The Value of Fetal Heart Evaluation in Fetuses with Rare Congenital Lymphangiomas: A Cohort Study from a Single Tertiary Center across Two Decades (Years 1999–2020)
Abstract
1. Introduction
2. Materials and Methods
2.1. General Part
2.2. Statistical Analysis
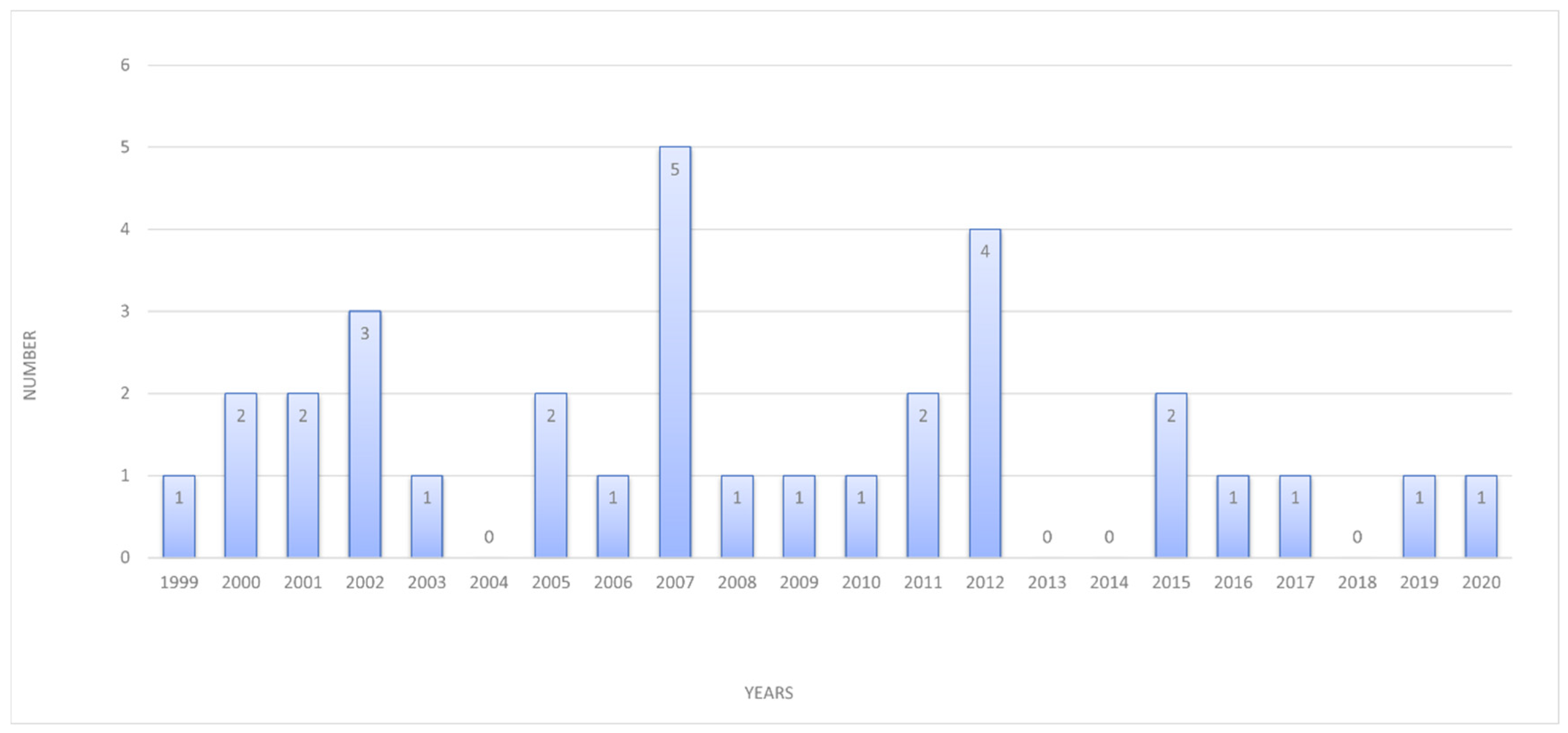
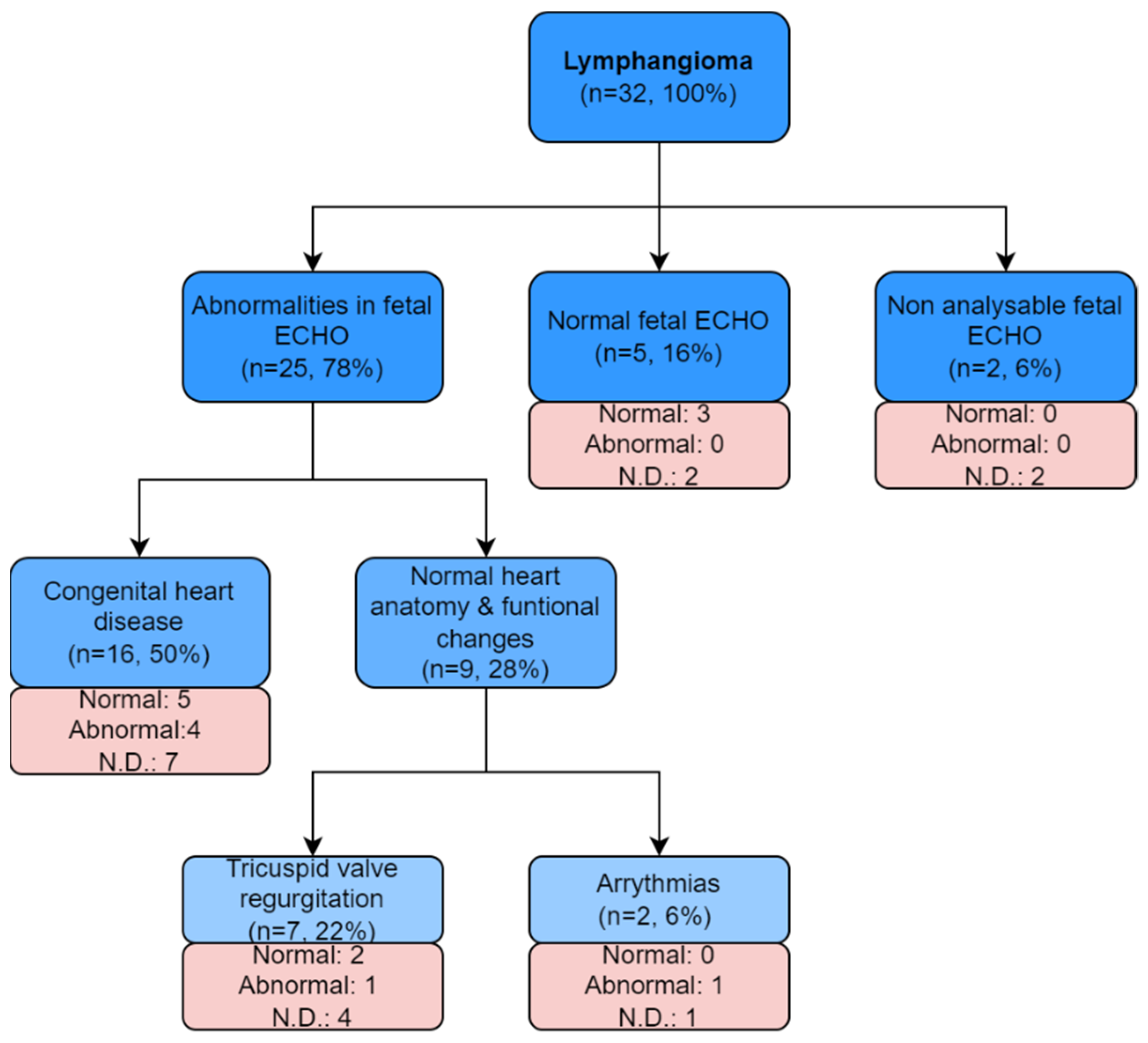
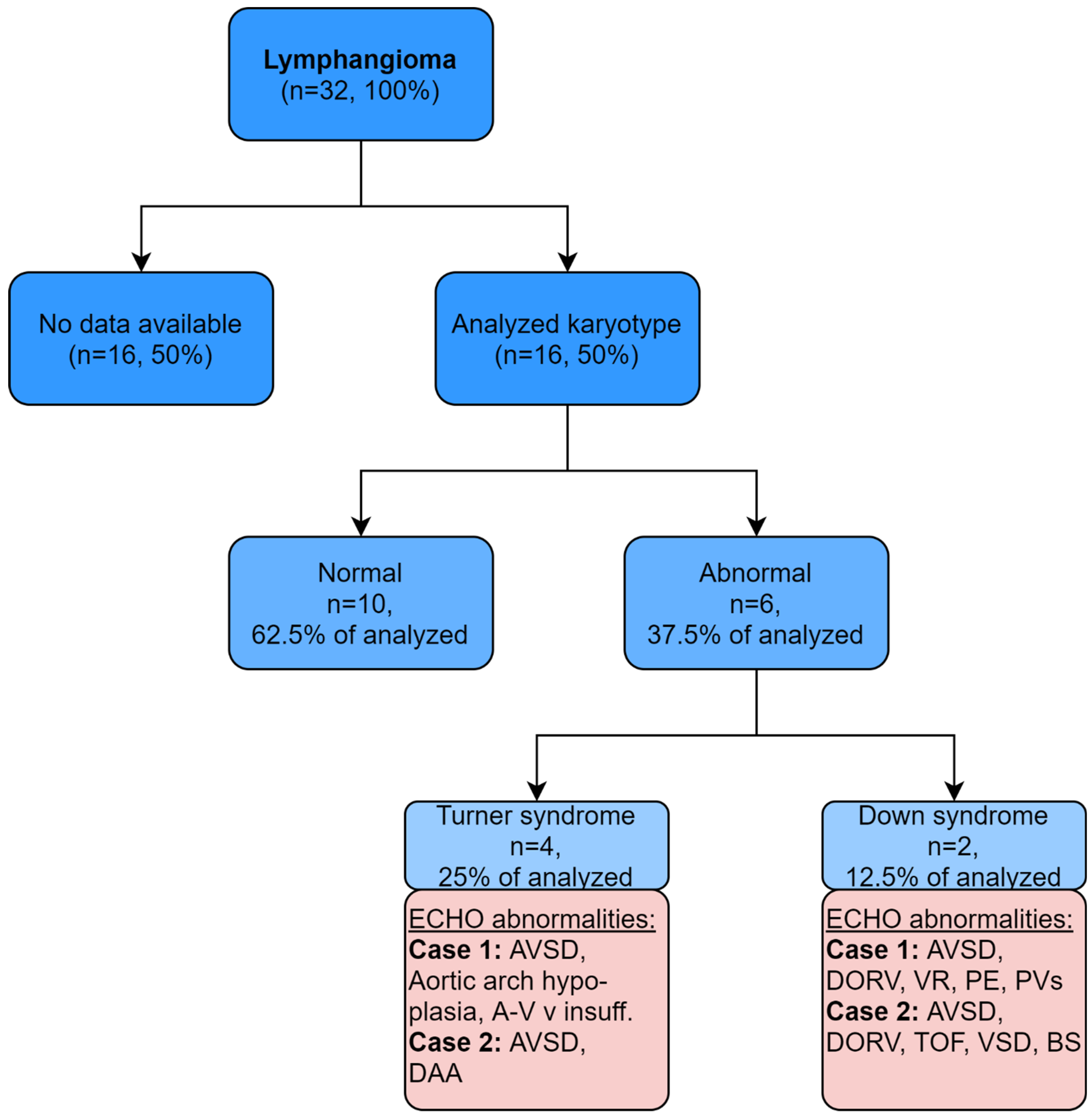
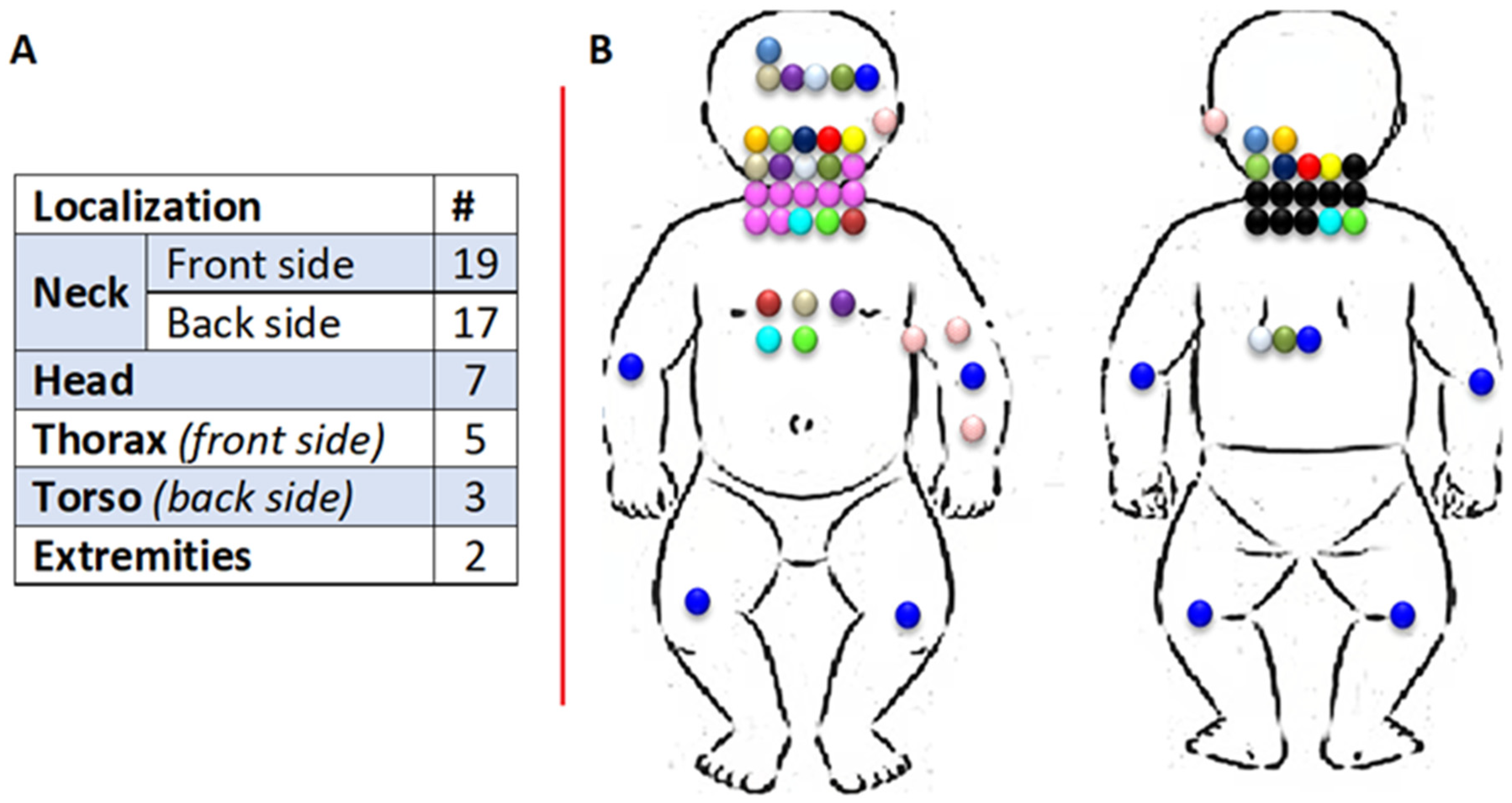
3. Results
- heart defect in 50% (n = 16);
- normal heart structure with functional changes in 28% (n = 9), including:
- ○
- tricuspid regurgitation in 22% (n = 7);
- ○
- fetal rhythm disturbances in 6% (n = 2).
Follow-Up
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moyle, R.D. Lymphangioma. J. R. Soc. Med. 1936, 29, 1290–1291. [Google Scholar] [CrossRef]
- Lo Magno, E.; Ermito, S.; Dinatale, A.; Cacciatore, A.; Pappalardo, E.M.; Militello, M.; Cavaliere, A.; Rossetti, D. Fetal cystic lymphangioma of the neck: A case report. J. Prenat. Med. 2009, 3, 12–14. [Google Scholar]
- Godart, S. Embryological significance of lymphangioma. Arch. Dis. Child. 1966, 41, 204–206. [Google Scholar] [CrossRef][Green Version]
- Rattan, K.N.; Kajal, P.; Kadian, Y.S.; Gupta, R. Haemorrhage in a scrotal lymphangioma in a child: A rarity. Afr. J. Paediatr. Surg. 2009, 6, 110–111. [Google Scholar] [CrossRef]
- Budhiraja, S.; Rattan, K.N.; Gupta, S.; Pandit, S.K. Abdomino-Scrotal Lymphangioma. Indian J. Pediatr. 1997, 64, 720–722. [Google Scholar] [CrossRef]
- Singla, S.L.; Rattan, K.N.; Singh, S. Cystic hygroma of the gluteal region. Indian J. Pediatr. 2000, 67, 779–780. [Google Scholar] [CrossRef]
- Comstock, C.H.; Lee, W.; Bronsteen, R.A.; Vettraino, I.; Wechter, D. Fetal mediastinal lymphangiomas. J. Ultrasound Med. 2008, 27, 145–148. [Google Scholar] [CrossRef]
- Tanaka, H.; Masumoto, K.; Aoyama, T.; Sanmoto, Y.; Ono, K.; Sakamoto, N.; Kawakami, H. Prenatally diagnosed large mediastinal lymphangioma: A case report. Clin. Case Rep. 2018, 6, 1880–1884. [Google Scholar] [CrossRef]
- The Fetal Medicine Foundation. Available online: https://fetalmedicine.org/education/fetal-abnormalities/tumors/lymphangioma (accessed on 8 February 2022).
- Fisher Exact Test Online. For Table Analysis, including the Median Test. Available online: https://sgapeio.es/INFORMEST/VICongreso/taller/applets/Sisa/fisher.htm (accessed on 14 August 2021).
- Borowski, D.; Pietryga, M.; Basta, P.; Cnota, W.; Czuba, B.; Dubiel, M.; Fuchs, T.; Huras, H.; Iciek, R.; Jaczynska, R.; et al. Practice guidelines of the Polish Society of Gynecologists and Obstetricians—ultrasound section for ultrasound screening in uncomplicated pregnancy—2020. Ginekol. Pol. 2020, 91, 490–501. [Google Scholar] [CrossRef]
- Farnaghi, S.; Kothari, A. The value of early recognition of fetal lymphangioma. Australas. J. Ultrasound Med. 2013, 16, 147–152. [Google Scholar] [CrossRef]
- Zanotti, S.D.; LaRusso, S.; Coulson, C. Prenatal sonographic diagnosis of axillary cystic lymphangiomas. J. Clin. Ultrasound 2001, 29, 112–115. [Google Scholar] [CrossRef]
- Jiao-Ling, L.; Hai-Ying, W.; Wei, Z.; Jin-Rong, L.; Kun-Shan, C.; Qian, F. Treatment and prognosis of fetal lymphangioma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 231, 274–279. [Google Scholar] [CrossRef]
- Chen, Y.N.; Chen, C.P.; Lin, C.J.; Chen, S.W. Prenatal Ultrasound Evaluation and Outcome of Pregnancy with Fetal Cystic Hygromas and Lymphangiomas. J. Med. Ultrasound 2017, 25, 12–15. [Google Scholar] [CrossRef]
- Arisoy, R.; Erdogdu, E.; Kumru, P.; Demirci, O.; Yuksel, M.A.; Pekin, O.; Tugrul, S.; Aydin, H. Prenatal diagnosis and outcome of lymphangiomas and its relationship with fetal chromosomal abnormalities. J. Matern. Neonatal Med. 2016, 29, 466–472. [Google Scholar] [CrossRef]
- Gedikbasi, A.; Gul, A.; Sargin, A.; Ceylan, Y. Cystic hygroma and lymphangioma: Associated findings, perinatal outcome and prognostic factors in live-born infants. Arch. Gynecol. Obstet. 2007, 276, 491–498. [Google Scholar] [CrossRef]
- Malone, F.D.; Ball, R.H.; Nyberg, D.A.; Comstock, C.H.; Saade, G.R.; Berkowitz, R.L.; Gross, S.J.; Dugoff, L.; Craigo, S.D.; Timor-Tritsch, I.E.; et al. First-trimester septated cystic hygroma: Prevalence, natural history, and pediatric outcome. Obstet. Gynecol. 2005, 106, 288–294. [Google Scholar] [CrossRef]
- Bennasar, M.; Arigita, M.; Salazar, L.; Puerto, B. Cystic hygroma. In Obstetric Imaging: Fetal Diagnosis and Care, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 331–333.e1. ISBN 9780323445481. [Google Scholar]
- Bondy, C.A. Congenital cardiovascular disease in turner syndrome. Congenit. Heart Dis. 2008, 3, 2–15. [Google Scholar] [CrossRef]
- Benhaourech, S.; Drighil, A.; El Hammiri, A. Congenital heart disease and down syndrome: Various aspects of a confirmed association. Cardiovasc. J. Afr. 2016, 27, 287–290. [Google Scholar] [CrossRef]
- Załuska, R.; Kazłowski, J.; Gumkowska, A.; Pikto-Pietkiewicz, W. Congenital disorders of the cardiovascular system and their complications in a 21-year-old woman with Turner syndrome—A case report. Kardiol. Pol. 2008, 66, 63–66. [Google Scholar]
- Bansal, N.; Haidar-El-Atrache, S.; Walters, H.L.; Kobayashi, D. Cardiac Lymphangioma Encasing Right Coronary Artery in an Infant. Ann. Thorac. Surg. 2017, 104, e279–e281. [Google Scholar] [CrossRef]
- Szmyd, B.; Biedrzycka, M.; Karuga, F.F.; Rogut, M.; Strzelecka, I.; Respondek-Liberska, M. Interventricular Septal Thickness as a Diagnostic Marker of Fetal Macrosomia. J. Clin. Med. 2021, 10, 949. [Google Scholar] [CrossRef] [PubMed]
- Strzelecka, I.; Biedrzycka, M.; Karuga, F.F.; Szmyd, B.; Batarowicz, K.; Respondek-Liberska, M. Seasonality of Hypoplastic Left Heart Syndrome and Single Ventricle Heart in Poland in the Context of Air Pollution. J. Clin. Med. 2021, 10, 3207. [Google Scholar] [CrossRef] [PubMed]
| The outcomes of all cases | |
| All cases: Termination of pregnancy 18% (n = 6) Death in utero: 44% (n = 14) Death after birth: 16% (n = 5) Alive: 22% (n = 7) | Isolated lymphangioma: Termination of pregnancy: 19% (n = 3) Death in utero: 31% (n = 5) Death after birth: 19% (n = 3) Alive: 31% (n = 5) |
| Non-isolated lymphangioma: Termination of pregnancy: 19% (n = 3) Death in utero: 56% (n = 9) Death after birth: 12.5% (n = 2) Alive: 12.5% (n = 2) | |
| The outcomes of euploid cases | |
| Euploid cases (n = 9) Termination of pregnancy: 44% (n = 4) Miscarriage: 11% (n = 1) Death after birth: 22% (n = 2) Alive: 22% (n = 2) | Isolated lymphangioma: Termination of pregnancy: 33% (n = 3) Death after birth: 11% (n = 1) Alive: 11% (n = 1) |
| Non-isolated lymphangioma: Termination of pregnancy 11% (n = 1) Miscarriage 11% (n = 1) Death after birth 11% (n = 1) Alive: 11% (n = 1) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kordjalik, P.; Szmyd, B.; Karuga, F.F.; Daszkiewicz, G.; Strzelecka, I.; Respondek-Liberska, M. The Value of Fetal Heart Evaluation in Fetuses with Rare Congenital Lymphangiomas: A Cohort Study from a Single Tertiary Center across Two Decades (Years 1999–2020). J. Clin. Med. 2022, 11, 1035. https://doi.org/10.3390/jcm11041035
Kordjalik P, Szmyd B, Karuga FF, Daszkiewicz G, Strzelecka I, Respondek-Liberska M. The Value of Fetal Heart Evaluation in Fetuses with Rare Congenital Lymphangiomas: A Cohort Study from a Single Tertiary Center across Two Decades (Years 1999–2020). Journal of Clinical Medicine. 2022; 11(4):1035. https://doi.org/10.3390/jcm11041035
Chicago/Turabian StyleKordjalik, Paulina, Bartosz Szmyd, Filip Franciszek Karuga, Gabriela Daszkiewicz, Iwona Strzelecka, and Maria Respondek-Liberska. 2022. "The Value of Fetal Heart Evaluation in Fetuses with Rare Congenital Lymphangiomas: A Cohort Study from a Single Tertiary Center across Two Decades (Years 1999–2020)" Journal of Clinical Medicine 11, no. 4: 1035. https://doi.org/10.3390/jcm11041035
APA StyleKordjalik, P., Szmyd, B., Karuga, F. F., Daszkiewicz, G., Strzelecka, I., & Respondek-Liberska, M. (2022). The Value of Fetal Heart Evaluation in Fetuses with Rare Congenital Lymphangiomas: A Cohort Study from a Single Tertiary Center across Two Decades (Years 1999–2020). Journal of Clinical Medicine, 11(4), 1035. https://doi.org/10.3390/jcm11041035








