Influence of Bone-Level Dental Implants Placement and of Cortical Thickness on Osseointegration: In Silico and In Vivo Analyses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Finite Element Analysis Methodology
2.2. Implantation in Animals (In Vivo Analysis)
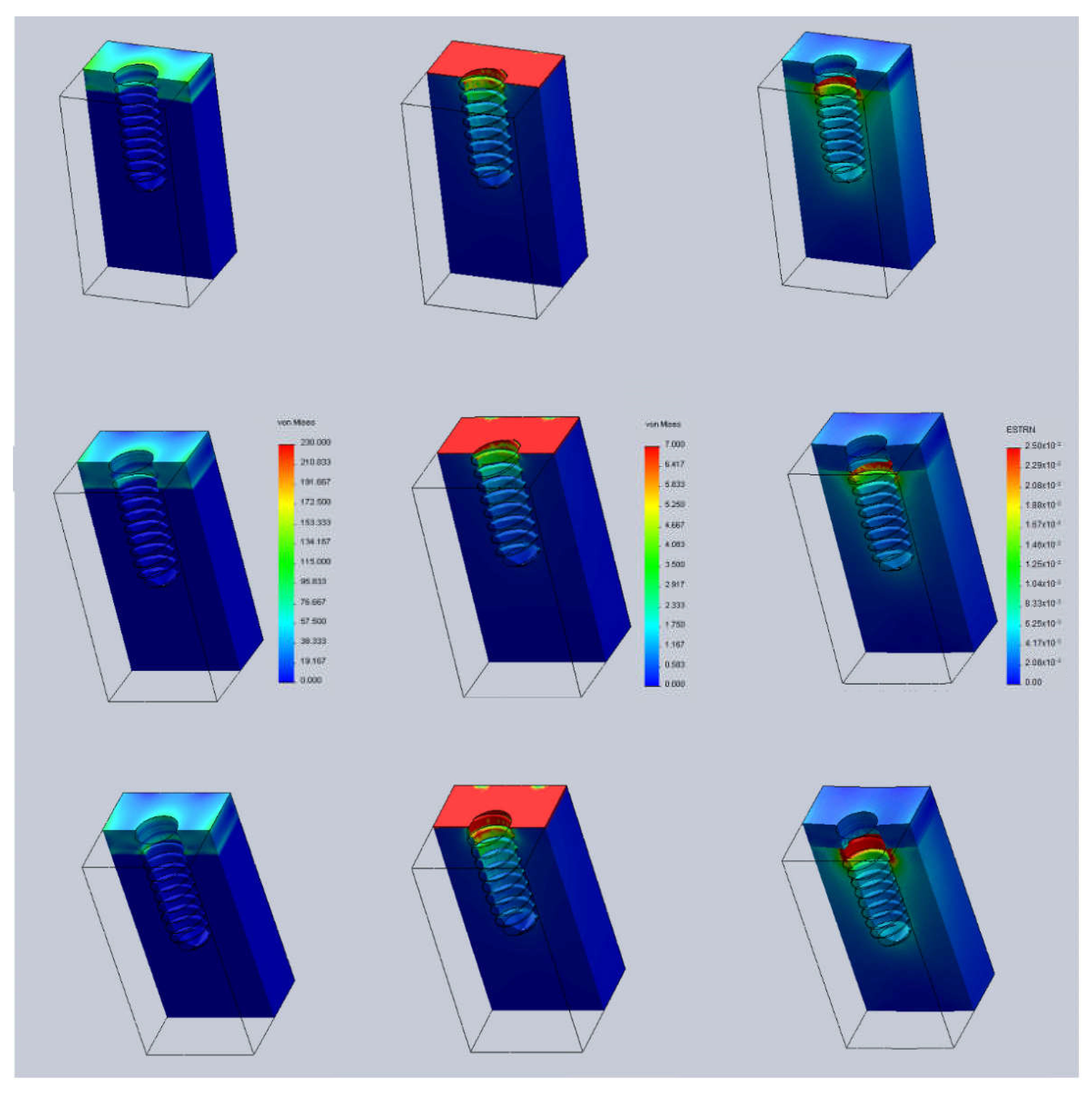
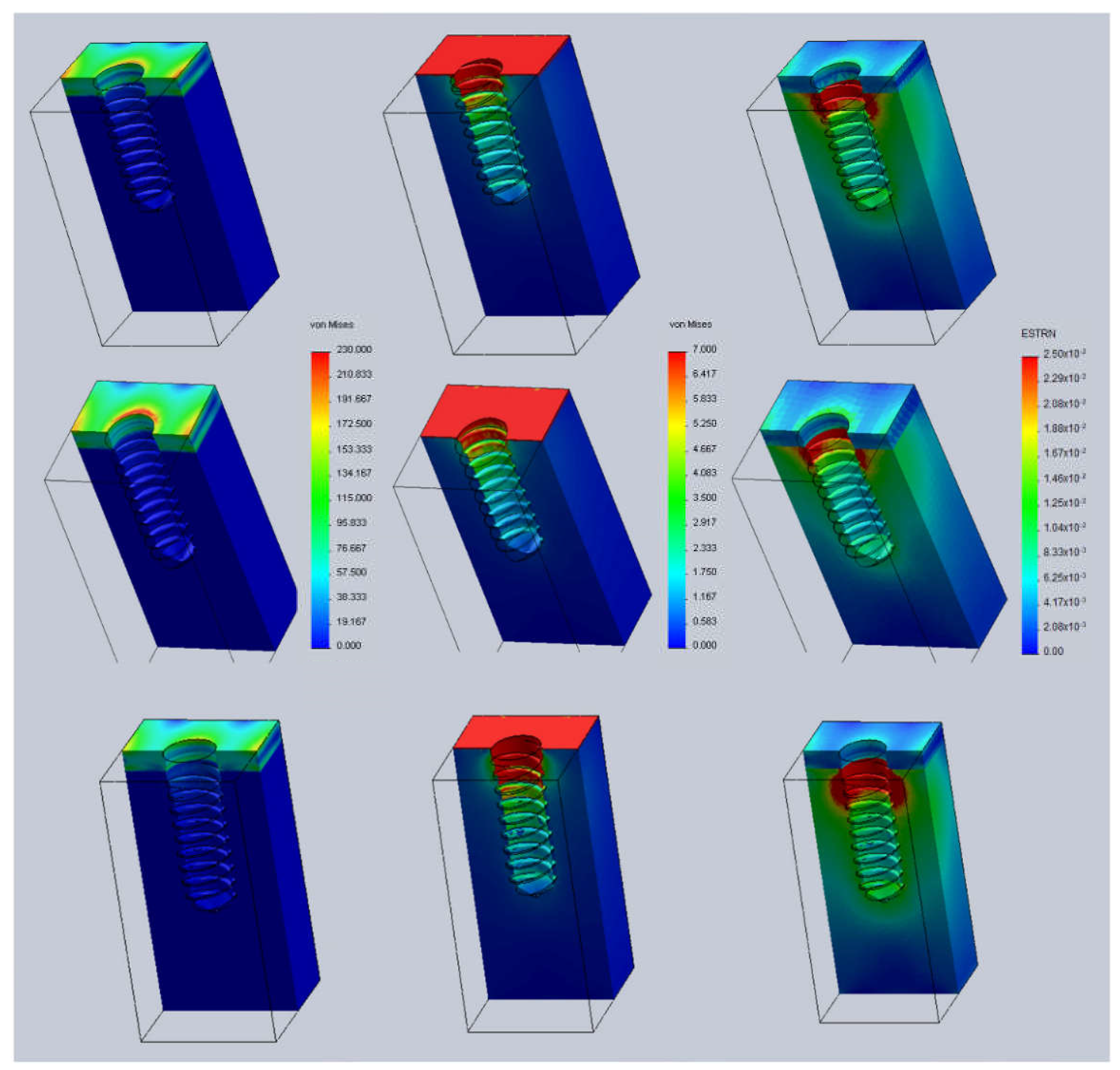
3. Results and Discussion
- Case A: 0.5 mm above the bone surface (h = +0.5).
- Case B: at the same level of the bone surface (h = 0).
- Case C: 0.5 mm below the bone surface (h = −0.5).
- t = 1.5 mm (cortical thickness).
- t = 2.5 mm (cortical thickness).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BAT | Total bone area |
| BIC | Bone–implant contact |
| FEA | Finite element analysis |
| FEM | Finite element method |
| GPa | GigaPascals |
| h | Height |
| mm | Millimeter |
| MPa | MegaPascals |
| ROI | Region of interest |
| SEM | Scanning electron microscopy |
| 3D | Three-dimensional |
References
- Canullo, L.; Micarelli, C.; Lembo-Fazio, L.; Iannello, G.; Clementini, M. Microscopical and microbiologic characterization of customized titanium abutments after different cleaning procedures. Clin. Oral Implant. Res. 2014, 25, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Gil, J.; Pérez, R.; Herrero-Climent, M.; Rizo-Gorrita, M.; Torres-Lagares, D.; Gutierrez, J.L. Benefits of residual aluminium oxide for sand blasting titanium dental implants: Osseointegration and bactericidal effects. Materials 2022, 15, 178. [Google Scholar] [CrossRef]
- Herrero-Climent, M.; López-Jarana, P.; Lemos, B.F.; Gil, F.J.; Falcao, C.; Rios-Santos, J.V.; Rios-Carrasco, B. Relevant design aspects to improve the stability of titanium dental implants. Materials 2020, 13, 1910. [Google Scholar] [CrossRef] [Green Version]
- Nicolas-Silvente, A.I.; Velasco-Ortega, E.; Ortiz-García, I.; Monsalve-Guil, L.; Gil, F.J.; Jimenez-Guerra, A. Influence of the Titanium implants surface treatment on the surface roughness and chemical composition. Materials 2020, 13, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Ciurana, X.; Vela-Nebot, X.; Segalà-Torres, M.; Calvo-Guirado, J.L.; Camba, J.; Méndez-Blando, V. The effect of interimplant distance on the height of the interimplant bone crest when using platform-switched implants. Int. J. Periodontics Restor. Dent. 2009, 29, 141–151. [Google Scholar]
- Linkevicius, T.; Apse, P.; Grybauskas, S.; Puisys, A. Th influence of soft tissue thickness on crestal bone changes around implants: A 1-year prospective controlled clinical trial. Int. J. Oral Maxillofac. Implant. 2009, 24, 712–719. [Google Scholar]
- Naert, I.; Duyck, J.; Vandamme, K. Occlusal overload and bone/implant loss. Clin. Oral Implant. Res. 2012, 23, 95–107. [Google Scholar] [CrossRef]
- Prosper, L.; Redaelli, S.; Pasi, M.; Zarone, F.; Radaelli, G.; Gherlone, E.F. A randomized prospective multicenter trial evaluating the platform-switching technique for the prevention of postrestorative crestal bone loss. Int. J. Oral Maxillofac. Implant. 2009, 24, 299–308. [Google Scholar]
- Canullo, L.; Fedele, G.R.; Iannello, G.; Jepsen, S. Platform switching and marginal bone-level alterations: The results of a randomized-controlled trial. Clin. Oral Implant. Res. 2010, 21, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Romanos, G.E. Wound healing in immediately loaded implants. Periodontology 2000 2015, 68, 153–167. [Google Scholar] [CrossRef]
- Hoyos, M.; Velasco, F.; Ginebra, M.P.; Manero, J.M.; Gil, F.J.; Mas-Moruno, C. Regenerating bone via multifunctional coatings: The blending of cell integration and bacterial inhibition properties on the Surface of biomaterials. ACS Appl. Mater. Interfaces 2019, 11, 36449–36457. [Google Scholar] [CrossRef]
- Hoyos-Nogués, M.; Buxadera-Palomero, J.; Ginebra, M.P.; Manero, J.M.; Gil, F.J.; Mas-Moruno, C. All-in-One trifunctional strategy: A cell adhesive, bacteriostatic and bactericidal coating for titanium implants. Colloids Surf. B Biointerfaces 2018, 169, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implant. 1986, 1, 11–25. [Google Scholar]
- Roos-Jansaker, A.M.; Lindahl, C.; Renvert, H.; Renvert, S. Nine-to fourteen-year follow-up of implant treatment. Part II: Presence of peri-implant lesions. J. Clin. Periodontol. 2006, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Jemt, T.; Lekholm, U. Single implants and buccal bone grafts in the anterior maxilla: Measurements of buccal crestal contours in a 6-year prospective clinical study. Clin. Implant Dent. Relat. Res. 2005, 7, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Pellicer-Chover, H.; Díaz-Sanchez, M.; Soto-Peñaloza, D.; Peñarrocha-Diago, M.A.; Canullo, L.; Peñarrocha-Oltra, D. Impact of crestal and subcrestal implant placement upon changes in marginal peri-implant bone level. A systematic review. Med. Oral Patol. Oral Y Cir. Bucal 2019, 24, 673–683. [Google Scholar] [CrossRef]
- Brånemark, P.I.; Adell, R.; Breine, U.; Hansson, B.O.; Lindström, J.; Ohlsson, A. Intra-Osseous anchorage of dental prostheses. I. Experimental studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Hermann, J.S.; Cochran, D.L.; Nummikoski, P.V.; Buser, D. Crestal bone changes around titanium implants. A radiographic evaluation of unloaded nonsubmerged and submerged implants in the canine mandible. J. Periodontol. 1997, 68, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Moreno, P.; León-Cano, A.; Ortega-Oller, I.; Monje, A.; O′Valle, F.; Catena, A. Marginal bone loss as success criterion in implant dentistry: Beyond 2 mm. Clin. Oral Implant. Res. 2015, 26, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Grusovin, M.G.; Tzanetea, E.; Piattelli, A.; Worthington, H.V. Interventions for replacing missing teeth: Treatment of perimplantitis. Cochrane Database Syst. Rev. 2010, 16, 4970. [Google Scholar] [CrossRef]
- Hämmerle, C.H.; Brägger, U.; Bürgin, W.; Lang, N.P. The effect of subcrestal placement of the polished surface of ITI implants on marginal soft and hard tissues. Clin. Oral Implant. Res. 1996, 7, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Cesaretti, G.; Lang, N.P.; Salata, L.A.; Schweikert, M.T.; Gutierrez Hernandez, M.E.; Botticelli, D. Sub-Crestal positioning of implants results in higher bony crest resorption: An experimental study in dogs. Clin. Oral Implant. Res. 2015, 26, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.E.; McGlmphy, E.A.; Johnston, W.M.; Larsen, P.E. Effects of implant design and surface roughness on crestal bone and soft tissue levels in the esthetic zone. Int. J. Oral Maxillofac. Implant. 2009, 24, 910–919. [Google Scholar]
- Broggini, N.; McManus, L.M.; Hermann, J.S.; Medina, R.U.; Oates, T.W.; Schenk, R.K. Persistent acute inflammation at the implant-abutment interface. J. Dent. Res. 2003, 82, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Piattelli, A.; Vrespa, G.; Petrone, G.; Iezzi, G.; Annibali, S.; Scarano, A. Role of the Microgap Between Implant and Abutment: A Retrospective Histologic Evaluation in Monkeys. J. Periodontol. 2003, 74, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C.; Xenoudi, P. Post-Treatment supportive care for the natural dentition and dental implants. Periodontology 2000 2016, 71, 164–184. [Google Scholar] [CrossRef] [PubMed]
- Punset, M.; Villarrasa, J.; Nart, J.; Manero, J.M.; Bosch, B.; Padrós, R.; Perez, R.A.; Gil, F.J. Citric Acid Passivation of Titanium Dental Implants for Minimizing Bacterial Colonization Impact. Coatings 2021, 11, 214. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Wang, Z.; Shen, Y.; Manero, J.M.; Gil, F.J.; Rodriguez, D.; Haapasalo, M. Antibacterial coatings on titanium surfaces: A comparison study between in vitro single-species and multispecies biofilm. ACS Appl. Mater. Interfaces 2015, 7, 5992–6001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godoy-Gallardo, M.; Manzanares-Céspedes, M.C.; Sevilla, P.; Nart, J.; Manzanares, N.; Manero, J.M.; Gil, F.J.; Boyd, S.K.; Rodríguez, D. Evaluation of bone loss in antibacterial coated dental implants: An experimental study in dogs. Mater. Sci. Eng. C 2016, 69, 538–545. [Google Scholar] [CrossRef] [Green Version]
- Gil, F.J.; Rodriguez, A.; Espinar, E.; Llamas, J.M.; Padulles, E.; Juarez, A. Effect of the oral bacteria on the mechanical behavior of titanium dental implants. Int. J. Oral Maxillofac. Implant. 2012, 27, 64–68. [Google Scholar] [CrossRef]
- Buser, D.; Weber, H.P.; Lang, N.P. Tissue integration of non-submerged implants. 1-year results of a prospective study with 100 ITI hollow-cylinder and hollow-screw implants. Clin. Oral Implant. Res. 1990, 1, 33–40. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T.; Andersson, B.; Krol, J.J. A histomorphometric and removal torque study of screw-shaped titanium implants with three different surface topographies. Clin. Oral Implant. Res. 1995, 6, 24–30. [Google Scholar] [CrossRef]
- Degidi, M.; Perrotti, V.; Shibli, J.A.; Novaes, A.B.; Piattelli, A.; Iezzi, G. Equicrestal and subcrestal dental implants: A histologic and histomorphometric evaluaton of nine retrieved human implants. J. Periodontol. 2011, 82, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Donovan, R.; Fetner, A.; Koutouzis, T.; Lundgren, T. Crestal bone changes around implants with reduced abutment diameter placed non-submerged and at subcrestal positions: A 1-year radiographic evaluation. J. Periodontol. 2010, 81, 428–434. [Google Scholar] [CrossRef]
- Aimetti, M.; Ferrarotti, F.; Mariani, G.M.; Ghelardoni, C.; Romano, F. Soft tissue and crestal bone changes around implants with platform-switched abutments placed nonsubmerged at subcrestal position: A 2-year clinical and radiographic evaluation. Int. J. Oral Maxillofac. Implant. 2015, 30, 1369–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, M.; Grusovin, M.G.; Polyzos, I.P.; Felice, P.; Worthington, H.V. Interventions for replacing missing teeth: Dental implants in fresh extraction sockets (immediate, immediate-delayed and delayed implants). Cochrane Database Syst. Rev. 2010, 8. [Google Scholar] [CrossRef]
- Sanz, I.; Garcia-Gargallo, M.; Herrera, D.; Martin, C.; Figuero, E.; Sanz, M. Surgical protocols for early implant placement in post-extraction sockets: A systematic review. Clin. Oral Implant. Res. 2012, 23 (Suppl. S5), 67–79. [Google Scholar] [CrossRef] [PubMed]
- Poiate, I.A.; Vasconcellos, A.B.; Mori, M.; Poiate, E., Jr. 2D and 3D finite element analysis of central incisor generated by computerized tomography. Comput. Methods Programs Biomed. 2011, 104, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Wennerberg, A. On osseointegration in relation to implant surfaces. Clin. Implant Dent. Relat. Res. 2019, 21 (Suppl. S1), 4–7. [Google Scholar] [CrossRef] [Green Version]
- Javed, F.; Ahmed, H.B.; Crespi, R.; Romanos, G.E. Role of primary stability for successful osseointegration of dental implants: Factors of influence and evaluation. Interv. Med. Appl. Sci. 2013, 5, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Tribst, J.P.M.; Dal Piva, A.M.D.O.; Lo Giudice, R.; Borges, A.L.S.; Bottino, M.A.; Epifania, E.; Ausiello, P. The Influence of Custom-Milled Framework Design for an Implant-Supported Full-Arch Fixed Dental Prosthesis: 3D-FEA Study. Int. J. Environ. Res. Public Health 2020, 17, 4040. [Google Scholar] [CrossRef]
- Penteado, M.M.; Tribst, J.P.M.; Dal Piva, A.M.; Ausiello, P.; Zarone, F.; Garcia-Godoy, F.; Borges, A.L. Mechanical behavior of conceptual posterior dental crowns with functional elasticity gradient. Am. J. Dent. 2019, 32, 165–168. [Google Scholar] [PubMed]
- Ausiello, P.; Tribst, J.P.M.; Ventre, M.; Salvati, E.; di Lauro, A.E.; Martorelli, M.; Lanzotti, A.; Watts, D.C. The role of cortical zone level and prosthetic platform angle in dental implant mechanical response: A 3D finite element analysis. Dent. Mater. 2021, 37, 1688–1697. [Google Scholar] [CrossRef] [PubMed]
- SolidWorks User’s Manual. Dassault Systèmes SolidWorks Corp. 2021. Available online: https://www.solidworks.com (accessed on 2 May 2021).
- Liebschner, M.A.K. Biomechanical consideration of animal models used in tissue engineering of bone. Biomaterials 2004, 25, 1697–1714. [Google Scholar] [CrossRef]
- van Eijden, T. Biomechanics of the mandible. Crit. Rev. Oral Biol. Med. 2000, 11, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, A.M. Primary stability and initial bone-to-implant contact: The effects on immediate placement and restoration of dental implants. J. Implant. Reconstr. Dent. 2009, 1, 35–41. [Google Scholar]
- Boyer, R.; Welsch, G.; Collings, E.W. Materials Properties Handbook: Titanium Alloys; ASM International: Almere, The Netherlands, 1994; Available online: https://books.google.es/books?id=x3rToHWOcD8C&printsec=frontcover&hl=ca#v=onepage&q&f=false (accessed on 17 May 2018).
- Middleton, J.; Jones, M.L.; Pande, G.N. Computer Methods in Biomechanics & Biomedical Engineering—2; Gordon and Breach Science Publishers: Philadelphia, PA, USA, 1998; Available online: https://www.taylorfrancis.com/books/edit/10.1201/9781003078289/computer-methods-biomechanics-biomedical-engineering-2-middleton-jones-pande (accessed on 17 May 2018).
- Zienkiewicz, O.C. The Finite Element Method; McGraw-Hill Ed.: London, UK, 1977. [Google Scholar]
- Gallagher, R.H. Finite Element Analysis: Fundamentals; Prentice-Hall: New York, NY, USA, 1974. [Google Scholar]
- Weblet Importer. Available online: http://www.cimne.com/comet/.Barcelona.Spain (accessed on 17 May 2018).
- Bassir, S.H.; El Kholy, K.; Chen, C.Y.; Lee, K.H.; INtini, G. Outcome of early dental implant placement versus other dental implant placement protocols: A systematic review and meta-analysis. J. Periodontol. 2019, 90, 493–506. [Google Scholar] [CrossRef] [PubMed]
- de Siqueira, R.A.C.; Junior, R.S.G.; dos Santos, P.G.F.; de Mattias Sartori, I.A.; Wang, H.-L.; Fontão, F.N.G.K. Effect of different implant placement depths on crestal bone levels and soft tissue behavior: A 5-year randomized clinical trial. Clin. Oral Implant. Res. 2020, 31, 282–293. [Google Scholar] [CrossRef]
- Al Amri, M.D.; Al-Johany, S.S.; Al Baker, A.M.; Al Rifaiy, M.Q.; Abduljabbar, T.S.; Al-Kheraif, A.A. Soft tissue changes and crestal bone loss around platform-switched implants placed at crestal and subcrestal levels: 36-month results from a prospective split-mouth clinical trial. Clin. Oral Implant. Res. 2017, 28, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Koh, R.U.; Oh, T.J.; Rudek, I.; Neiva, G.F.; Misch, C.E.; Rothman, E.D.; Wang, H.L. Hard and soft tissue changes after crestal and subcrestal immediate implant placement. J. Periodontol. 2011, 82, 1112–1120. [Google Scholar] [CrossRef] [Green Version]
- Koutouzis, T.; Neiva, R.; Nair, M.; Nonhoff, J.; Lundgren, T. Cone beam computed tomographic evaluation of implants withplatform-switched Morse taper connection with the implant-abutment interface at different levels in relation to the alveolar crest. Int. J. Oral Maxillofac. Implant. 2014, 29, 1157–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palaska, I.; Tsaousoglou, P.; Vouros, I.; Konstantinidis, A.; Menexes, G. Influence of placement depth and abutment connection pattern on bone remodeling around 1-stage implants: A prospective randomized controlled clinical trial. Clin. Oral Implant. Res. 2016, 27, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.R.; Novaes, A.B., Jr.; Muglia, V.A.; Iezzi, G.; Piattelli, A. Influence of interimplant distances and placement depth on peri-implant bone remodeling of adjacent and immediately loaded Morse cone connection implants: A histomorphometric study in dogs. Clin. Oral Implant. Res. 2010, 21, 371–378. [Google Scholar] [CrossRef]
- Monje, A.; Galindo-Moreno, P.; Tozum, T.F.; Suarez-Lopez del Amo, F.; Wang, H.L. Into the paradigm of local factors as contributors for peri-implant disease: Short communication. Int. J. Oral Maxillofac. Implant. 2016, 31, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Novaes, A.B., Jr.; Barros, R.R.; Muglia, V.A.; Borges, G.J. Influence of interimplant distances and placement depth on papilla formation and crestal resorption: A clinical and radiographic study in dogs. J. Oral Implantol. 2009, 35, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Novoa, L.; Batalla, P.; Caneiro, L.; Pico, A.; Linares, A.; Blanco, J. Influence of abutment height on maintenance of peri-implant crestal bone at bone-level implants: A 3-year follow-up study. Int. J. Periodontics Restor. Dent. 2017, 37, 721–727. [Google Scholar] [CrossRef]
- Schwarz, F.; Becker, K.; Sahm, N.; Horstkemper, T.; Rousi, K.; Becker, J. The prevalence of peri-implant diseases for two-piece implants with an internal tube-in-tube connection: A cross-sectional analysis of 512 implants. Clin. Oral Implant. Res. 2017, 28, 24–28. [Google Scholar] [CrossRef]
- Vervaeke, S.; Matthys, C.; Nassar, R.; Christiaens, V.; Cosyn, J.; De Bruyn, H. Adapting the vertical position of implants with a conical connection in relation to soft tissue thickness prevents early implant surface exposure: A 2-year prospective intra-subject comparison. J. Clin. Periodontol. 2018, 45, 605–612. [Google Scholar] [CrossRef]
- Bennardo, F.; Barone, S.; Vocaturo, C.; Nucci, L.; Antonelli, A.; Giudice, A. Usefulness of Magnetic Mallet in Oral Surgery and Implantology: A Systematic Review. J. Pers. Med. 2022, 12, 108. [Google Scholar] [CrossRef]
- Dávila, E.; Ortiz-Hernández, M.; Perez, R.A.; Herrero-Climent, M.; Cerrolaza, M.; Gil, F.J. Crestal module design optimization of dental implants: Finite element analysis and in vivo studies. J. Mater. Sci. Mater. Med. 2019, 30, 90. [Google Scholar] [CrossRef]
- Tribst, J.P.M.; de Morais, D.C.; Melo de Matos, J.D.; Lopes, G.D.R.S.; Dal Piva, A.M.; Souto Borges, A.L.; Bottino, M.A.; Lanzotti, A.; Martorelli, M.; Ausiello, P. Influence of Framework Material and Posterior Implant Angulation in Full-Arch All-on-4 Implant-Supported Prosthesis Stress Concentration. Dent. J. 2022, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Laurell, L.; Lundgren, D. Marginal bone level changes at dental implants after 5 years in function: A meta-analysis. Clin. Implant. Dent. Relat. Res. 2011, 13, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Cosola, S.; Marconcini, S.; Boccuzzi, M.; Menchini Fabris, G.B.; Covani, U.; Peñarrocha-Diago, M.; Peñarrocha-Oltra, D. Radiological Outcomes of Bone-Level and Tissue-Level Dental Implants: Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 6920. [Google Scholar] [CrossRef] [PubMed]
- Vouros, I.D.; Kalpidis, C.D.R.; Horvath, A.; Petrie, A.; Donos, N. Systematic Assessment of Clinical Outcomes in Bone-Level and Tissue-Level Endosseous Dental Implants. Int. J. Oral Maxillofac. Implant. 2012, 27, 1359–1374. [Google Scholar]
- Eser, A.; Tonuk, E.; Akca, K.; Dard, M.; Cehleri, M. Predicting bone remodeling around tissue-and bone-level dental implants used in reduced bone width. J. Biomech. 2013, 46, 2250–2257. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Sánchez, M.; Soto-Peñaloza, D.; Peñarrocha-Oltra, D.; Peñarrocha-Diago, M. Influence of supracrestal tissue attachment thickness on radiographic bone level around dental implants: A systematic review and meta-analysis. J. Periodontal Res. 2019, 54, 573–588. [Google Scholar] [CrossRef]
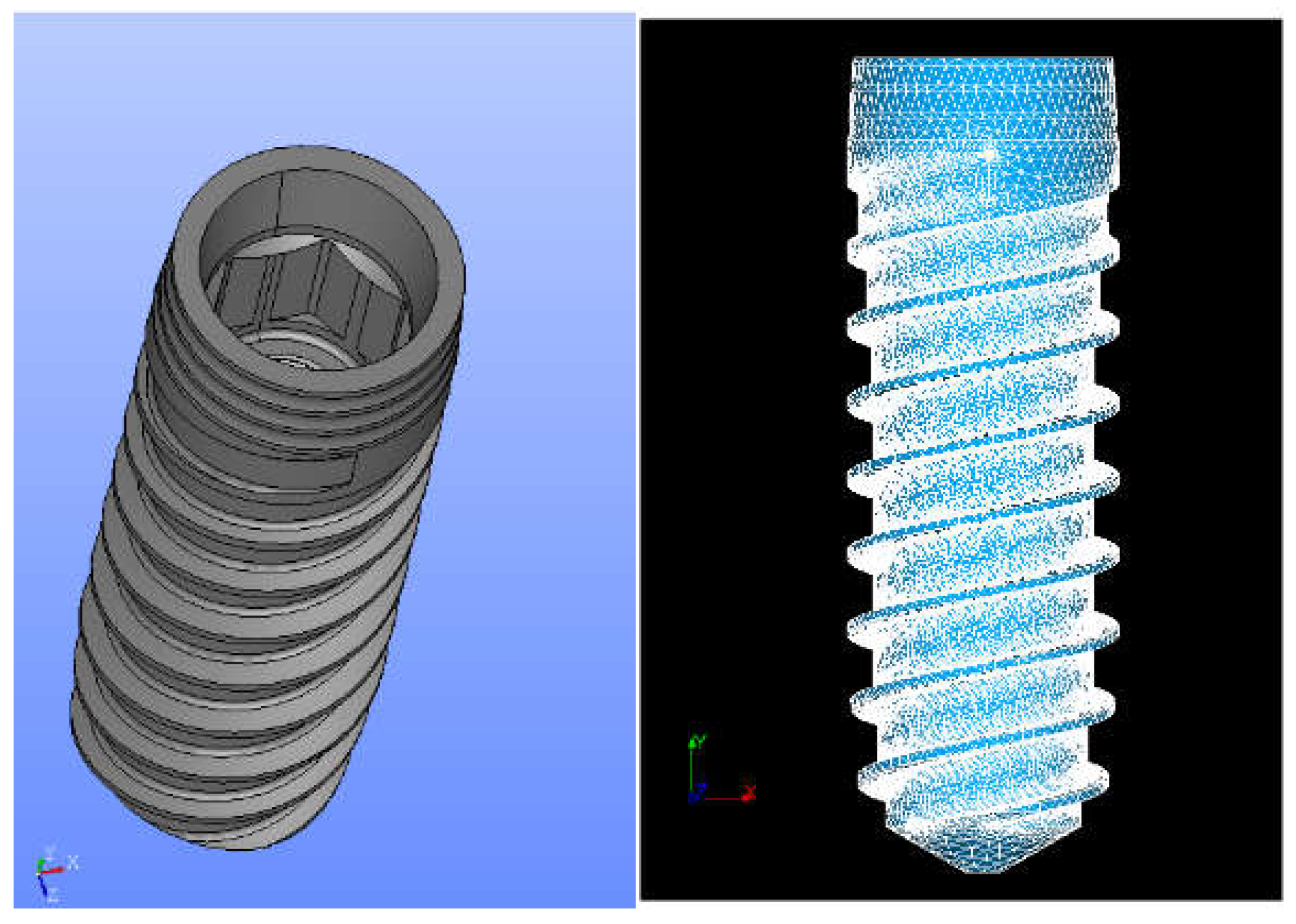
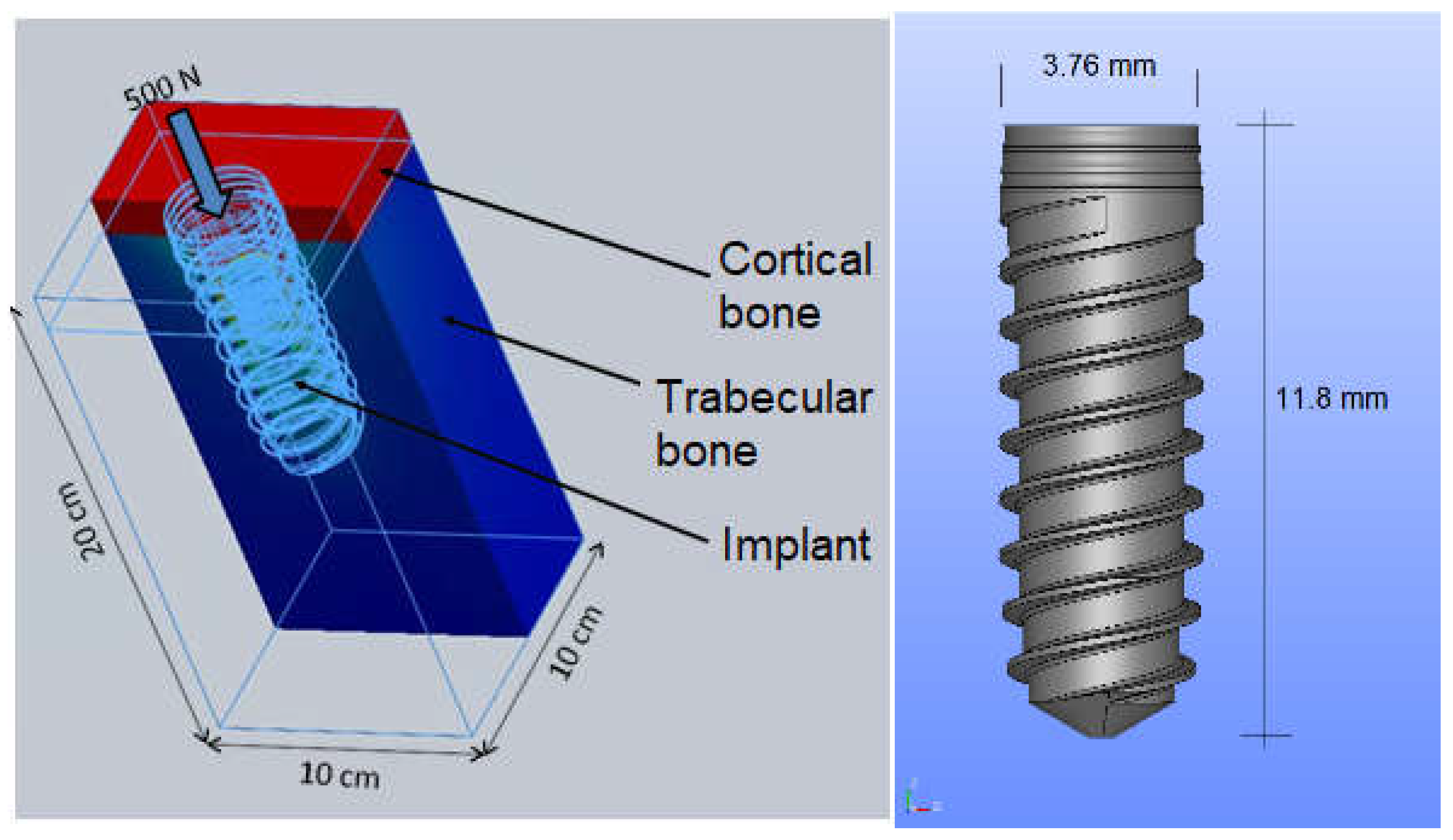
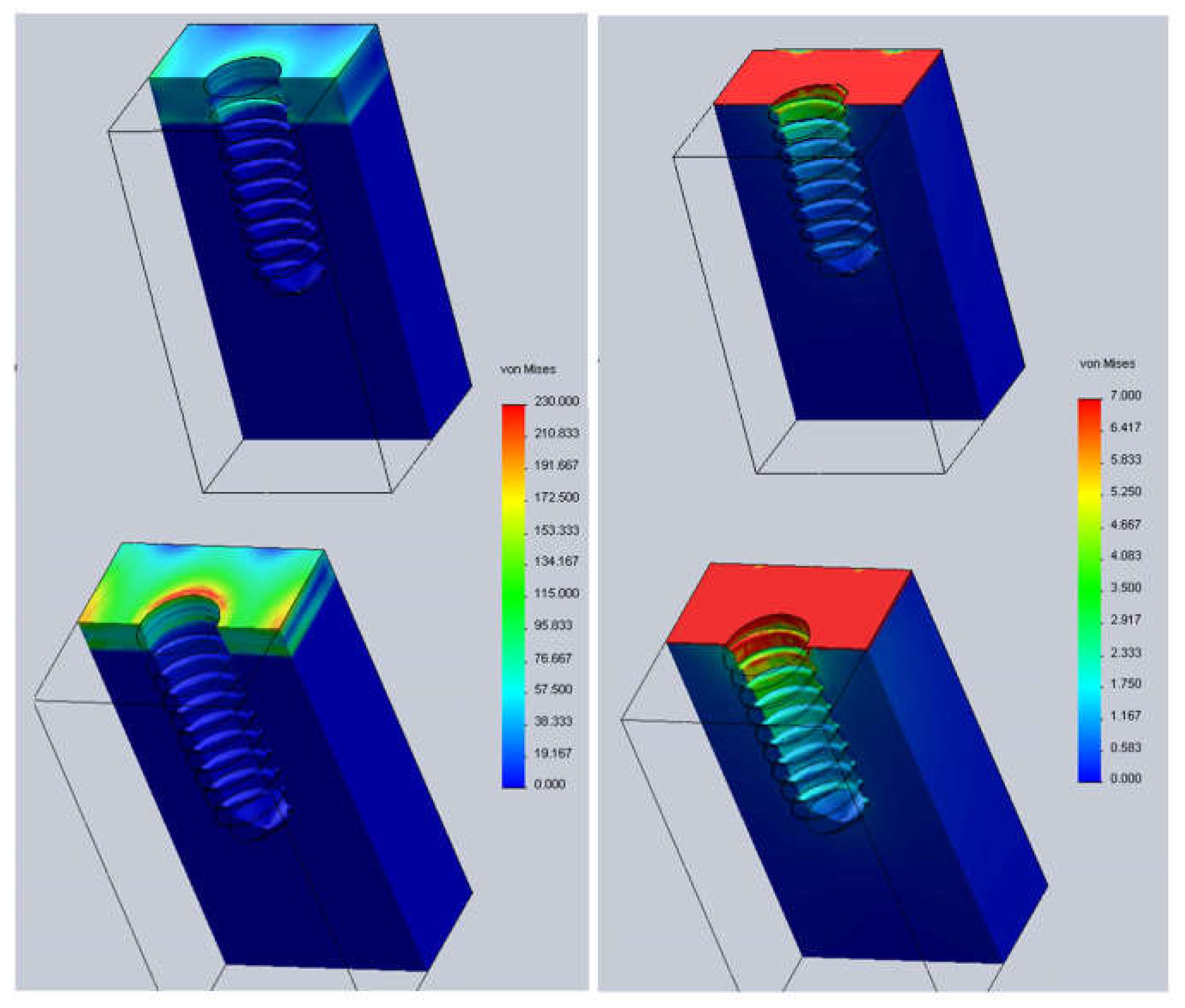
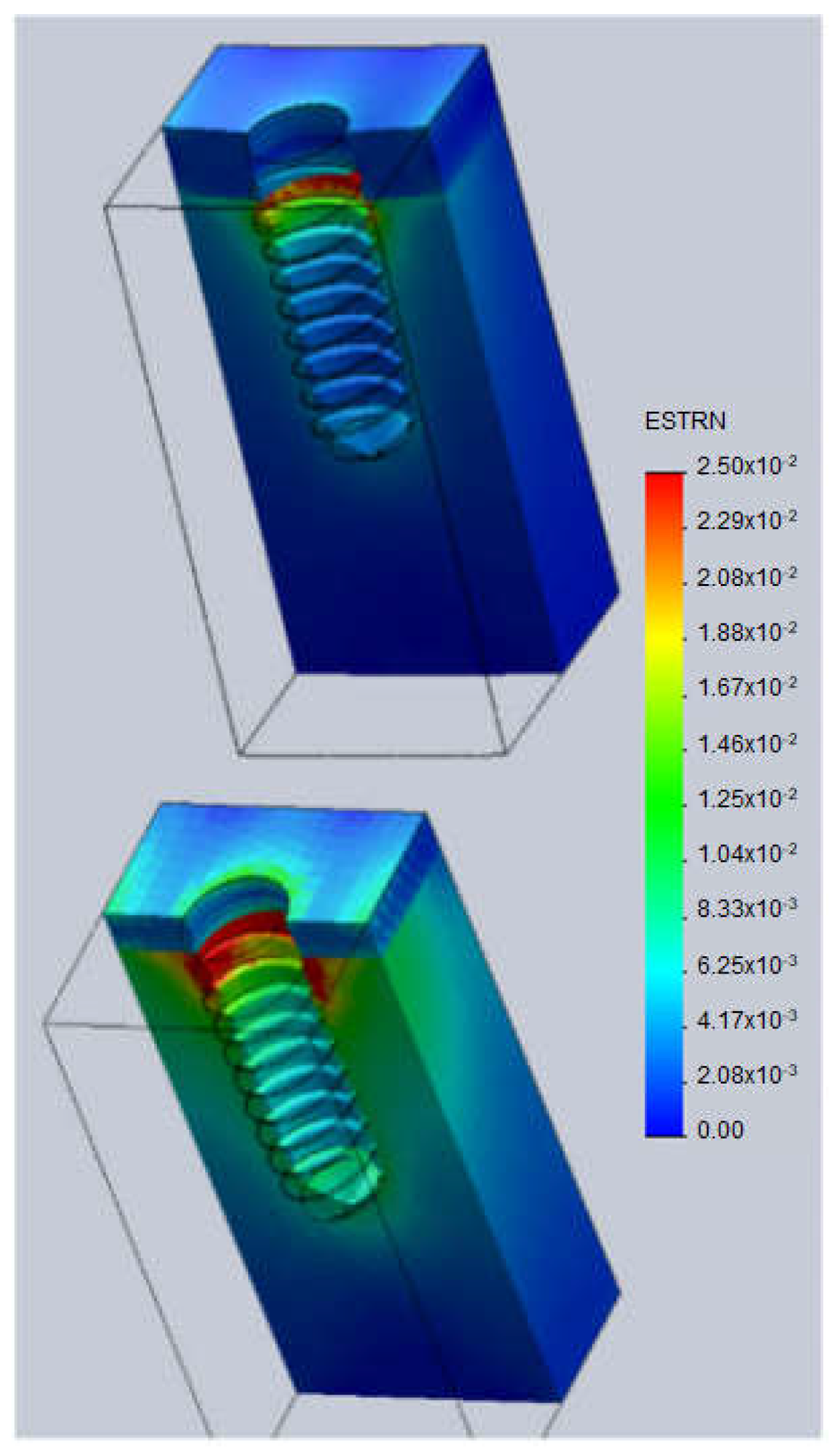



| Material | Young’s Modulus (MPa) | Poisson’s Ratio |
|---|---|---|
| Implant | 110,000 | 0.34 |
| Cortical bone | 19,400 | 0.30 |
| Trabecular bone | 5600 | 0.28 |
| BIC | 3 Weeks | 6 Weeks |
| Above 0.5 mm | 22% ± 5% | 27% ± 8% |
| Bone level | 29% ± 6% | 33% ± 9% |
| Below 0.5 mm | 35% ± 6% * | 55% ± 8% * |
| BAT | 3 Weeks | 6 Weeks |
| Above 0.5 mm | 26% ± 4% | 32% ± 7% |
| Bone level | 32% ± 9% | 40% ± 8% |
| Below 0.5 mm | 40% ± 6% * | 62% ± 5% * |
| ROI | 3 Weeks | 6 Weeks |
| Above 0.5 mm | 18 ± 7% | 25% ± 6% |
| Bone level | 24% ± 6% | 38% ± 8% |
| Below 0.5 mm | 30% ± 5% * | 55% ± 7% * |
| BIC | 3 Weeks | 6 Weeks |
| Above 0.5 mm | 12% ± 3% | 19% ± 5% |
| Bone level | 18% ± 6% | 25% ± 9% |
| Below 0.5 mm | 29% ± 4% * | 39% ± 9% * |
| BAT | 3 Weeks | 6 Weeks |
| Above 0.5 mm | 16% ± 4% | 27% ± 8% |
| Bone level | 26% ± 6% | 35% ± 9% |
| Below 0.5 mm | 35% ± 5% * | 48% ± 5% * |
| ROI | 3 Weeks | 6 Weeks |
| Above 0.5 mm | 20 ± 5% | 26% ±4% |
| Bone level | 25% ± 6% | 32% ± 5% |
| Below 0.5 mm | 29% ± 8% * | 45% ± 8% * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil, J.; Sandino, C.; Cerrolaza, M.; Pérez, R.; Herrero-Climent, M.; Rios-Carrasco, B.; Rios-Santos, J.V.; Brizuela, A. Influence of Bone-Level Dental Implants Placement and of Cortical Thickness on Osseointegration: In Silico and In Vivo Analyses. J. Clin. Med. 2022, 11, 1027. https://doi.org/10.3390/jcm11041027
Gil J, Sandino C, Cerrolaza M, Pérez R, Herrero-Climent M, Rios-Carrasco B, Rios-Santos JV, Brizuela A. Influence of Bone-Level Dental Implants Placement and of Cortical Thickness on Osseointegration: In Silico and In Vivo Analyses. Journal of Clinical Medicine. 2022; 11(4):1027. https://doi.org/10.3390/jcm11041027
Chicago/Turabian StyleGil, Javier, Clara Sandino, Miguel Cerrolaza, Román Pérez, Mariano Herrero-Climent, Blanca Rios-Carrasco, Jose Vicente Rios-Santos, and Aritza Brizuela. 2022. "Influence of Bone-Level Dental Implants Placement and of Cortical Thickness on Osseointegration: In Silico and In Vivo Analyses" Journal of Clinical Medicine 11, no. 4: 1027. https://doi.org/10.3390/jcm11041027
APA StyleGil, J., Sandino, C., Cerrolaza, M., Pérez, R., Herrero-Climent, M., Rios-Carrasco, B., Rios-Santos, J. V., & Brizuela, A. (2022). Influence of Bone-Level Dental Implants Placement and of Cortical Thickness on Osseointegration: In Silico and In Vivo Analyses. Journal of Clinical Medicine, 11(4), 1027. https://doi.org/10.3390/jcm11041027











