Did the COVID-19 Pandemic Truly Adversely Affect Disease Progress and Therapeutic Options in Breast Cancer Patients? A Single-Centre Analysis
Abstract
1. Introduction
2. Material and Methods
2.1. Patient Group
2.2. Evaluated Clinical Data
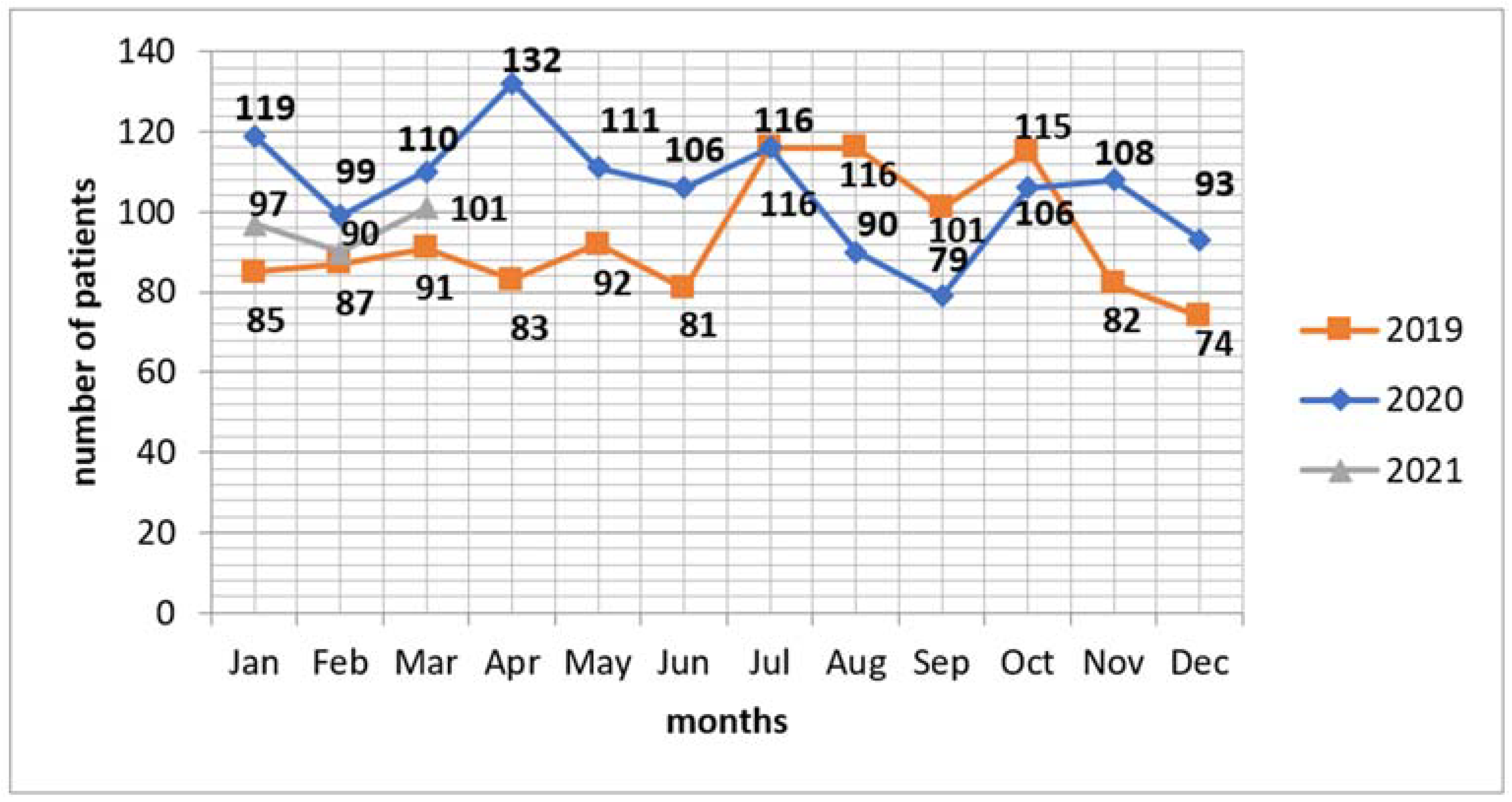
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Cucinotta, D.; Vanelli, M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Barclay, M.E.; Abel, G.A.; Greenberg, D.C.; Rous, B.; Lyratzopoulos, G. Socio-demographic variation in stage at diagnosis of breast, bladder, colon, endometrial, lung, melanoma, prostate, rectal, renal and ovarian cancer in England and its population impact. Br. J. Cancer 2021, 124, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Sud, A.; Torr, B.; Jones, M.; Broggio, J.; Scott, S.; Loveday, C.; Garrett, A.; Gronthoud, F.; Nicol, D.L.; Jhanji, S.; et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: A modelling study. Lancet Oncol. 2020, 21, 1035–1044. [Google Scholar] [CrossRef]
- Baxter, M.A.; Murphy, J.; Cameron, D.; Jordan, J.; Crearie, C.; Lilley, C.; Sadozye, A.; Maclean, M.; Hall, P.; Phillips, A.; et al. The impact of COVID-19 on systemic anticancer treatment delivery in Scotland. Br. J. Cancer 2021, 124, 1353–1356. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.G.; Pasea, L.; Banerjee, A.; Hall, G.; Denaxas, S.; Chang, W.H.; Katsoulis, M.; Williams, B.; Pillay, D.; Noursadeghi, M.; et al. Estimated impact of the COVID-19 pandemic on cancer services and excess 1-year mortality in people with cancer and multimorbidity: Near real-time data on cancer care, cancer deaths and a population-based cohort study. BMJ Open 2020, 10, e043828. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Piper-Vallillo, A.J.; Bindal, P.; Wischhusen, J.; Patel, J.M.; Costa, D.B.; Peters, M. Time to SARS-CoV-2 Clearance Among Patients with Cancer and COVID-19. medRxiv Prepr. Serv. Health Sci. 2020, 7, 23.20161000. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Blair, S.L.; Burstein, H.J.; Dang, C.; Elias, A.D.; et al. NCCN Guidelines® Insights: Breast Cancer, Version 4.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Anil, I.; Arnold, R.; Benkwitz-Beford, S. The UK Coronavirus cancer monitoring project: Protecting patients with cancer in the era of COVID-19. Lancet Oncol. 2020, 21, 622–624. [Google Scholar] [CrossRef]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.-Y.; Desai, A.; de Lima Lopes, G., Jr.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef]
- Curigliano, G.; Burstein, H.; Winer, E.; Gnant, M.; Dubsky, P.; Loibl, S.; Colleoni, M.; Regan, M.; Piccart-Gebhart, M.; Senn, H.-J.; et al. Correction to: De-escalating and escalating treatments for early-stage breast cancer: The St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann. Oncol. 2018, 29, 2153. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast Cancer Version 4. 2021. Available online: https://www.nccn.org/professionals/physician_gls/ (accessed on 24 April 2021).
- Giuliano, A.E.; Edge, S.B.; Hortobagyi, G.N. Eighth Edition of the AJCC Cancer Staging Manual: Breast Cancer. Ann. Surg. Oncol. 2018, 25, 1783–1785. [Google Scholar] [CrossRef] [PubMed]
- Kozierkiewicz, A.; Topor-Madry, R.; Śliwczynski, A.; Pakulski, M.; Jassem, J. Effectiveness and costs of breast cancer therapy in Poland: A regional approach. Nowotwory. J. Oncol. 2014, 64, 24–32. [Google Scholar] [CrossRef]
- Gathani, T.; Clayton, G.; MacInnes, E.; Horgan, K. The COVID-19 pandemic and impact on breast cancer diagnoses: What happened in England in the first half of 2020. Br. J. Cancer 2020, 124, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Elsheikh, M.; Gilmour, K.; Cohen, V.; Sagoo, M.S.; Damato, B.; Anguita, R.; Heimann, H.; Hussain, R.; Cauchi, P.; et al. Impact of COVID-19 pandemic on eye cancer care in United Kingdom. Br. J. Cancer 2021, 124, 1357–1360. [Google Scholar] [CrossRef] [PubMed]
- Zapała, P.; Ślusarczyk, A.; Rajwa, P.; Przydacz, M.; Krajewski, W.; Dybowski, B.; Kubik, P.; Kuffel, B.; Przudzik, M.; Osiecki, R.; et al. Not as black as it is painted? The impact of the first wave of COVID-19 pandemic on surgical treatment of urological cancer patients in Poland—A cross-country experience. Arch. Med. Sci. 2021, 1–6. [Google Scholar] [CrossRef]
- Rutter, M.D.; Brookes, M.; Lee, T.J.; Rogers, P.; Sharp, L. Impact of the COVID-19 pandemic on UK endoscopic activity and cancer detection: A National Endoscopy Database Analysis. Gut 2020, 70, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Drinka, E.; Allen, P.K.; McBride, A.; Buchholz, T.; Sahin, A. Metastatic Tumor Volume and Extranodal Tumor Extension: Clinical Significance in Patients with Stage II Breast Cancer. Arch. Pathol. Lab. Med. 2015, 139, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- McCormack, V.; Aggarwal, A. Early cancer diagnosis: Reaching targets across whole populations amidst setbacks. Br. J. Cancer 2021, 124, 1181–1182. [Google Scholar] [CrossRef] [PubMed]
| Clinical Data Analysed | Group I (Pre-Pandemic Period) | Group II (Pandemic Period) | p |
|---|---|---|---|
| n = 1355 | n = 1262 | ||
| Age (range) | 59.2 ± 12.2 (27–91) | 58.8 ± 12.3 (24–90) | 0.392 |
| Histological form of cancer: | 0.369 | ||
| -Invasive | 1261 (93.1) | 1189 (94.2) | |
| -Preinvasive (DCIS, LCIS) | 94 (6.9) | 73 (5.8) | |
| Palpability of a tumour | 891 (65.9) | 924 (73.3) | <0.001 |
| Tumour size—clinical evaluation [mm] (range) | 26.0 ± 18.9 (5–200) | 27.3 ± 19.0 (4–150) | 0.033 |
| Tumour size—clinical evaluation: | |||
| -cT0 | 2 (0.2) | 0 | 0.172 |
| -cT1 | 662 (48.9) | 528 (41.8) | <0.001 |
| -cT2 | 528 (39.0) | 565 (44.8) | 0.003 |
| -cT3 | 78 (5.8) | 100 (7.9) | 0.028 |
| -cT4 | 82 (6.1) | 66 (5.2) | 0.363 |
| Metastatic lesions—clinical evaluation: | |||
| -cN0 | 1119 (82.6) | 1043 (82.6) | 0.966 |
| -cN1 | 204 (15.1) | 194 (15.4) | 0.821 |
| -cN2 | 24 (1.8) | 21 (1.7) | 0.833 |
| -cN3 | 8 (0.6) | 4 (0.3) | 0.301 |
| Clinical stage (cTNM): | |||
| -I (I A) | 621 (45.8) | 505 (40.0) | 0.003 |
| -II: | 610 (45.0) | 647 (51.3) | 0.001 |
| II A | 453 (33.4) | 469 (37.2) | 0.046 |
| II B | 157 (11.6) | 178 (14.1) | 0.054 |
| -III: | 116 (8.6) | 103 (8.2) | 0.713 |
| III A | 34 (2.5) | 41 (3.3) | 0.257 |
| III B | 75 (5.5) | 59 (4.7) | 0.319 |
| III C | 7 (0.5) | 3 (0.2) | 0.248 |
| -IV | 8 (0.6) | 7 (0.6) | 0.904 |
| Tumour size—pathological evaluation: | n = 1006 | n = 911 | |
| -pT0 | 1 (0.1) | 0 | 0.341 |
| -pTis | 94 (9.3) | 73 (8.0) | 0.302 |
| -pT1 | 510 (50.7) | 460 (50.5) | 0.930 |
| pT1mic | 16 (1.6) | 27 (3.0) | 0.043 |
| pT1a | 53 (5.3) | 36 (4.0) | 0.171 |
| pT1b | 149 (14.8) | 113 (12.4) | 0.126 |
| pT1c | 292 (29.0) | 284 (31.2) | 0.306 |
| -pT2 | 370 (36.8) | 341 (37.4) | 0.768 |
| -pT3 | 13 (1.3) | 25 (2.7) | 0.023 |
| -pT4 | 18 (1.8) | 12 (1.3) | 0.406 |
| Tumour size—pathological evaluation [mm] (range) | 20.2 ± 19.0 (1–260) | 21.1 ± 14.4 (1–110) | 0.048 |
| Metastatic lesions—pathological evaluation: | |||
| -pN0 | 651 (64.7) | 622 (68.3) | 0.099 |
| -pN1mi | 24 (2.4) | 19 (2.1) | 0.658 |
| -pN1a | 161 (16.0) | 145 (15.9) | 0.958 |
| -pN2a | 60 (6.0) | 38 (4.2) | 0.075 |
| -pN3a | 37 (3.7) | 28 (3.1) | 0.465 |
| -pNx | 73 (7.3) | 59 (6.5) | 0.501 |
| Neoadjuvant treatment | 349 (25.8) | 351 (27.8) | 0.235 |
| BCT | 890 (65.7) | 760 (60.2) | 0.004 |
| Mastectomy | 465 (34.3) | 502 (39.8) | |
| ACT | 913 (67.4) | 850 (67.4) | 0.988 |
| ALND | 442 (32.6) | 412 (32.6) | |
| Tumour diagnosed during screening mammography * | 334/763 (43.8) | 287/711 (40.4) | 0.185 |
| Patient place of residence (voivodeship): | 0.626 | ||
| -Kujawsko-Pomorskie | 1138 (84.0) | 1051 (83.3) | |
| -Other | 217 (16.0) | 211 (16.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowikiewicz, T.; Szymankiewicz, M.; Drzewiecka, M.; Głowacka-Mrotek, I.; Tarkowska, M.; Nowikiewicz, M.; Zegarski, W. Did the COVID-19 Pandemic Truly Adversely Affect Disease Progress and Therapeutic Options in Breast Cancer Patients? A Single-Centre Analysis. J. Clin. Med. 2022, 11, 1014. https://doi.org/10.3390/jcm11041014
Nowikiewicz T, Szymankiewicz M, Drzewiecka M, Głowacka-Mrotek I, Tarkowska M, Nowikiewicz M, Zegarski W. Did the COVID-19 Pandemic Truly Adversely Affect Disease Progress and Therapeutic Options in Breast Cancer Patients? A Single-Centre Analysis. Journal of Clinical Medicine. 2022; 11(4):1014. https://doi.org/10.3390/jcm11041014
Chicago/Turabian StyleNowikiewicz, Tomasz, Maria Szymankiewicz, Marta Drzewiecka, Iwona Głowacka-Mrotek, Magdalena Tarkowska, Magdalena Nowikiewicz, and Wojciech Zegarski. 2022. "Did the COVID-19 Pandemic Truly Adversely Affect Disease Progress and Therapeutic Options in Breast Cancer Patients? A Single-Centre Analysis" Journal of Clinical Medicine 11, no. 4: 1014. https://doi.org/10.3390/jcm11041014
APA StyleNowikiewicz, T., Szymankiewicz, M., Drzewiecka, M., Głowacka-Mrotek, I., Tarkowska, M., Nowikiewicz, M., & Zegarski, W. (2022). Did the COVID-19 Pandemic Truly Adversely Affect Disease Progress and Therapeutic Options in Breast Cancer Patients? A Single-Centre Analysis. Journal of Clinical Medicine, 11(4), 1014. https://doi.org/10.3390/jcm11041014






