The Link between Fibromyalgia Syndrome and Anger: A Systematic Review Revealing Research Gaps
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis
3. Results
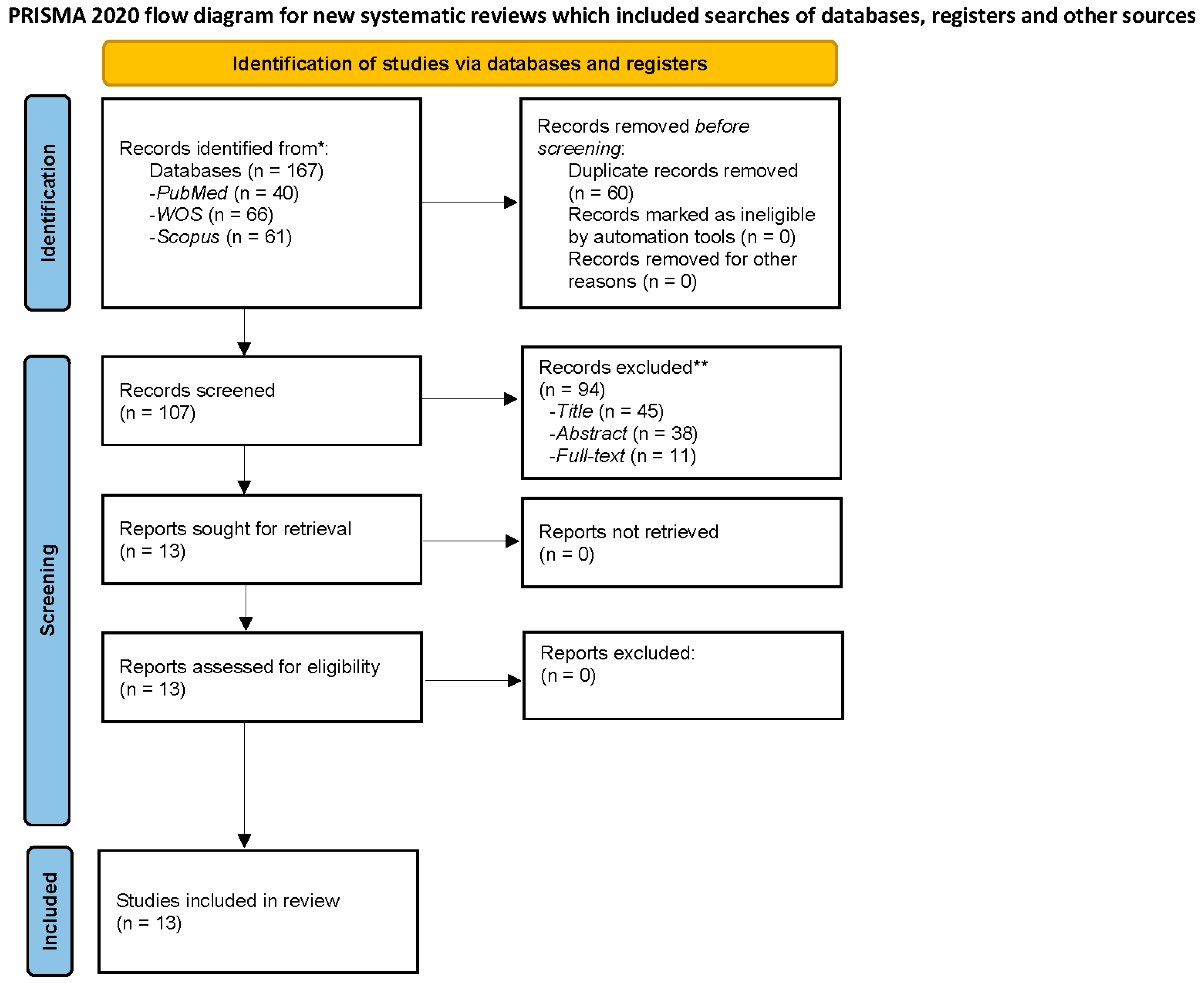
3.1. Literature Search and Study Characteristics
| First Author (Publication Year), Study Name, Country | Objective | Study Design | Sample Size [Mean ± Age (SD)] | FMS Diagnostic Criteria | Instruments | Variables and Results |
|---|---|---|---|---|---|---|
| Amir et al., 2000 [30]. Coping styles, anger, social support, and suicide risk of women with Fibromyalgia Syndrome. Israel. | To examine some personal dispositions in FMS patients. | Cross-sectional. | N = 220 female participants. 51 FMS patients (48.96 ± 8.41). 51 RA patients (46.25 ± 13.61). 50 CLBP patients (47.12 ± 11.61). 50 HW (45.66 ± 13.11). | 1990 ACR. | Coping Inventory for Stressful Situations. STAXI. Suicide Risk Scale. Social Support Scale. | CP patients: similar personality traits. High coping style of avoidance and anger (especially: state anger and anger-in). FMS patients: not significant differences from the other patient groups on any variables. Not have a characteristic personality pattern. |
| Sayar et al., 2004 [27]. Alexithymia and anger in patients with Fibromyalgia. Turkey. | To delineate the relevance of the personality construct alexithymia and anger-in in patients with FMS. | Cross-sectional. | N = 112 female participants. 50 FMS patients (40.50 ± 8.80). 20 RA patients (45.60 ± 14.90). 42 HW (38.80 ± 10.40). | 1990 ACR. | FIQ. BDI. BAI. STAXI. VAS. TAS. | FMS patients: higher anger-in than in RA patients. Anger-out and anxiety predicted the level of pain severity. In spite of anger-in is higher in FMS, it is the behavioral expression of anger, together with anxiety, that predicts the pain severity. |
| Shelley-Tremblay et al., 2009 [28]. The effects of sucrose consumption on left-frontal asymmetry and anger in persons with Fibromyalgia Syndrome. United States of America. | To determine whether FMS patients differ from age-matched healthy normal controls in their reaction to 75 g of sucrose by measuring the time course of self-report and electrophysiological responses. | Cross-sectional. | N = 18 female participants. 8 FMS patients (48.05 ± 6.14). 10 HW (47.05 ± 7.08). | 1990 ACR. | Demographic Questionnaire. FIQ. HADS. POMS. Carbohydrate Addict’s Scale. Sucrose test meal beverage. Apparatus: Biopac MP30. Scan 4.2. An Accu-Chek Advantage. | FMS patients: higher levels of depression, anger, and other indicators of distress at all time points and increased rLFA than HW. Correlation between anger an increased rLFA. |
| Van Middendorp et al., 2010 [44]. The effects of anger and sadness on clinical pain reports and experimentally-induced pain thresholds in women with and without fibromyalgia. Netherlands. | To examine the effects of experimentally induced anger and sadness on self-reported clinical and experimentally-induced pain in a sample of women with and without fibromyalgia. | Cross-sectional. | N = 121 female participants. 62 FMS patients (46.30 ± 10.80). 59 HW (48.90 ± 11.40). | 1990 ACR. | PANAS-X. VAS. Emotion induction procedure: autobiographical recall procedure. Experimentally-induced pain (electrical pain induction) measures: Sensory threshold. Pain threshold. Pain tolerance. | FMS patients: anger and sadness amplify pain in women with and without FMS. A stronger emotion-induced pain response was associated with more emotional reactivity. Anger and sadness reactivity to the emotion inductions were associated with greater increases in clinical pain responses. No convincing evidence was found for a larger sensitivity to anger and sadness in women with FMS than in women without FMS, or for a larger sensitivity to anger than to sadness in FMS. |
| Van Middendorp et al., 2010 [29]. Effects of anger and anger regulation styles on pain in daily life of women with fibromyalgia: a diary study. Netherlands. | To examine, among patients with fibromyalgia, whether anger during everyday life amplifies pain and whether general and situational anger inhibition and anger expression modulate the anger–pain link. | Cross-sectional. | N = 333 female participants. 333 FMS patients (47.00 ± 12.01). | 1990 ACR. | Diary. SECS. Daily anger questions. VAS. | FMS patients: state anger predicted higher end-of-day pain in half of the patients, but lower pain in one-quarter of patients. State anger inhibition was unrelated to pain. Trait anger inhibition was related to more pain. Lowest pain level among patients with high trait anger expression who actually expressed their anger in an anger-arousing situation. |
| González-Roldán et al., 2013 [41]. Altered psychophysiological responses to the view of others’ pain and anger faces in fibromyalgia patients. Spain. | To examined brain activity, corrugator muscle electromyography (EMG), and heart rate (HR) responses to others’ faces expressing pain in patients with fibromyalgia. | Cross-sectional. | N = 40 female participants. 20 FMS patients (53.40 ± 8.10). 20 HW (52.70 ± 9.90). | 1990 ACR. | Semistandardized interview. BDI. STAI. PANAS. EHI. WHYMPI (only in FMS). Emotional Face Task. Psychophysiological Recordings: Corrugator EMG activity, HR, and EEG signals. | FMS patients: greater cardiac deceleration to all facial expressions than pain-free controls, and enhanced N100 amplitudes to pain and anger faces in comparison with neutral faces. Greater theta power in response to pain and anger faces, as well as more reduced alpha power than pain-free controls to all faces. |
| Amutio et al., 2015 [46]. Mindfulness training for reducing anger, anxiety, and depression in Fibromyalgia patients. Spain. | To verify whether the application of a mindfulness-based training program was effective in modifying anger, anxiety, and depression levels in a group of women diagnosed with FMS. | Longitudinal Study. | N = 32 female patients. 32 FMS patients (51.82 ± 10.18): 14 experimental group and 18 control group (waiting list). | BDI. STAI. STAXI-2. Mindfulness intervention program. | FMS patients: a significant reduction of anger (trait) levels, internal expression of anger, state anxiety, and depression as well as a significant increase in internal control of anger. Mindfulness-based treatment was effective after 7 weeks. Results were maintained 3 months after the end of the intervention. | |
| Ricci et al., 2016 [42]. Worry and anger rumination in Fibromyalgia Syndrome. Italy. | (1) To investigate the psychological profile of patients with FMS as compared to patients with other chronic pain syndromes (CP) and healthy subjects (HS) and (2) To examine the associations between anxiety, depression, worry and angry rumination in FMS patients. | Cross-sectional. | N = 90 female participants. 30 FMS patients (54.00 ± 12.00). 30 (59.00 ± 13.00) women with other type of CP (osteoporosis and osteoarthritis). 30 HW (age not specified). | 1990 and 2010 ACR. | Socio-demographic information form. STAI. PSWQ. BDI-I. ARS. | FMS patients: higher levels of state and trait anxiety, worry and angry rumination than CP patients and HS. Worry and angry rumination were strongly associated in FMS. |
| Di Tella et al., 2017 [40]. Alexithymia, not fibromyalgia, predicts the attribution of pain to anger-related facial expressions. Italy. | To test the hypothesis that the attribution of pain to emotional facial expressions (other than pain) is greater in patients with FMS. | Cross-sectional. | N = 123 female participants. 41 FMS patients (50.80 ± 10.20). 82 HW (51.70 ± 8.40). | Expert rheumatologist. | HADS. TAS-20. Emotional Pain Estimation and Emotional Pain Ascription Task: modified version of the Ekman 60 Faces Test. | FMS patients: not increased attribution of pain to facial expressions of emotions. Alexithymic individuals demonstrated no specific problem in the recognition of basic emotions, but attributed significantly more pain to angry facial expression. |
| Offenbaecher et al., 2017 [24]. Struggling with adversities of life: the role of forgiveness in patients suffering from Fibromyalgia. Germany. | (1) To compare the magnitude and direction of associations between forgiveness and pain, mental and physical health, quality of life, and anger in a sample of FMS participants and healthy controls, and (2) To compare FMS and controls on mean levels of these variables. | Cross-sectional | N = 254 participants. 173 FMS patients (161 women and 9 men) (58.00 ± 8.80). 81 HP (76 women and 5 men) (47.20 ± 14.20). | Not specified. | Initial survey. Demographic questions: age, education, religion, sex and marital status. Two questions about the degree of religiosity and spirituality. Forgiveness of self and others scales. HADS. SF-12. SF-16. STAI-II. VAS. Regional pain scale. | FMS patients: higher pain and anger and poorer health and quality of life. Lower levels of both forgiveness of self and others. |
| El Tassa et al., 2018 [43]. Mood states, depressive symptoms, and physical function in women with Fibromyalgia. Brazil. | To investigate the relationship between mood states, depressive symptoms, and physical performance in women with FMS. | Cross-sectional case-control study. | N = 45 female participants. 28 FMS patients (44.80 ± 5.50). 17 HW (43.40 ± 4.70). | ACR 1990. | BRUMS. BDI. HAQ. VAS. Threshold painful sensibility: dial algometer. Physical Function: 6-min Walk Test; Sit and Reach Test; 8-ft Up Go Test; and 30-s Chair Stand Test. Knee flexion and extension maximum isometric voluntary contractions (MIVC). | FMS patients: tension and anger showed a positive correlation with tests that demand strength in knee extension. |
| Andrade et al., 2019 [47]. Acute effect of strength training on mood of patients with fibromyalgia syndrome. Brazil. | To analyze the acute effect of strength training (ST) sessions on the mood states of patients with fibromyalgia. | Clinical trial. | N = 28 female participants. 28 FMS patients (51.88 ± 10.22). | 1990 and 2016 ACR. | Sociodemographic and clinical data: self-reported instrument. BRUMS. Strength training program. | FMS patients: the ST practice had positive effects on the patients’ mood states after a single session. Reductions in anger, mental confusion, mood depression, fatigue, and tension, due to ST program. |
| Toussaint et al., 2019 [45]. Anger rumination mediates differences between fibromyalgia patients and healthy controls on mental health and quality of life. Germany. | To examine differences between fibromyalgia patients and healthy controls on anger rumination, mental health and quality of life and tested anger rumination as a mediator of patient-control differences in mental health and quality of life. | Cross-sectional. | N = 116 female participants. 58 FMS patients (58.80 ± 8.80). 58 HW (47.00 ± 14.20). | Having an FMS diagnosis (any criteria specified). | Socio-demographics data: age, sex, and educational level. ARS. HADS. SF-12. SF-16. | FMS patients: higher anger rumination scales and depression and anxiety and lower on quality of life. All anger rumination scales were related to poorer mental health and quality of life. Patient–control differences on mental health and quality of life were mediated by anger rumination. The only subscale with mediating effects was anger memories. |
3.2. FMS and Anger
3.2.1. Anger in the Context of Personality Research
3.2.2. The Association between Anger and Other Relevant Variables in FMS
3.2.3. Interventions Aimed at Reducing or Better Coping Anger in FMS
3.3. Quality of Selected Studies
4. Discussion
4.1. Anger in the Context of Personality Studies
4.2. The Relationships between Anger and Clinical, Emotional and Cognitive Variables in FMS
4.3. Interventions Focus on Reducing or Managing Anger in FMS
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gilam, G.; Hendler, T. Deconstructing Anger in the Human Brain. Behav. Neurobiol. Schizophr. Its Treatment. 2015, 30, 257–273. [Google Scholar] [CrossRef]
- Averill, J.R. Studies on anger and aggression: Implications for theories of emotion. Am. Psychol. 1983, 38, 1145–1160. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, L. On the formation and regulation of anger and aggression: A cognitive-neoassociationistic analysis. Am. Psychol. 1990, 45, 494–503. [Google Scholar] [CrossRef]
- Berkowitz, L.; Harmon-Jones, E. Toward an Understanding of the Determinants of Anger. Emotion 2004, 4, 107–130. [Google Scholar] [CrossRef] [PubMed]
- Bear, M.F.; Connors, B.W.; Paradiso, M.A. Neuroscience: Exploring the Brain; Wolter Kluwer: Philadelphia, PA, USA, 2016. [Google Scholar]
- Trost, Z.; Vangronsveld, K.; Linton, S.J.; Quartana, P.J.; Sullivan, M.J. Cognitive dimensions of anger in chronic pain. Pain 2012, 153, 515–517. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Jacobs, G.; Russel, S.; Crane, R.S. Assessment of anger: The state-trait anger scale. In Advances in Personality Assessment; Butcher, J.N., Spielberger, C.D., Eds.; Hillsdale: Lawrence Erlbaum, NJ, USA, 1983; Volume 2, pp. 159–186. [Google Scholar]
- Bruehl, S.; Burns, J.W.; Chung, O.Y.; Ward, P.; Johnson, B. Anger and pain sensitivity in chronic low back pain patients and pain-free controls: The role of endogenous opioids. Pain 2002, 99, 223–233. [Google Scholar] [CrossRef]
- Alia-Klein, N.; Gan, G.; Gilam, G.; Bezek, J.; Bruno, A.; Denson, T.F.; Hendler, T.; Lowe, L.; Mariotti, V.; Muscatello, M.R.; et al. The feeling of anger: From brain networks to linguistic expressions. Neurosci. Biobehav. Rev. 2020, 108, 480–497. [Google Scholar] [CrossRef] [PubMed]
- Okifuji, A.; Turk, D.C.; Curran, S.L. Anger in chronic pain: Investigations of anger targets and intensity. J. Psychosom. Res. 1999, 47, 1–12. [Google Scholar] [CrossRef]
- Janssen, S.; Spinhoven, P.; Brosschot, J.F. Experimentally induced anger, cardiovascular reactivity, and pain sensitivity. J. Psychosom. Res. 2001, 51, 479–485. [Google Scholar] [CrossRef]
- Fernandez, E.; Turk, D.C. The scope and significance of anger in the experience of chronic pain. Pain 1995, 61, 165–175. [Google Scholar] [CrossRef]
- Porter, L.S.; Stone, A.A.; Schwartz, J.E. Anger expression and ambulatory blood pressure: A comparison of state and trait measures. Psychosom. Med. 1999, 61, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Corbishley, M.; Hendrickson, R.; Beutler, L. Behavior, affect, and cognition among psychogenic pain patients in group ex-pressive psychotherapy. J. Pain Symptoms Manag. 1990, 5, 241–248. [Google Scholar] [CrossRef]
- Moldofsky, H.; Chester, W. Pain and mood patterns in patients with rheumatoid arthritis: A Prospective study. Psychosom. Med. 1970, 32, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Kerns, R.D.; Rosenberg, R.; Jacob, M.C. Anger expression and chronic pain. J. Behav. Med. 1994, 17, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Gaskin, M.; Greene, A.; Robinson, M.; Geisser, M. Negative affect and the experience of chronic pain. J. Psychosom. Res. 1992, 36, 707–713. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology Preliminary Diagnostic Criteria for Fibromyalgia and Measurement of Symptom Severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef]
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.M.; Abeles, M.; Clark, P.; et al. The american college of rheumatology. Criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990, 33, 160–172. [Google Scholar] [CrossRef]
- van Middendorp, H.; Lumley, M.A.; Jacobs, J.W.; van Doornen, L.J.; Bijlsma, J.W.; Geenen, R. Emotions and emotional approach and avoidance strategies in fibromyalgia. J. Psychosom. Res. 2008, 64, 159–167. [Google Scholar] [CrossRef]
- Galvez-Sánchez, C.M.; Montoro, C.I.; Duschek, S.; Del Paso, G.A.R. Pain catastrophizing mediates the negative influence of pain and trait-anxiety on health-related quality of life in fibromyalgia. Qual. Life Res. 2020, 29, 1871–1881. [Google Scholar] [CrossRef]
- Galvez-Sánchez, C.M.; Montoro, C.I.; Duschek, S.; del Paso, G.A.R. Depression and trait-anxiety mediate the influence of clinical pain on health-related quality of life in fibromyalgia. J. Affect. Disord. 2020, 265, 486–495. [Google Scholar] [CrossRef]
- Montoro, C.I.; del Paso, G.A.R. Personality and fibromyalgia: Relationships with clinical, emotional, and functional variables. Pers Individ 2015, 85, 236–244. [Google Scholar] [CrossRef]
- Offenbaecher, M.; Dezutter, J.; Kohls, N.; Sigl, C.; Vallejo, M.A.; Rivera, J.; Bauerdorf, F.; Schelling, J.; Vincent, A.; Hirsch, J.K.; et al. Struggling With Adversities of Life: The Role of Forgiveness in Patients Suffering from Fibromyalgia. Clin. J. Pain 2017, 33, 528–534. [Google Scholar] [CrossRef]
- Wolfe, F.; Walitt, B.; Perrot, S.; Rasker, J.J.; Häuser, W. Fibromyalgia diagnosis and biased assessment: Sex, prevalence and bias. PLoS ONE 2018, 13, e0203755. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Maloney, E.; Wright, B.; Kennedy, M.; Kallail, K.J.; Rasker, J.J.; Häuser, W.; Wolfe, F. The Problematic Nature of Fibromyalgia Diagnosis in the Community. ACR Open Rheumatol. 2019, 1, 43–51. [Google Scholar] [CrossRef]
- Sayar, K.; Gulec, H.; Topbas, M. Alexithymia and anger in patients with fibromyalgia. Clin. Rheumatol. 2004, 23, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Shelley-Tremblay, J.; Ernst, A.; Kline, J.P. The effects of sucrose consumption on left-frontal asymmetry and anger in persons with Fibromyalgia Syndrome. J. Musculoskelet. Pain 2009, 17, 334–349. [Google Scholar] [CrossRef]
- van Middendorp, H.; Lumley, M.A.; Moerbeek, M.; Jacobs, J.W.; Bijlsma, J.W.; Geenen, R. Effects of anger and anger regulation styles on pain in daily life of women with fibromyalgia: A diary study. Eur. J. Pain 2010, 14, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Amir, M.; Neumann, L.; Bor, O.; Shir, Y.; Rubinow, A.; Buskila, D. Coping Styles, Anger, Social Support, and Suicide Risk of Women with Fibromyalgia Syndrome. J. Musculoskelet. Pain 2000, 8, 7–20. [Google Scholar] [CrossRef]
- Quartana, P.J.; Burns, J.W. Painful consequences of anger suppression. Emotion 2007, 7, 400–414. [Google Scholar] [CrossRef]
- Quartana, P.J.; Yoon, K.L.; Burns, J.W. Anger Suppression, Ironic Processes and Pain. J. Behav. Med. 2007, 30, 455–469. [Google Scholar] [CrossRef]
- Burns, J.W.; Quartana, P.J.; Bruehl, S. Anger inhibition and pain: Conceptualizations, evidence and new directions. J. Behav. Med. 2008, 31, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Bruehl, S.; Chung, O.Y.; Burns, J.W. Anger Expression and Pain: An Overview of Findings and Possible Mechanisms. J. Behav. Med. 2006, 29, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.W.; Kubilus, A.; Bruehl, S. Emotion induction moderates effects of anger management style on acute pain sensitivity. Pain 2003, 106, 109–118. [Google Scholar] [CrossRef]
- Burns, J.W. Arousal of negative emotions and symptom-specific reactivity in chronic low back pain patients. Emotion 2006, 6, 309–319. [Google Scholar] [CrossRef]
- Engebretson, T.O.; Matthews, K.A.; Scheier, M.F. Relations between anger expression and cardiovascular reactivity: Reconciling inconsistent findings through a matching hypothesis. J. Pers Soc. Psychol. 1989, 57, 513–521. [Google Scholar] [CrossRef]
- Brosschot, J.F.; Thayer, J.F. Anger inhibition, cardiovascular recovery, and vagal function: A model of the link between hostility and cardiovascular disease. Ann. Behav. Med. 1998, 20, 326–332. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Di Tella, M.; Enrici, I.; Castelli, L.; Colonna, F.; Fusaro, E.; Ghiggia, A.; Romeo, A.; Tesio, V.; Adenzato, M. Alexithymia, not fibromyalgia, predicts the attribution of pain to anger-related facial expressions. J. Affect. Disord. 2018, 227, 272–279. [Google Scholar] [CrossRef]
- González-Roldán, A.M.; Muñoz, M.A.; Cifre, I.; Sitges, C.; Montoya, P. Altered Psychophysiological Responses to the View of Others’ Pain and Anger Faces in Fibromyalgia Patients. J. Pain 2013, 14, 709–719. [Google Scholar] [CrossRef]
- Ricci, A.; Bonini, S.; Continanza, M.; Turano, M.; Puliti, E.; Finocchietti, A.; Bertolucci, D. Worry and anger rumination in fibromyalgia syndrome. Reumatismo 2016, 68, 195–198. [Google Scholar] [CrossRef]
- El Tassa, K.O.M.; Leite, N.; Goes, S.M.; Homann, D.; Rodacki, A.L.F.; Titski, A.C.K.; Stefanello, J.M.F. Mood States, Depressive Symptoms, and Physical Function in Women with Fibromyalgia. J. Exerc. Physiol. Online 2018, 21, 119–132. [Google Scholar]
- Van Middendorp, H.; Lumley, M.A.; Jacobs, J.W.G.; Bijlsma, J.W.J.; Geenen, R. The effects of anger and sadness on clinical pain reports and experimentally-induced pain thresholds in women with and without fibromyalgia. Arthritis Care Res. 2010, 62, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, L.; Sirois, F.; Hirsch, J.; Kohls, N.; Weber, A.; Schelling, J.; Vajda, C.; Offenbäecher, M. Anger rumination mediates differences between fibromyalgia patients and healthy controls on mental health and quality of life. Pers Ment. Heal. 2019, 13, 119–133. [Google Scholar] [CrossRef]
- Amutio, A.; Franco, C.; de Pérez-Fuentes, M.C.; Gázquez, J.J.; Mercader, I. Mindfulness training for reducing anger, anxiety, and depression in fibromyalgia patients. Front Psychol. 2015, 5, 1572. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.; Steffens, R.D.A.K.; Sieczkowska, S.M.; Coimbra, D.R.; Vilarino, G.T. Acute effect of strength training on mood of patients with fibromyalgia syndrome. Reumatismo 2019, 71, 141–147. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef]
- Galvez-Sánchez, C.M.; Del Paso, G.A.R. Diagnostic Criteria for Fibromyalgia: Critical Review and Future Perspectives. J. Clin. Med. 2020, 9, 1219. [Google Scholar] [CrossRef]
- Galvez-Sánchez, C.M.; Duschek, S.; del Paso, G.A.R. Psychological impact of fibromyalgia: Current perspectives. Psychol. Res. Behav. Manag. 2019, 12, 117–127. [Google Scholar] [CrossRef]
- Ahmed, S.; Aggarwal, A.; Lawrence, A. Performance of the American College of Rheumatology 2016 criteria for fibromyalgia in a referral care setting. Rheumatol. Int. 2019, 39, 1397–1403. [Google Scholar] [CrossRef]
- Suls, J.; David, J.P.; Harvey, J.H. Personality and Coping: Three Generations of Research. J. Pers. 1996, 64, 711–735. [Google Scholar] [CrossRef]
- Tschannen, T.A.; Duckro, P.N.; Margolis, R.B.; Tomazic, T.J. The Relationship of Anger, Depression, and Perceived Disability Among Headache Patients. Headache J. Head Face Pain 1992, 32, 501–503. [Google Scholar] [CrossRef]
- Affleck, G.; Tennen, H.; Urrows, S.; Higgins, P. Individual differences in the day-to-day experience of chronic pain: A prospective daily study of rheumatoid arthritis patients. Health Psychol. 1991, 10(6), 419–426. [Google Scholar] [CrossRef]
- Greenwood, K.; Thurston, R.; Rumble, M.; Waters, S.J.; Keefe, F.J. Anger and persistent pain: Current status and future directions. Pain 2003, 103, 1–5. [Google Scholar] [CrossRef]
- Davydov, D.M.; Galvez-Sánchez, C.M.; Montoro, C.I.; de Guevara, C.M.L.; Reyes del Paso, G.A. Personalized behavior management as a replacement for medications for pain control and mood regulation. Sci. Rep. 2021, 11, 20297. [Google Scholar] [CrossRef] [PubMed]
- Coan, J.; Allen, J.J. Frontal EEG asymmetry as a moderator and mediator of emotion. Biol. Psychol. 2004, 67, 7–50. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Mori, T.; Suzuki, H.; Endo, S.; Kawano, K. EEG changes in odor effects after the stress of long monotonous work. J. Int. Soc. Life Inform. Sci. 2001, 19, 271–274. [Google Scholar]
- Harmon-Jones, E. Contributions from research on anger and cognitive dissonance to understanding the motivational functions of asymmetrical frontal brain activity. Biol. Psychol. 2004, 67, 51–76. [Google Scholar] [CrossRef]
- Elkfury, J.L.; Antune, L.C.; Angoleri, L.D.M.; Sipmann, R.B.; de Souza, A.; Torres, I.L.D.S.; Caumo, W. Dysfunctional eating behavior in fibromyalgia and its association with serum biomarkers of brain plasticity (BDNF and S100B): An exploratory study. Arch. Endocrinol. Metab. 2021, 65, 713–722. [Google Scholar] [CrossRef]
- Carnes, A.; Alcántara, A.; Bueno, M.; Castan, E.; Lecube, A. Fibromyalgia and eating disorders in morbid obesity. Endocrinología y Nutrición 2014, 61, 555–556. [Google Scholar] [CrossRef]
- Schmidt-Wilcke, T.; Luerding, R.; Weigand, T.; Jurgens, T.; Schuierer, G.; Leinisch, E. Striatal grey matter increased in patients suffering from fibromyalgia A voxel based morphometry study. Pain 2007, 132, S109–S116. [Google Scholar] [CrossRef]
- Chen, J.J.H.; Wang, J.Y.; Chang, Y.M.; Su, S.Y.; Chang, C.T.; Sun, S.S.; Kao, C.H.; Lee, C.C. Regional cerebral blood flow between primary and concomitant fibromyalgia patients: A possible way to differentiate concomitant fibromyalgia from the primary disease. Scand. J. Rheumatol. 2007, 36, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Wik, G.; Wik, G.; Fischer, H.; Bragee, B.; Finer, B.; Fredrikson, M. Functional anatomy of hypnotic analgesia: A PET study of patients with fibromyalgia. Eur. J. Pain 1999, 3, 7–12. [Google Scholar] [CrossRef]
- Aldrich, S.; Eccleston, C.; Crombez, G. Worrying about chronic pain: Vigilance to threat and misdirected problem solving. Behav. Res. Ther. 2000, 38, 457–470. [Google Scholar] [CrossRef]
- Asmundson, G.J.; Kuperos, J.L.; Norton, G.R. Do patients with chronic pain selectively attend to pain-related information? Preliminary evidence for the mediating role of fear. Pain 1997, 72, 27–32. [Google Scholar] [CrossRef]
- Vlaeyen, J.W.S.; Linton, S.J. Fearavoidance and its consequences in chronic musculoskeletal pain: A state of the art. Pain 2000, 85, 317–332. [Google Scholar] [CrossRef]
- Bartley, E.J.; Rhudy, J.L.; Williams, A.E. Experimental Assessment of Affective Processing in Fibromyalgia. J. Pain 2009, 10, 1151–1160. [Google Scholar] [CrossRef]
- Montoya, P.; Sitges, C.; García-Herrera, M.; Izquierdo, R.; Truyols, M.; Blay, N.; Collado, D. Abnormal Affective Modulation of Somatosensory Brain Processing Among Patients With Fibromyalgia. Psychosom. Med. 2005, 67, 957–963. [Google Scholar] [CrossRef]
- Sitges, C.; García-Herrera, M.; Pericás, M.; Collado, D.; Truyols, M.; Montoya, P. Abnormal brain processing of affective and sensory pain descriptors in chronic pain patients. J. Affect. Disord. 2007, 104, 73–82. [Google Scholar] [CrossRef]
- Montoro, C.I.; Duschek, S.; Reyes del Paso, G.A. Alexithymia in fibromyalgia síndrome. Pers. Individ. 2016, 102, 170–179. [Google Scholar] [CrossRef]
- Rainville, P.; Bao, Q.V.; Chretien, P. Pain-related emotions modulate experimental pain perception and autonomic responses. Pain 2005, 118, 306–318. [Google Scholar] [CrossRef]
- Bruehl, S.; Burns, J.W.; Chung, O.Y.; Chont, M. Pain-related effects of trait anger expression: Neural substrates and the role of endogenous opioid mechanisms. Neurosci. Biobehav. Rev. 2009, 33, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Smyth, J.M.; Stone, A.A.; Hurewitz, A.; Kaell, A. Effects of writing about stressful experiences on symptom reduction in patients with asthma or rheumatoid arthritis: A randomized trial. J. Am. Med. Assoc. 1999, 281, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Pennebaker, J.W.; Zech, E.; Rimé, B. Disclosing and sharing emotion: Psychological, social, and health consequences. In Handbook of Bereavement Research: Consequences, Coping, and Care; Stroebe, M.S., Hansson, R.O., Stroebe, W., Schut, H., Eds.; American Psychological Association: Washington, DC, USA, 2001. [Google Scholar]
- Gillis, M.E.; Lumley, M.A.; Mosley-Williams, A.; Leisen, J.C.C.; Roehrs, T. The health effects of at-home written emotional disclosure in fibromyalgia: A randomized trial. Ann. Behav. Med. 2006, 32, 135–146. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bushman, B.J.; Bonacci, A.M.; Pedersen, W.C.; Vasquez, E.A.; Miller, N. Chewing on it can chew you up: Effects of rumination on triggered displaced aggression. J. Pers. Soc. Psychol. 2005, 88, 969–983. [Google Scholar] [CrossRef]
- Denson, T.F. The Multiple Systems Model of Angry Rumination. Pers. Soc. Psychol. Rev. 2012, 17, 103–123. [Google Scholar] [CrossRef]
- Zautra, A.; Smith, B.; Affleck, G.; Tennen, H. Examinations of chronic pain and affect relationships: Applications of a dynamic model of affect. J. Consult. Clin. Psychol. 2001, 69, 786–795. [Google Scholar] [CrossRef]
- Staud, R.; Robinson, M.E.; Vierck, C.J., Jr.; Cannon, R.C.; Mauderli, A.P.; Price, D.D. Ratings of experimental pain and pain-related negative affect predict clinical pain in patients with fibromyalgia syndrome. Pain 2003, 105, 215–222. [Google Scholar] [CrossRef]
- Castelli, L.; Tesio, V.; Colonna, F.; Molinaro, S.; Leombruni, P.; Bruzzone, M.; Fusaro, E.; Sarzi-Puttini, P.; Torta, R. Alexithymia and psychological distress in fibromyalgia: Prevalence and relation with quality of life. Clin. Exp. Rheumatol. 2012, 30, 70–77. [Google Scholar]
- Di Tella, M.; Castelli, L. Alexithymia in Chronic Pain Disorders. Curr. Rheumatol. Rep. 2016, 18, 41. [Google Scholar] [CrossRef]
- Di Tella, M.; Ghiggia, A.; Tesio, V.; Romeo, A.; Colonna, F.; Fusaro, E.; Torta, R.; Castelli, L. Pain experience in Fibromyalgia Syndrome: The role of alexithymia and psychological distress. J. Affect. Disord. 2017, 208, 87–93. [Google Scholar] [CrossRef]
- Ghiggia, A.; Romeo, A.; Tesio, V.; Di Tella, M.; Colonna, F.; Geminiani, G.C.; Fusaro, E.; Castelli, L. Alexithymia and de-pression in patients with fibromyalgia: When the whole is greater than the sum of its parts. Psychiatry Res. 2017, 255, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Sánchez, C.M.; del Paso, G.A.R.; Duschek, S. Cognitive Impairments in Fibromyalgia Syndrome: Associations With Positive and Negative Affect, Alexithymia, Pain Catastrophizing and Self-Esteem. Front. Psychol. 2018, 9, 377. [Google Scholar] [CrossRef] [PubMed]
- Sifneos, P.E. Short-Term Psychotherapy and Emotional Crisis; Harvard University Press: Cambridge, MA, USA, 1972. [Google Scholar]
- Taylor, G.J.; Bagby, R.M.; Parker, J.D.A. Disorders of Affect Regulation: Alexithymia in Medical and Psychiatric Illness; Cambridge University Press: Cambridge, MA, USA, 1999. [Google Scholar]
- Aftanas, L.; Varlamov, A.; Reva, N.; Pavlov, S. Disruption of early event-related theta synchronization of human EEG in alexithymics viewing affective pictures. Neurosci. Lett. 2003, 340, 57–60. [Google Scholar] [CrossRef]
- Berthoz, S.; Artiges, E.; Poline, J.-B.; Rouquette, S.; Consoli, S.M.; Martinot, J.-L.; Van De Moortele, P.-F. Effect of Impaired Recognition and Expression of Emotions on Frontocingulate Cortices: An fMRI Study of Men With Alexithymia. Am. J. Psychiatry 2002, 159, 961–967. [Google Scholar] [CrossRef]
- Kano, M.; Fukudo, S.; Gyoba, J.; Kamachi, M.; Tagawa, M.; Mochizuki, H.; Itoh, M.; Hongo, M.; Yanai, K. Specific brain pro-cessing of facial expressions in people with alexithymia: An H2 15O-PET study. Brain 2003, 126, 1474–1484. [Google Scholar] [CrossRef]
- Vermeulen, N.; Luminet, O.; Corneille, O. Alexithymia and the automatic processing of affective information: Evidence from the affective priming paradigm. Cogn. Emot. 2006, 20, 64–91. [Google Scholar] [CrossRef]
- Blair, R.J.R.; Morris, J.S.; Frith, C.; Perrett, D.; Dolan, R. Dissociable neural responses to facial expressions of sadness and anger. Brain 1999, 122 (Pt. 5), 883–893. [Google Scholar] [CrossRef]
- Carson, J.W.; Keefe, F.J.; Goli, V.; Fras, A.M.; Lynch, T.R.; Thorp, S.R.; Buechler, J.L. Forgiveness and chronic low back pain: A preliminary study examining the relationship of forgiveness to pain, anger, and psychological distress. J. Pain 2005, 6, 84–91. [Google Scholar] [CrossRef]
- Worthington, E.L., Jr.; Lavelock, C.; van Oyen Witvliet, C. Measures of forgiveness: Self-report, physiological, chemical, and behavioral indicators. In Measures of Personality and Social Psychological Constructs; Boyle, G.J., Saklofske, D.H., Matthews, G., Eds.; Academic Press: Oxford, UK, 2014; pp. 474–502. [Google Scholar]
- Toussaint, L.; Shields, G.; Dorn, G.; Slavich, G.M. Effects of lifetime stress exposure on mental and physical health in young adulthood: How stress degrades and forgiveness protects health. J. Health Psychol. 2016, 21, 1004–1014. [Google Scholar] [CrossRef]
- Whited, M.C.; Wheat, A.L.; Larkin, K.T. The influence of forgiveness and apology on cardiovascular reactivity and recovery in response to mental stress. J. Behav. Med. 2010, 33, 293–304. [Google Scholar] [CrossRef]
- Witvliet, C.V.; Ludwig, T.E.; Vander Laan, K.L. Granting forgiveness or harboring grudges: Implications for emotion physiology, and health. Psychol. Sci. 2001, 12, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, L.L.; Owen, A.D.; Cheadle, A. Forgive to Live: Forgiveness, Health, and Longevity. J. Behav. Med. 2011, 35, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Rippentrop, E.A.; Altmaier, E.M.; Chen, J.J.; Found, E.M.; Keffala, V. The relationship between religion/spirituality and physical health, mental health, and pain in a chronic pain population. Pain 2005, 116, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Kabat-Zinn, J. Full Catastrophe Living: Using the Wisdom of your Body and Mind to Face Stress, Pain and Illness; Delacorte: New York, NY, USA, 1990. [Google Scholar]
- Bishop, S.R.; Lau, M.; Shapiro, S.; Carlson, L.; Anderson, N.D.; Carmody, J.; Segal, Z.V.; Abbey, S.; Speca, M.; Velting, D.; et al. Mindfulness: A proposed operational definition. Clin. Psychol. Sci. Pract. 2004, 11, 230–241. [Google Scholar] [CrossRef]
- Parrish, B.P.; Zautra, A.J.; Davis, M.C. The role of positive and negative interpersonal events on daily fatigue in women with fibromyalgia, rheumatoid arthritis, and osteoarthritis. Health Psychol. 2008, 27, 694–702. [Google Scholar] [CrossRef]
- Zautra, A.J.; Johnson, L.M.; Davis, M.C. Positive Affect as a Source of Resilience for Women in Chronic Pain. J. Consult. Clin. Psychol. 2005, 73, 212–220. [Google Scholar] [CrossRef]
- Rainville, P.; Bechara, A.; Naqvi, N.; Damasio, A.R. Basic emotions are associated with distinct patterns of cardiorespiratory activity. Int. J. Psychophysiol. 2006, 61, 5–18. [Google Scholar] [CrossRef]
- Brosseau, L.; A Wells, G.; Tugwell, P.; Egan, M.; Wilson, K.G.; Dubouloz, C.-J.; Casimiro, L.; A Robinson, V.; McGowan, J.; Busch, A.; et al. Ottawa Panel Evidence-Based Clinical Practice Guidelines for Aerobic Fitness Exercises in the Management of Fibromyalgia: Part 1. Phys. Ther. 2008, 88, 857–871. [Google Scholar] [CrossRef]
- Brosseau, L.; A Wells, G.; Tugwell, P.; Egan, M.; Wilson, K.G.; Dubouloz, C.-J.; Casimiro, L.; A Robinson, V.; McGowan, J.; Busch, A.; et al. Ottawa Panel Evidence-Based Clinical Practice Guidelines for Strengthening Exercises in the Management of Fibromyalgia: Part 2. Phys. Ther. 2008, 88, 873–886. [Google Scholar] [CrossRef]
- Kelley, G.A.; Kelley, K.S.; Jones, D.L. Efficacy and Effectiveness of Exercise on Tender Points in Adults with Fibromyalgia: A Meta-Analysis of Randomized Controlled Trials. Arthritis 2011, 2011, 125485. [Google Scholar] [CrossRef]
- Busch, A.J.; Webber, S.; Richards, R.S.; Bidonde, J.; Schachter, C.L.; A Schafer, L.; Danyliw, A.; Sawant, A.; Bello-Haas, V.D.; Rader, T.; et al. Resistance exercise training for fibromyalgia. Cochrane Database Syst. Rev. 2013, 2013, CD010884. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, J.D.; McMillan, V.; Figueroa, A. The Effects of 12 Weeks of Resistance Exercise Training on Disease Severity and Autonomic Modulation at Rest and After Acute Leg Resistance Exercise in Women with Fibromyalgia. Arch. Phys. Med. Rehabil. 2010, 91, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.; Vilarino, G.T.; Serafim, T.T.; Júnior, A.A.P.; Souza, C.A.; Sieczkowska, S.M. Modulation of Autonomic Function by Physical Exercise in Patients with Fibromyalgia Syndrome: A Systematic Review. PM&R 2019, 11, 1121–1131. [Google Scholar] [CrossRef]
- Andrade, A.; Steffens, R.D.A.K.; Sieczkowska, S.M.; Tartaruga, L.A.P.; Vilarino, G.T. A systematic review of the effects of strength training in patients with fibromyalgia: Clinical outcomes and design considerations. Adv. Rheumatol. 2018, 58, 36. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.; Sieczkowska, S.M.; Vilarino, G. Resistance Training Improves Quality of Life and Associated Factors in Patients With Fibromyalgia Syndrome. PM&R 2019, 11, 703–709. [Google Scholar] [CrossRef]
- Gavi, M.B.R.O.M.; Vassalo, D.V.; Amaral, F.T.; Macedo, D.C.; Gava, P.L.; Dantas, E.M.; Valim, V. Strengthening exercises improve symptoms and quality of life but do not change autonomic modulation in fibromyalgia: A randomized clinical trial. PLoS ONE 2014, 9, 8. [Google Scholar] [CrossRef]
- Ericsson, A.; Palstam, A.; Larsson, A.; Löfgren, M.; Bileviciute-Ljungar, I.; Bjersing, J.; Gerdle, B.; Kosek, E.; Mannerkorpi, K. Resistance exercise improves physical fatigue in women with fibromyalgia: A randomized controlled trial. Arthritis Res. Ther. 2016, 18, 176. [Google Scholar] [CrossRef]
- Castelli, L.; Tesio, V. Commentary: Mindfulness training for reducing anger, anxiety, and depression in fibromyalgia patients. Front. Psychol. 2016, 7, 740. [Google Scholar] [CrossRef]
- Menzies, V. CE: Fibromyalgia syndrome: Current considerations in symptom management. Am. J. Nurs. 2016, 116, 24–32. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galvez-Sánchez, C.M.; Reyes del Paso, G.A.; Duschek, S.; Montoro, C.I. The Link between Fibromyalgia Syndrome and Anger: A Systematic Review Revealing Research Gaps. J. Clin. Med. 2022, 11, 844. https://doi.org/10.3390/jcm11030844
Galvez-Sánchez CM, Reyes del Paso GA, Duschek S, Montoro CI. The Link between Fibromyalgia Syndrome and Anger: A Systematic Review Revealing Research Gaps. Journal of Clinical Medicine. 2022; 11(3):844. https://doi.org/10.3390/jcm11030844
Chicago/Turabian StyleGalvez-Sánchez, Carmen M., Gustavo A. Reyes del Paso, Stefan Duschek, and Casandra I. Montoro. 2022. "The Link between Fibromyalgia Syndrome and Anger: A Systematic Review Revealing Research Gaps" Journal of Clinical Medicine 11, no. 3: 844. https://doi.org/10.3390/jcm11030844
APA StyleGalvez-Sánchez, C. M., Reyes del Paso, G. A., Duschek, S., & Montoro, C. I. (2022). The Link between Fibromyalgia Syndrome and Anger: A Systematic Review Revealing Research Gaps. Journal of Clinical Medicine, 11(3), 844. https://doi.org/10.3390/jcm11030844







