Squamous Cell Carcinoma of the Vulva: A Survival and Epidemiologic Study with Focus on Surgery and Radiotherapy
Abstract
:1. Introduction
2. Materials and Methods
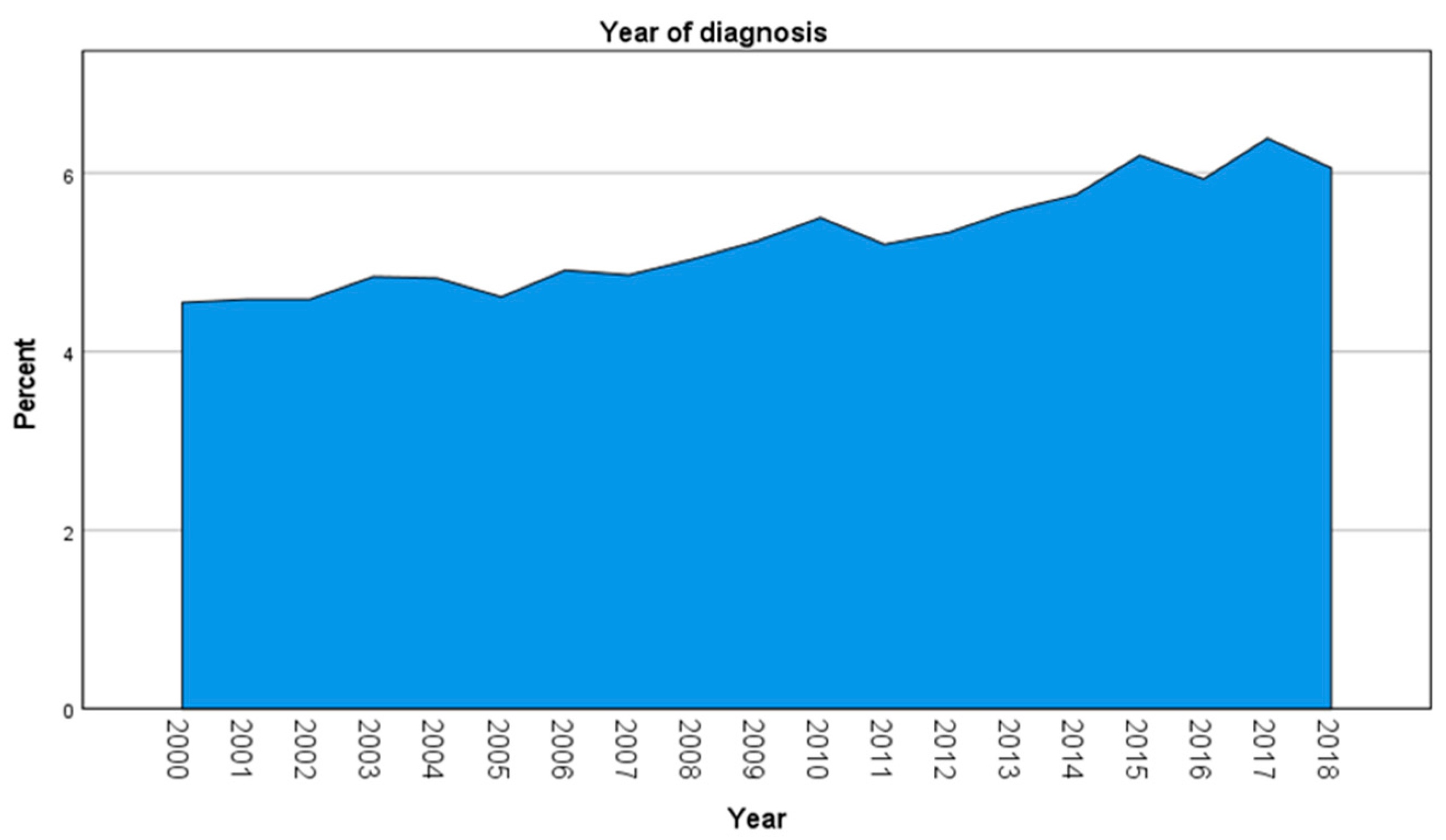
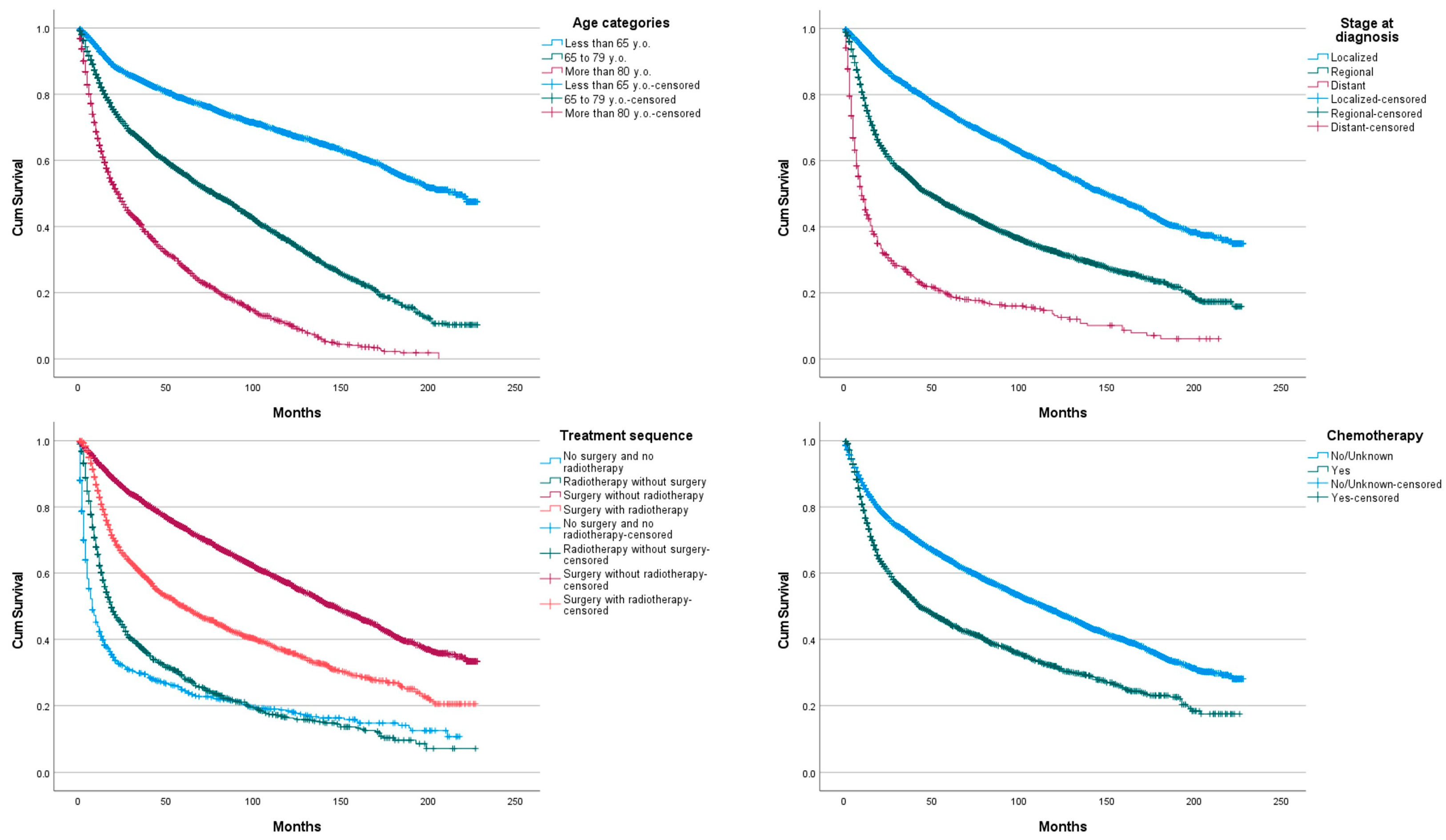
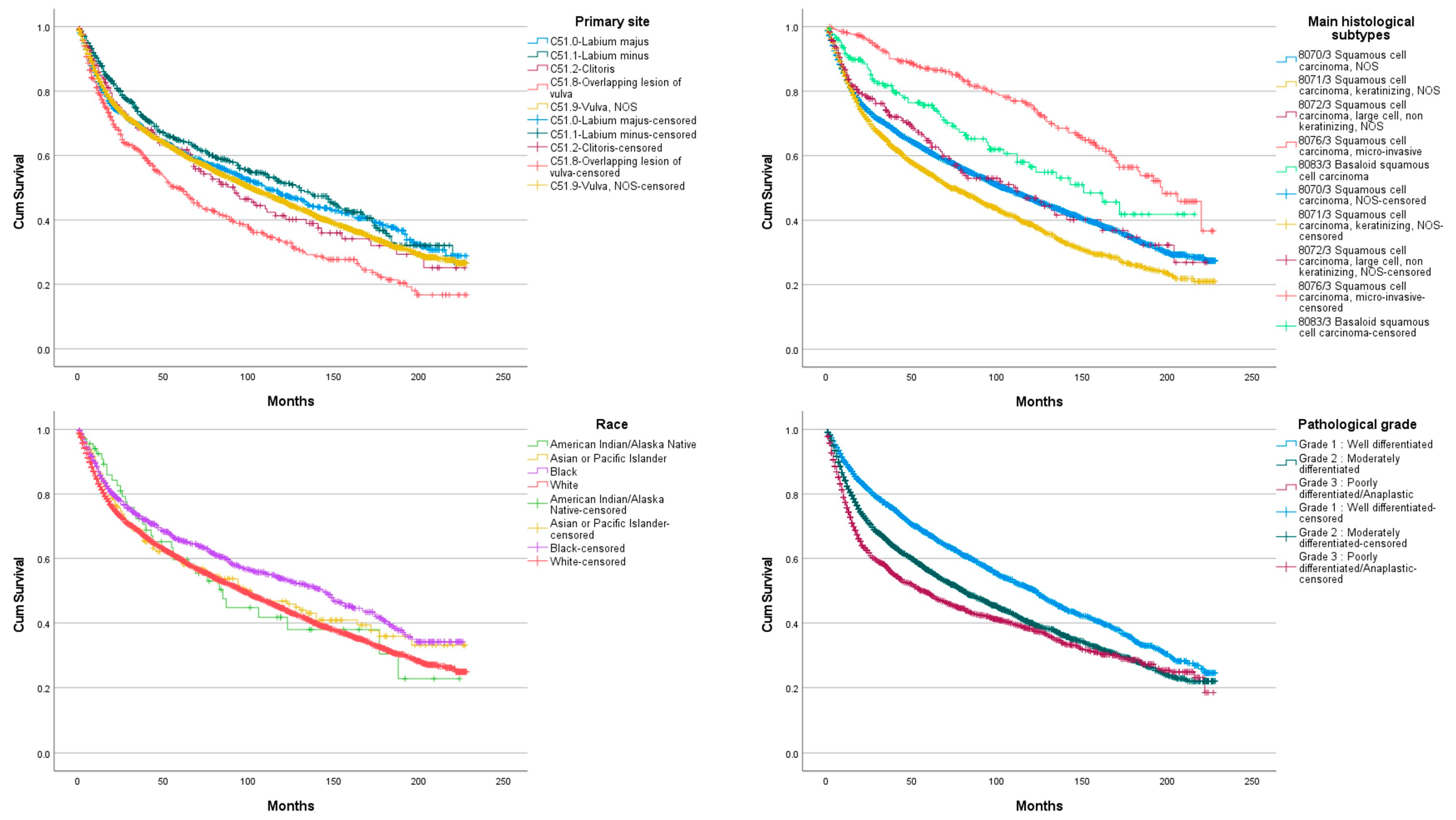
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, W.; Wistuba, I.I.; Emmert-Buck, M.R.; Erickson, H.S. Squamous Cell Carcinoma–Similarities and Differences among Anatomical Sites. Am. J. Cancer Res. 2011, 1, 275. [Google Scholar] [PubMed]
- Cancer of the Vulva-Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/vulva.html (accessed on 4 September 2021).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA. Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Bieber, A.K.; Stein, J.A.; Pomeranz, M.K. Diagnosis and Management of Vulvar Cancer: A Review. J. Am. Acad. Dermatol 2019, 81, 1387–1396. [Google Scholar] [CrossRef]
- Singh, N.; Gilks, C.B. Vulval Squamous Cell Carcinoma and Its Precursors. Histopathology 2020, 76, 128–138. [Google Scholar] [CrossRef]
- Hinten, F.; Molijn, A.; Eckhardt, L.; Massuger, L.F.A.G.; Quint, W.; Bult, P.; Bulten, J.; Melchers, W.J.G.; de Hullu, J.A. Vulvar Cancer: Two Pathways with Different Localization and Prognosis. Gynecol. Oncol. 2018, 149, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, J.; Bogliatto, F.; Haefner, H.K.; Stockdale, C.K.; Preti, M.; Bohl, T.G.; Reutter, J.; ISSVD Terminology Committee. The 2015 International Society for the Study of Vulvovaginal Disease (ISSVD) Terminology of Vulvar Squamous Intraepithelial Lesions. J. Low Genit. Tract Dis. 2016, 20, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.-J.; Greer, B.E.; Abu-Rustum, N.R.; Campos, S.M.; Cho, K.R.; Chon, H.S.; Chu, C.; Cohn, D.; Crispens, M.A.; Dizon, D.S.; et al. Vulvar Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2017, 15, 92–120. [Google Scholar] [CrossRef] [Green Version]
- Aerts, L.; Enzlin, P.; Vergote, I.; Verhaeghe, J.; Poppe, W.; Amant, F. Sexual, Psychological, and Relational Functioning in Women after Surgical Treatment for Vulvar Malignancy: A Literature Review. J. Sex. Med. 2012, 9, 361–371. [Google Scholar] [CrossRef]
- About the SEER Program. Available online: https://seer.cancer.gov/about/overview.html (accessed on 10 April 2021).
- Stroup, A.M.; Harlan, L.C.; Trimble, E.L. Demographic, Clinical, and Treatment Trends among Women Diagnosed with Vulvar Cancer in the United States. Gynecol. Oncol. 2008, 108, 577–583. [Google Scholar] [CrossRef] [Green Version]
- Alhatem, A.; Lambert, W.C.; Karanfilian, K.; Behbahani, S.; Heller, D. Impact of Partnership Status on Clinical Outcomes of Patients with Vulvar Squamous Cell Carcinoma and Performance of Sentinel Lymph Node Biopsy. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2020, 30, 583–589. [Google Scholar] [CrossRef]
- Lei, L.; Tan, L.; Zhao, X.; Zeng, F.; Xu, D. A Prognostic Nomogram Based on Lymph Node Ratio for Postoperative Vulvar Squamous Cell Carcinoma from the Surveillance, Epidemiology, and End Results Database: A Retrospective Cohort Study. Ann. Transl. Med. 2020, 8, 1382. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulos, E.P.; Grover, S.; Puri, P.M.; Corradetti, M.N.; Heilbroner, S.P.; Mitra, N.; Simone, C.B.; Lin, L.L. Survival Benefit of Adjuvant Radiation Therapy in Node-Positive Vulvar Cancer. Am. J. Clin. Oncol. 2018, 41, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Summary Staging Manual-2018. Available online: https://seer.cancer.gov/tools/ssm/index.html (accessed on 5 February 2022).
- Hellman, K.; Holmberg, E.; Bjurberg, M.; Borgfeldt, C.; Dahm-Kähler, P.; Flöter Rådestad, A.; Hjerpe, E.; Högberg, T.; Marcickiewicz, J.; Rosenberg, P.; et al. Primary Treatment and Relative Survival by Stage and Age in Vulvar Squamous Cell Carcinoma: A Population-Based SweGCG Study. Gynecol. Oncol. 2020, 159, 663–671. [Google Scholar] [CrossRef]
- Tanaka, Y.; Ueda, Y.; Kakuda, M.; Yagi, A.; Okazawa, A.; Egawa-Takata, T.; Matsuzaki, S.; Kobayashi, E.; Yoshino, K.; Fukui, K.; et al. Trends in Incidence and Long-Term Survival of Japanese Women with Vulvar Cancer: A Population-Based Analysis. Int. J. Clin. Oncol. 2019, 24, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, M.S.; van den Einden, L.C.G.; Massuger, L.F.A.G.; Kiemeney, L.A.; van der Aa, M.A.; de Hullu, J.A. Trends in Incidence and Survival of Dutch Women with Vulvar Squamous Cell Carcinoma. Eur. J. Cancer Oxf. Engl. 2013, 49, 3872–3880. [Google Scholar] [CrossRef] [PubMed]
- Meltzer-Gunnes, C.J.; Småstuen, M.C.; Kristensen, G.B.; Tropé, C.G.; Lie, A.K.; Vistad, I. Vulvar Carcinoma in Norway: A 50-Year Perspective on Trends in Incidence, Treatment and Survival. Gynecol. Oncol. 2017, 145, 543–548. [Google Scholar] [CrossRef]
- Rottmann, M.; Beck, T.; Burges, A.; Dannecker, C.; Kiechle, M.; Mayr, D.; Schlesinger-Raab, A.; Schubert-Fritschle, G.; Engel, J. Trends in Surgery and Outcomes of Squamous Cell Vulvar Cancer Patients over a 16-Year Period (1998–2013): A Population-Based Analysis. J. Cancer Res. Clin. Oncol. 2016, 142, 1331–1341. [Google Scholar] [CrossRef]
- Gill, B.S.; Bernard, M.E.; Lin, J.F.; Balasubramani, G.K.; Rajagopalan, M.S.; Sukumvanich, P.; Krivak, T.C.; Olawaiye, A.B.; Kelley, J.L.; Beriwal, S. Impact of Adjuvant Chemotherapy with Radiation for Node-Positive Vulvar Cancer: A National Cancer Data Base (NCDB) Analysis. Gynecol. Oncol. 2015, 137, 365–372. [Google Scholar] [CrossRef]
- Stecklein, S.R.; Frumovitz, M.; Klopp, A.H.; Gunther, J.R.; Eifel, P.J. Effectiveness of Definitive Radiotherapy for Squamous Cell Carcinoma of the Vulva with Gross Inguinal Lymphadenopathy. Gynecol. Oncol. 2018, 148, 474–479. [Google Scholar] [CrossRef]
- Rao, Y.J.; Chin, R.-I.; Hui, C.; Mutch, D.G.; Powell, M.A.; Schwarz, J.K.; Grigsby, P.W.; Markovina, S. Improved Survival with Definitive Chemoradiation Compared to Definitive Radiation Alone in Squamous Cell Carcinoma of the Vulva: A Review of the National Cancer Database. Gynecol. Oncol. 2017, 146, 572–579. [Google Scholar] [CrossRef]
- Reade, C.J.; Eiriksson, L.R.; Mackay, H. Systemic Therapy in Squamous Cell Carcinoma of the Vulva: Current Status and Future Directions. Gynecol. Oncol. 2014, 132, 780–789. [Google Scholar] [CrossRef]
- Zapardiel, I.; Iacoponi, S.; Coronado, P.J.; Zalewski, K.; Chen, F.; Fotopoulou, C.; Dursun, P.; Kotsopoulos, I.C.; Jach, R.; Buda, A.; et al. Prognostic Factors in Patients with Vulvar Cancer: The VULCAN Study. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2020, 30, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, M.; Pizzuti, L.; Krasniqi, E.; Di Lisa, F.S.; Cappuzzo, F.; Landi, L.; Sergi, D.; Pelle, F.; Cappelli, S.; Botti, C.; et al. Role of Chemotherapy in Vulvar Cancers: Time to Rethink Standard of Care? Cancers 2021, 13, 4061. [Google Scholar] [CrossRef] [PubMed]
- Van de Nieuwenhof, H.P.; van Kempen, L.C.L.T.; de Hullu, J.A.; Bekkers, R.L.M.; Bulten, J.; Melchers, W.J.G.; Massuger, L.F.A.G. The Etiologic Role of HPV in Vulvar Squamous Cell Carcinoma Fine Tuned. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2009, 18, 2061–2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, C.L.; Sand, F.L.; Hoffmann Frederiksen, M.; Kaae Andersen, K.; Kjaer, S.K. Does HPV Status Influence Survival after Vulvar Cancer? Int. J. Cancer 2018, 142, 1158–1165. [Google Scholar] [CrossRef] [Green Version]
- McAlpine, J.N.; Leung, S.C.Y.; Cheng, A.; Miller, D.; Talhouk, A.; Gilks, C.B.; Karnezis, A.N. Human Papillomavirus (HPV)-Independent Vulvar Squamous Cell Carcinoma Has a Worse Prognosis than HPV-Associated Disease: A Retrospective Cohort Study. Histopathology 2017, 71, 238–246. [Google Scholar] [CrossRef]

| N (%) | |
|---|---|
| Age | |
| Median | 65 (range 17–99) |
| <65 years old | 5440 (57.9) |
| 65 to 79 years old | 3409 (30) |
| ≥80 years old | 2511 (22.1) |
| Race | |
| White | 9895 (87.1) |
| Black | 1045 (9.2) |
| American Indian/Alaska Native | 69 (0.6) |
| Asian or Pacific Islander | 255 (2.2) |
| Unknown | 96 (0.8) |
| Marital status | |
| Married | 4179 (36.8) |
| Not Married | 3587 (31.6) |
| Widowed | 2745 (24.2) |
| Unknown | 849 (7.5) |
| Primary site | |
| C51.0 Labium majus | 965 (8.5) |
| C51.1 Labium minus | 536 (4.7) |
| C51.2 Clitoris | 211 (1.9) |
| C51.8 Overlapping lesion of vulva | 521 (4.6) |
| C51.9 Vulva, NOS * | 9127 (80.3) |
| Histologic subtypes | |
| 8070/3 Squamous cell carcinoma, NOS * | 7740 (68.1) |
| 8071/3 Squamous cell carcinoma, keratinizing, NOS * | 2889 (25.4) |
| 8072/3 Squamous cell carcinoma, large cell, non-keratinizing, NOS * | 248 (2.2) |
| 8076/3 Squamous cell carcinoma, micro-invasive | 266 (2.3) |
| 8083/3 Basaloid squamous cell carcinoma | 173 (1.5) |
| Pathologic grade | |
| G1: Well differentiated | 2900 (25.5) |
| G2: Moderately differentiated | 4317 (38) |
| G3: Poorly differentiated/Anaplastic | 1761 (15.5) |
| Unknown | 2382 (21) |
| Stage at diagnosis | |
| Localized | 6425 (56.6) |
| Regional | 3897 (34.3) |
| Distant | 664 (5.8) |
| Unknown | 374 (3.3) |
| No surgery | 2122 (18.7) |
| Surgery | 9204 (80.9) |
| Local destruction | 13 (0.1) |
| Local excision | 1591 (14) |
| Simple/partial surgical removal of primary site | 3831 (33.7) |
| Total surgical removal of primary site | 1405 (12.4) |
| Debulking surgery | 25 (0.2) |
| En bloc resection | 2277 (20) |
| Surgery, NOS * | 62 (0.5) |
| Unknown | 34 (0.3) |
| Radiotherapy | |
| Yes | 3473 (30.6) |
| No/unknown | 7886 (69.4) |
| Treatment | |
| No surgery and no radiotherapy | 745 (6.6) |
| Radiotherapy without surgery | 1353 (11.9) |
| Surgery without radiotherapy | 7028 (61.9) |
| Surgery with radiotherapy | 2115 (18.6) |
| Chemotherapy | |
| Yes | 2079 (18.3) |
| No/unknown | 9281 (81.7) |
| B | Sig. | Exp(B) | 95% CI for Exp(B) Low/Up | |
|---|---|---|---|---|
| Age: 3 subgroups | ||||
| Less than 65 y.o. | 0.000 | |||
| 65 to 79 y.o. | 0.936 | 0.000 | 2.550 | 2.360–2.756 |
| More than 80 y.o. | 1.647 | 0.000 | 5.193 | 4.790–5.631 |
| Primary site | ||||
| Labium majus | 0.472 | |||
| Labium minus | 0.066 | 0.446 | 1.068 | 0.902–1.264 |
| Clitoris | 0.139 | 0.235 | 1.149 | 0.914–1.444 |
| Overlapping lesion of vulva | 0.132 | 0.102 | 1.141 | 0.974–1.337 |
| Vulva, NOS | 0.084 | 0.123 | 1.087 | 0.978–1.209 |
| Main histological subtype | ||||
| 8070/3 Squamous cell carcinoma, NOS | 0.000 | |||
| 8071/3 Squamous cell carcinoma, keratinizing, NOS | 0.123 | 0.000 | 1.131 | 1.057–1.211 |
| 8072/3 Squamous cell carcinoma, large cell, non-keratinizing, NOS | −0.325 | 0.003 | 0.722 | 0.583–0.895 |
| 8076/3 Squamous cell carcinoma, micro-invasive | −0.246 | 0.189 | 0.782 | 0.541–1.129 |
| 8083/3 Basaloid squamous cell carcinoma | −0.273 | 0.075 | 0.761 | 0.564–1.028 |
| Pathological grade | ||||
| Grade 1: Well differentiated | 0.000 | |||
| Grade 2: Moderately differentiated | 0.153 | 0.000 | 1.165 | 1.083–1.253 |
| Grade 3: Poorly differentiated/Anaplastic | 0.285 | 0.000 | 1.330 | 1.215–1.456 |
| Stage at diagnosis | ||||
| Localized | 0.000 | |||
| Regional | 0.524 | 0.000 | 1.689 | 1.568–1.820 |
| Distant | 1.196 | 0.000 | 3.308 | 2.913–3.755 |
| Race | ||||
| American Indian/Alaska Native | 0.000 | |||
| Asian or Pacific Islander | −0.671 | 0.001 | 0.511 | 0.339–0.772 |
| Black | −0.423 | 0.028 | 0.655 | 0.450–0.955 |
| White | −0.274 | 0.136 | 0.760 | 0.530–1.090 |
| Treatment sequence | ||||
| No surgery and no radiotherapy | 0.000 | |||
| Radiotherapy without surgery | −0.577 | 0.000 | 0.562 | 0.484–0.652 |
| Surgery without radiotherapy | −1.482 | 0.000 | 0.227 | 0.200–0.258 |
| Surgery with radiotherapy | −1.162 | 0.000 | 0.313 | 0.273–0.359 |
| Chemotherapy | ||||
| No/Unknown | ||||
| Yes | −0.190 | 0.000 | 0.827 | 0.750–0.910 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scampa, M.; Kalbermatten, D.F.; Oranges, C.M. Squamous Cell Carcinoma of the Vulva: A Survival and Epidemiologic Study with Focus on Surgery and Radiotherapy. J. Clin. Med. 2022, 11, 1025. https://doi.org/10.3390/jcm11041025
Scampa M, Kalbermatten DF, Oranges CM. Squamous Cell Carcinoma of the Vulva: A Survival and Epidemiologic Study with Focus on Surgery and Radiotherapy. Journal of Clinical Medicine. 2022; 11(4):1025. https://doi.org/10.3390/jcm11041025
Chicago/Turabian StyleScampa, Matteo, Daniel F. Kalbermatten, and Carlo M. Oranges. 2022. "Squamous Cell Carcinoma of the Vulva: A Survival and Epidemiologic Study with Focus on Surgery and Radiotherapy" Journal of Clinical Medicine 11, no. 4: 1025. https://doi.org/10.3390/jcm11041025
APA StyleScampa, M., Kalbermatten, D. F., & Oranges, C. M. (2022). Squamous Cell Carcinoma of the Vulva: A Survival and Epidemiologic Study with Focus on Surgery and Radiotherapy. Journal of Clinical Medicine, 11(4), 1025. https://doi.org/10.3390/jcm11041025







