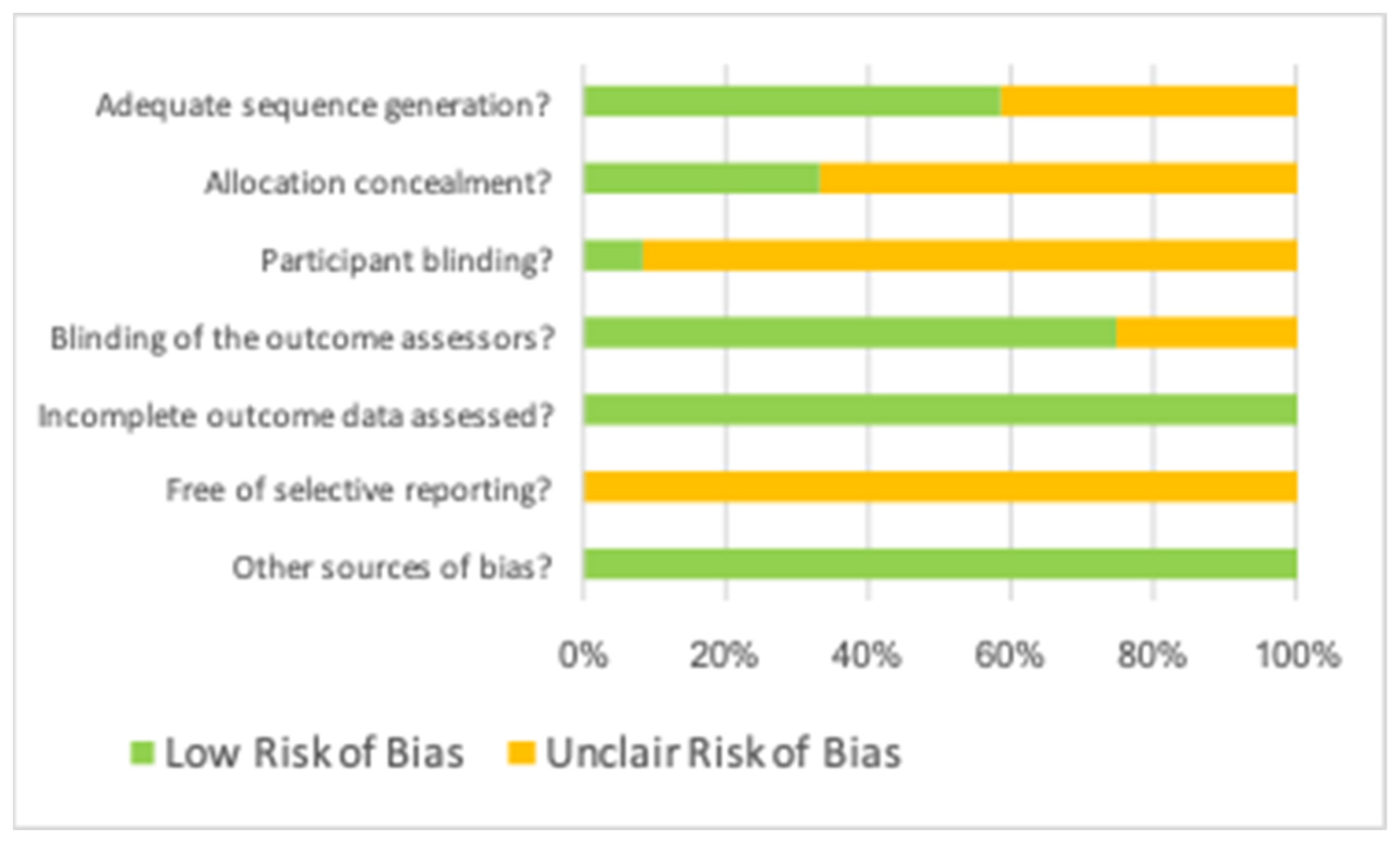
| Author, Year | Adequate Sequence Generation? | Allocation Concealment? | Participant Blinding? | Blinding of Outcome Assessors? | Incomplete Outcome Data Assessed? | Free of Selective Reporting? | Other Sources of Bias? |
|---|---|---|---|---|---|---|---|
| Dhamija, 2020 | Low | Low | Unclear | Low | Low | Unclear | Low |
| Dhiamn, 2015 | Unclear | Low | Unclear | Low | Low | Unclear | Low |
| Goyal, 2011 | Low | Low | Unclear | Low | Low | Unclear | Low |
| Marin Botero, 2006 | Low | Low | Unclear | Low | Low | Unclear | Low |
| Parmar, 2019 | Low | Unclear | Low | Low | Low | Unclear | Low |
| Pecora, 2002 | Low | Low | Unclear | Low | Low | Unclear | Low |
| Stassen, 1994 | Unclear | Unclear | Unclear | Low | Low | Unclear | Low |
| Taschieri, 2007a | Low | Unclear | Unclear | Low | Low | Unclear | Low |
| Taschieri, 2007b | Low | Unclear | Unclear | Low | Low | Unclear | Low |
| Taschieri, 2008 | Low | Unclear | Unclear | Low | Low | Unclear | Low |
| Tobon, 2002 | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Low |
| Author/Year | Study Type | Sample (n) | Follow-Up Time (Months) | Measurement Procedure | GTR Technique | Complete Healing Rate | Periapical Healing Results |
|---|---|---|---|---|---|---|---|
| Dhiamn, 2015 | RCT | 26 | 12 | Clinical and radiographic | Control | 8/11 | Control: 53.3% complete healing |
| PL | 8/15 | PL: 53.33% complete healing | |||||
| Goyal, 2011 | RCT | 25 | 3 | Clinical and radiographic | MB | NAv | MB: 38.7 ± 22.3% periapical size reduction |
| PL | NAv | PL: 39.2 ± 11.7% periapical size reduction | |||||
| PL + MB | NAv | PL + MB: 45.6 ± 14.2% periapical size reduction | |||||
| 25 | 6 | MB | NAv | MB: 67.9 ± 16.3% periapical size reduction | |||
| PL | NAv | PL: 84.9 ± 10.4% periapical size reduction | |||||
| PL + MB | NAv | PL + MB: 75.9 ± 12.2% periapical size reduction | |||||
| 25 | 9 | MB | NAv | MB: 88.6 ± 10.1% periapical size reduction | |||
| PL | NAv | PL: 93.3 ± 3.0% periapical size reduction | |||||
| PL + MB | NAv | PL + MB: 90.3 ± 6.9% periapical size reduction | |||||
| 25 | 12 | MB | 7/10 | MB: 97.0 ± 3.2 periapical size reduction | |||
| PL | 5/6 | PL: 96.3 ± 3.0% periapical size reduction | |||||
| PL + MB | 7/9 | PL + MB: 97.3 ± 3.3% periapical size reduction | |||||
| Marin Botero, 2006 | RCT | 30 | 12 | Clinical and radiographic | Control | 9/15 | Control: 91.1 ± 18.1% periapical size reduction |
| Mb | 6/15 | MB: 87.0 ± 18.6% periapical size reduction | |||||
| Os | 50/68 | ||||||
| Parmar, 2019 | RCT | 30 | 12 | Radiographic 2D | Control | 12/15 | Control: 12 ± 21mm2 (92 ± 12% reduction) |
| MB | 11/15 | MB: 31 ± 30 mm2 (86 ± 14% reduction) | |||||
| 30 | 12 | Radiographic 3D | Control | 9/15 | Control: 174 ± 264 mm3 (85 ± 19% reduction) | ||
| MB | 8/15 | MB: 324 ± 364 mm3 (82 ± 13% reduction) | |||||
| Pecora, 2002 | RCT | 20 | 6 | Clinical and radiographic | Control | 3/10 | Significant reduction in periapical defects (p ˂ 0.05) |
| Os | 8/10 | ||||||
| 18 | 12 | Control | 3/9 | ||||
| Os | 7/9 | ||||||
| Dhamija, 2020 | RTC | 32 | 12 | Clinical and radiographic | PL | 9/16 | Significant reduction in periapical defects (p ˂ 0.05) |
| Control | 5/16 | ||||||
| Stassen, 1994 | RTC | 101 | 24 | Clinical and radiographic | Control | 50/56 | No significant reduction in periapical defects (p = 0.057) |
| Os | 29/45 | ||||||
| Taschieri, 2007 | RTC | 59 | 12 | Radiographic 4-wall defects | Control | 18/22 | Control: 80.0–83.3% complete healing |
| MB + Os | 14/16 | MB + Os: 81.8–100% complete healing | |||||
| 59 | 12 | Radiographic through-and-through | Control | 8/13 | Control: 55.6–75.0% complete healing | ||
| MB + Os | 6/8 | MB + Os: 75.0% complete healing | |||||
| Taschieri, 2008 | RTC | 31 | 12 | Clinical and radiographic | Control | 8/14 | Control: 57.1% complete healing |
| MB + Os | 15/17 | Os: 88.2% complete healing | |||||
| Taschieri, 2008 | RTC | 69 | 12 | Clinical and radiographic 2-wall defects | Control | 9/14 | Statistically significant differences (p = 0.02) |
| MB + Os | 15/17 | ||||||
| Clinical and radiographic 4-wall defects | Control | 18/22 | No statistically significant differences (p = 0.21). | ||||
| MB + Os | 14/16 | ||||||
| Tobon, 2002 | RTC | 26 | 12 | Radiographic | Control | 4/9 | Control: 44.4% complete healing |
| MB | 6/9 | MB: 66.6% complete healing | |||||
| MB + Os | 8/8 | MB + Os: 100% complete healing |
| Control | MB | MB + Os | MB + PL | Os | PL | |
|---|---|---|---|---|---|---|
| Control | 1 | 0.95 0.50; 1.78 | 0.27 * 0.10; 0.73 | 0.63 0.07; 5.52 | 2.04 0.88; 4.66 | 0.82 0.31; 2.21 |
| MB | 1.06 0.56; 1.99 | 1 | 0.29 * 0.09; 0.94 | 0.66 0.08; 5.30 | 2.14 0.76; 6.11 | 0.87 0.29; 2.68 |
| MB + Os | 3.67 * 1.36; 9.90 | 3.47 * 1.07; 11.3 | 1 | 2.31 0.21; 25.1 | 7.46 2.04; 27.2 | 3.04 0.75; 12.3 |
| MB + PL | 1.58 0.18; 13.9 | 1.50 0.19; 11.9 | 0.43 0.04; 4.69 | 1 | 3.22 0.32; 32.9 | 1.31 0.12; 13.8 |
| Os | 0.49 0.21; 1.13 | 0.47 0.16; 1.32 | 0.13 0.04; 0.49 | 0.31 0.03; 3.16 | 1 | 0.41 0.11; 1.47 |
| PL | 1.21 0.45; 3.23 | 1.14 0.37; 3.49 | 0.33 0.08; 1.33 | 0.76 0.07; 8.04 | 2.46 0.68; 8.32 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubizarreta-Macho, Á.; Tosin, R.; Tosin, F.; Velasco Bohórquez, P.; San Hipólito Marín, L.; Montiel-Company, J.M.; Mena-Álvarez, J.; Hernández Montero, S. Influence of Guided Tissue Regeneration Techniques on the Success Rate of Healing of Surgical Endodontic Treatment: A Systematic Review and Network Meta-Analysis. J. Clin. Med. 2022, 11, 1062. https://doi.org/10.3390/jcm11041062
Zubizarreta-Macho Á, Tosin R, Tosin F, Velasco Bohórquez P, San Hipólito Marín L, Montiel-Company JM, Mena-Álvarez J, Hernández Montero S. Influence of Guided Tissue Regeneration Techniques on the Success Rate of Healing of Surgical Endodontic Treatment: A Systematic Review and Network Meta-Analysis. Journal of Clinical Medicine. 2022; 11(4):1062. https://doi.org/10.3390/jcm11041062
Chicago/Turabian StyleZubizarreta-Macho, Álvaro, Roberta Tosin, Fabio Tosin, Pilar Velasco Bohórquez, Lara San Hipólito Marín, José María Montiel-Company, Jesús Mena-Álvarez, and Sofía Hernández Montero. 2022. "Influence of Guided Tissue Regeneration Techniques on the Success Rate of Healing of Surgical Endodontic Treatment: A Systematic Review and Network Meta-Analysis" Journal of Clinical Medicine 11, no. 4: 1062. https://doi.org/10.3390/jcm11041062
APA StyleZubizarreta-Macho, Á., Tosin, R., Tosin, F., Velasco Bohórquez, P., San Hipólito Marín, L., Montiel-Company, J. M., Mena-Álvarez, J., & Hernández Montero, S. (2022). Influence of Guided Tissue Regeneration Techniques on the Success Rate of Healing of Surgical Endodontic Treatment: A Systematic Review and Network Meta-Analysis. Journal of Clinical Medicine, 11(4), 1062. https://doi.org/10.3390/jcm11041062







