Motor-Independent Cognitive Testing in Motor Degenerative Diseases
Abstract
1. Introduction
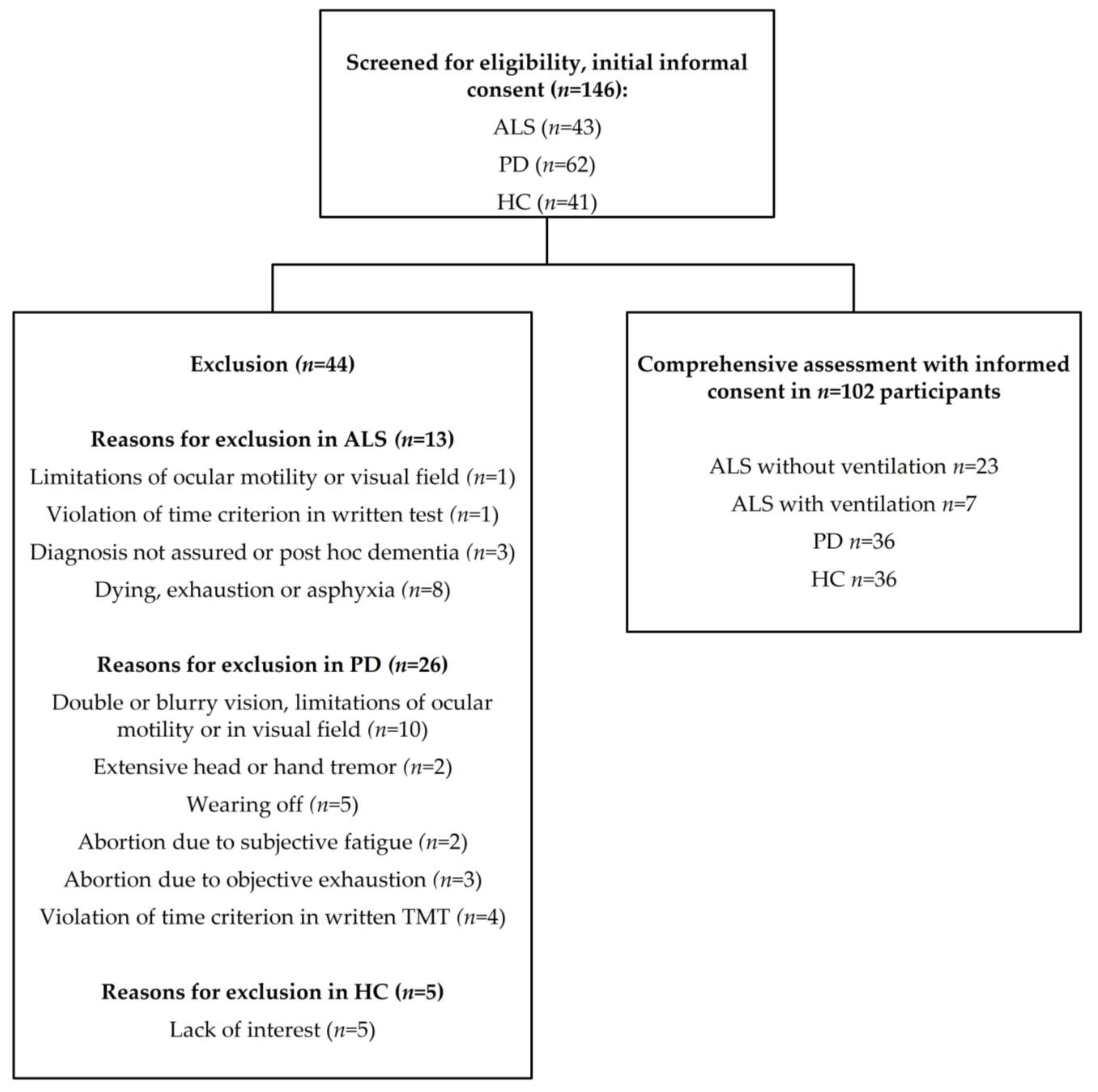
2. Materials and Methods
3. Results
3.1. Sociodemographic and Clinical Data
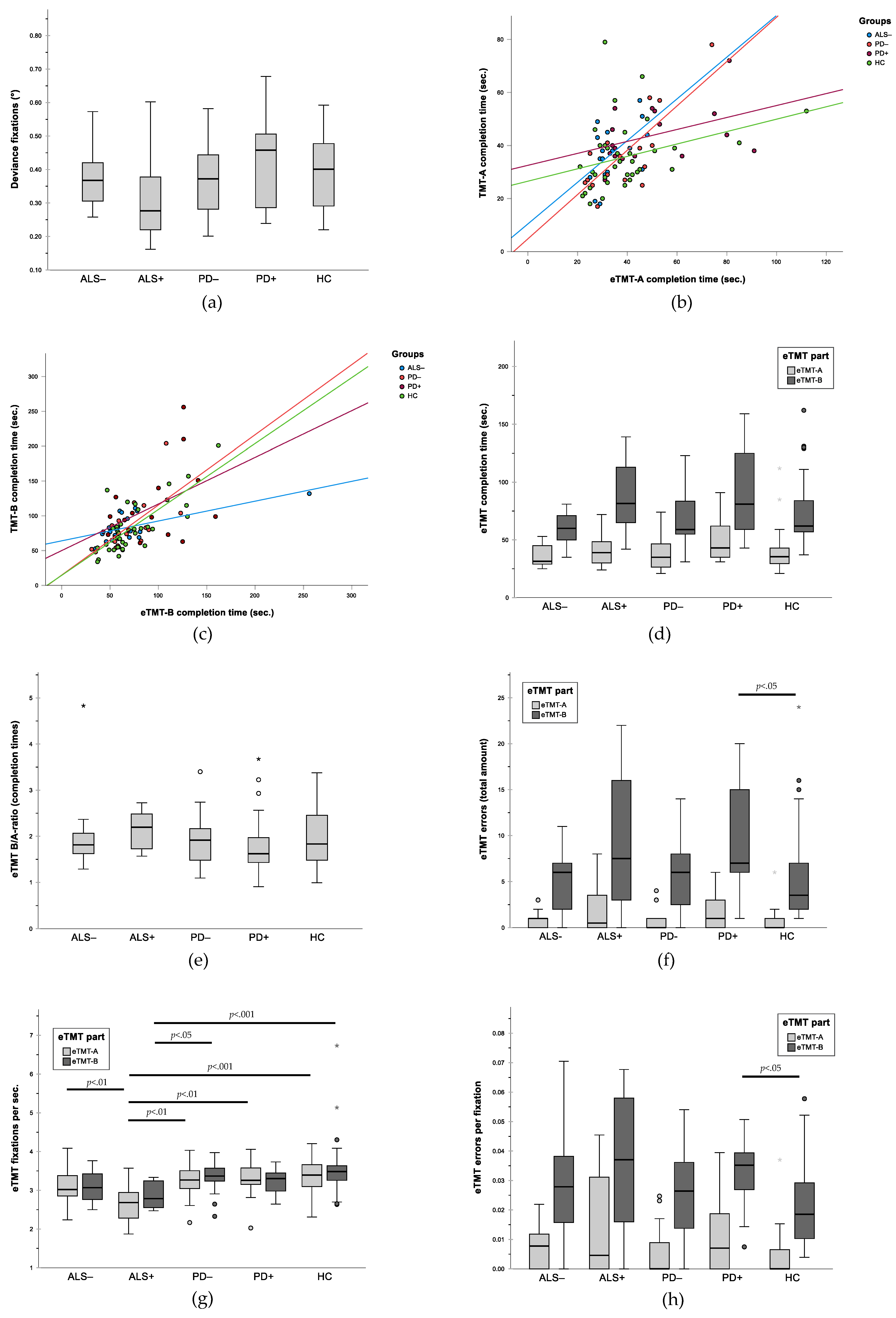
3.2. Gaze Accuracy
3.3. Validity of eTMT: Correlation between eTMT and Written TMT
3.4. Group Comparison for Performance in Written TMT
3.5. Group Comparison for Performance in eTMT
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lakerveld, J.; Kotchoubey, B.; Kubler, A. Cognitive function in patients with late stage amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 25–29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beeldman, E.; Raaphorst, J.; Twennaar, M.K.; De Visser, M.; Schmand, B.A.; De Haan, R.J. The cognitive profile of ALS: A systematic review and meta-analysis update. J. Neurol. Neurosurg. Psychiatry 2015, 87, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Krimly, A.; Bauer, L.; Schulenburg, S.; Böhm, S.; Aho-Özhan, H.E.A.; Uttner, I.; Gorges, M.; Kassubek, J.; Pinkhardt, E.H.; et al. A first approach to a neuropsychological screen-ing tool using eye-tracking for bedside cognitive testing based on the Edinburgh Cognitive and Behavioural ALS Screen. Amyotroph. Lateral Scler. Front. Degener. 2017, 18, 443–450. [Google Scholar] [CrossRef] [PubMed]
- BenBrika, S.; Desgranges, B.; Eustache, F.; Viader, F. Cognitive, Emotional and Psychological Manifestations in Amyotrophic Lateral Sclerosis at Baseline and Overtime: A Review. Front. Neurosci. 2019, 13, 951. [Google Scholar] [CrossRef]
- Abrahams, S.; Newton, J.; Niven, E.; Foley, J.; Bak, T.H. Screening for cognition and behaviour changes in ALS. Amyotroph. Lateral Scler. Front. Degener. 2013, 15, 9–14. [Google Scholar] [CrossRef]
- Chou, K.L.; Amick, M.M.; Brandt, J.; Camicioli, R.; Frei, K.; Gitelman, D.; Goldman, J.; Growdon, J.; Hurtig, H.I.; Levin, B.; et al. A recommended scale for cognitive screening in clini-cal trials of Parkinson’s disease. Mov. Disord. 2010, 25, 2501–2507. [Google Scholar] [CrossRef]
- Rojas, P.; Ramírez, A.I.; Fernández-Albarral, J.A.; López-Cuenca, I.; Salobrar-García, E.; Cadena, M.; Elvira-Hurtado, L.; Salazar, J.J.; de Hoz, R.; Ramirez, J.M. Amyotrophic Lateral Scle-rosis: A Neurodegenerative Motor Neuron Disease with Ocular Involvement. Front. Neurosci. 2020, 14, 976. [Google Scholar] [CrossRef]
- Poletti, B.; Carelli, L.; Solca, F.; Lafronza, A.; Pedroli, E.; Faini, A.; Ticozzi, N.; Ciammola, A.; Meriggi, P.; Cipresso, P.; et al. An eye-tracker controlled cognitive battery: Overcoming verbal-motor limitations in ALS. J. Neurol. 2017, 264, 1136–1145. [Google Scholar] [CrossRef]
- Linse, K.; Rüger, W.; Joos, M.; Schmitz-Peiffer, H.; Storch, A.; Hermann, A. Usability of eyetracking computer systems and impact on psychological wellbeing in patients with advanced amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2017, 19, 212–219. [Google Scholar] [CrossRef]
- Reitan, R.M. Validity of the Trail Making Test as an Indicator of Organic Brain Damage. Percept. Mot. Ski. 1958, 8. [Google Scholar] [CrossRef]
- Hicks, S.L.; Sharma, R.; Khan, A.N.; Berna, C.M.; Waldecker, A.; Talbot, K.; Kennard, C.; Turner, M.R. An Eye-Tracking Version of the Trail-Making Test. PLoS ONE 2013, 8, e84061. [Google Scholar] [CrossRef]
- Proudfoot, M.; Menke, R.A.L.; Sharma, R.; Berna, C.M.; Hicks, S.L.; Kennard, C.; Talbot, K.; Turner, M.R. Eye-tracking in amyotrophic lateral sclerosis: A longitudinal study of saccadic and cognitive tasks. Amyotroph. Lateral Scler. Front. Degener. 2016, 17, 101–111. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2000, 1, 293–299. [Google Scholar] [CrossRef]
- Cedarbaum, J.M.; Stambler, N.; Malta, E.; Fuller, C.; Hilt, D.; Thurmond, B.; Nakanishi, A. The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. J. Neurol. Sci. 1999, 169, 13–21. [Google Scholar] [CrossRef]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression, and mortality. Neurology 1967, 17, 427. [Google Scholar] [CrossRef]
- Wass, S.V.; Smith, T.; Johnson, M. Parsing eye-tracking data of variable quality to provide accurate fixation duration estimates in infants and adults. Behav. Res. Methods 2012, 45, 229–250. [Google Scholar] [CrossRef]
- Orquin, J.L.; Holmqvist, K. Threats to the validity of eye-movement research in psychology. Behav. Res. Methods 2017, 50, 1645–1656. [Google Scholar] [CrossRef]
- Linse, K.; Aust, E.; Joos, M.; Hermann, A. Communication Matters—Pitfalls and Promise of Hightech Communication Devices in Palliative Care of Severely Physically Disabled Patients with Amyotrophic Lateral Sclerosis. Front. Neurol. 2018, 9, 603. [Google Scholar] [CrossRef]
- Wojtala, J.; Heber, I.A.; Neuser, P.; Heller, J.; Kalbe, E.; Rehberg, S.P.; Storch, A.; Linse, K.; Schneider, C.; Gräber, S.; et al. Cognitive decline in Parkinson’s disease: The impact of the motor phenotype on cognition. J. Neurol. Neurosurg. Psychiatry 2018, 90, 171–179. [Google Scholar] [CrossRef]
- Kudlicka, A.; Clare, L.; Hindle, J.V. Executive functions in Parkinson’s disease: Systematic review and meta-analysis. Mov. Disord. 2011, 26, 2305–2315. [Google Scholar] [CrossRef] [PubMed]
- Karagulle Kendi, A.T.; Lehericy, S.; Luciana, M.; Ugurbil, K.; Tuite, P. Altered Diffusion in the Frontal Lobe in Parkinson Disease. Am. J. Neuroradiol. 2008, 29, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Ashendorf, L.; Jefferson, A.L.; O’Connor, M.K.; Chaisson, C.; Green, R.C.; Stern, R.A. Trail Making Test errors in normal aging, mild cognitive impairment, and dementia. Arch. Clin. Neuropsychol. 2008, 23, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.; Ahern, D.C.; Rabinowitz, A.R.; Farrer, T.J.; Watts, A.K.S.; Salloway, S.; Malloy, P.F.; Deoni, S.C. Lowering the Floor on Trail Making Test Part B: Psychometric Evidence for a New Scoring Metric. Arch. Clin. Neuropsychol. 2015, 30, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Gorges, M.; Müller, H.-P.; Lulé, D.; Del Tredici, K.; Brettschneider, J.; Keller, J.; Pfandl, K.; Ludolph, A.C.; Kassubek, J.; Pinkhardt, E.H. Eye Movement Deficits Are Consistent with a Staging Model of pTDP-43 Pathology in Amyotrophic Lateral Sclerosis. PLoS ONE 2015, 10, e0142546. [Google Scholar] [CrossRef]
- Hutton, S.B.; Ettinger, U. The antisaccade task as a research tool in psychopathology: A critical review. Psychophysiology 2006, 43, 302–313. [Google Scholar] [CrossRef]
| Characteristic | ALS− (n = 18) | ALS+ (n = 12) | PD− (n = 19) | PD+ (n = 17) | HC (n = 36) | Total (n = 102) | p Value 1 |
|---|---|---|---|---|---|---|---|
| Age, years | 60.2 ± 9.4 | 56.3 ± 8.5 | 61.7 ± 9.1 | 65.6 ± 8.8 | 62.3 ± 8.8 | 61.7 ± 9.1 | n. s. |
| Sex, male | 61.1% | 50.0% | 63.2% | 76.5% | 50.0% | 58.8% | n. s. |
| Education, years | 13.4 ± 2.2 | 15.4 ± 2.7 | 14.8 ± 2.3 | 14.9 ± 3.2 | 14.2 ± 3.1 | 14.4 ± 2.8 | n. s. |
| Right-handedness | 88.9% | 100% | 100% | 100% | 91.7% | 97% | n. s. |
| Employed | 16.7% | 0% | 26.3% | 0% | 44.4% | 23.5% | <0.001 |
| Disease duration (years) | 2.9 ± 2.8 | 6.2 ± 3.7 | 7.4 ± 5.5 | 13.2 ± 6.4 | - | - | <0.001 |
| ALS-type, sporadic | 83.3% | 100% | - | - | - | - | n. s. |
| ALS-onset, spinal:bulbar | 50%:50% | 75%:25% | - | - | - | - | n. s. |
| ALS-ventilated | 0% | 58.3% | - | - | - | - | <0.001 |
| ALSFRS-R score a | 36.4 ± 5.7 | 5.8 ± 6.0 | - | - | - | - | <0.001 |
| PD-equivalent | - | - | 31.6% | 47.1% | - | - | n. s |
| PD-hypo-kinetic-rigid | - | - | 42.1% | 41.2% | - | - | n. s. |
| PD-tremor dominant | - | - | 26.3% | 11.8% | - | - | n. s. |
| H&Y score b: Median (Range) | - | - | 2.0 (1.5 – 2.5) | 3.0 (3.0 – 4.0) | - | - | <0.001 |
| UPDRS III-score c | - | - | 18.1 ± 8.5 | 27.9 ± 7.2 | - | - | <0.01 |
| DBS (%) | - | - | 10.5% | 52.9% | - | - | <0.01 |
| Written TMT | |||||||
|---|---|---|---|---|---|---|---|
| ALS− (n = 18) | (ALS+) † (n = 2) | PD− (n = 19) | PD+ (n = 17) | HC (n = 36) | Total (n = 90) | p Value | |
| Completion time TMT-A | 38.1 ± 11.4 | (70.0 ± 8.5) † | 36.2 ± 14.4 | 43.9 ± 10.7 | 35.7 ±12.9 | 38.6 ±13.5 | 0.157 1 |
| Completion time TMT-B | 84.5 ± 20.7 | (109.0 ± 53.7) † | 83.3 ±36.0 | 110.1 ± 53.6 | 83.8 ± 36.6 | 89.3 ± 38.8 | 0.093 1 |
| Ratio TMT-B/-A | 2.3 ± 0.7 | (1.5 ± 0.6) † | 2.4 ± 0.7 | 2.7 ± 1.5 | 2.4 ± 0.8 | 2.4 ± 0.9 | 0.777 1 |
| Electronic TMT (eTMT) | |||||||
| ALS− (n = 18) | ALS+ (n = 12) | PD− (n = 19) | PD+ (n = 17) | HC (n = 36) | Total (n = 102) | p Value | |
| Fixation deviance (°) | 0.374 ± 0.078 | 0.325 ± 0.142 | 0.375 ± 0.108 | 0.423 ± 0.135 | 0.392 ± 0.108 | 0.383 ± 114 | 0.228 1 |
| Completion time eTMT-A | 35.22 ± 8.71 | 42.00 ± 15.71 | 37.53 ± 13.55 | 50.65 ± 19.91 | 39.39 ±17.61 | 40.49 ± 16.35 | 0.061 2 |
| Completion time eTMT-B | 71.61 ± 47.99 | 88.33 ± 29.68 | 67.95 ± 23.28 | 89.65 ± 36.07 | 73.22 ± 29.44 | 76.47 ± 33.97 | 0.076 2 |
| Ratio completion time eTMT-B/A | 1.96 ± 0.77 | 2.15 ± 0.40 | 1.89 ± 0.58 | 1.88 ± 0.78 | 1.95 ± 0.58 | 1.95 ± 0.63 | 0.399 2 |
| Amount of errors eTMT-A | 0.83 ± 0.92 | 1.92 ± 2.81 | 0.89 ± 1.33 | 2.00 ± 2.12 | 0.64 ± 1.42 | 1.10 ± 1.74 | 0.085 2 |
| Amount of errors eTMT-B | 8.17 ± 12.58 | 9.67 ± 7.34 | 5.58 ± 3.79 | 9.76 ± 5.76 a | 6.00 ± 6.09 | 7.36 ± 7.50 | <0.05 2 |
| Ratio errors eTMT-B/A | 7.43 ± 7.87 | 6.22 ± 6.06 | 4.29 ± 4.69 | 4.05 ± 4.96 | 6.19 ± 6.51 | 5.61 ± 6.04 | 0.199 2 |
| eTMT-A: Fixations total amount | 112.7 ± 36.9 | 110.4 ± 40.6 | 124.5 ± 51.3 | 163.1 ± 63.1 | 132.8 ± 62.0 | 130.1 ± 56.0 | 0.055 2 |
| eTMT-B: Fixations total amount | 223.0 ± 154.8 | 246.4 ± 67.7 | 226.0 ± 81.5 | 289.7 ± 120.3 | 271.2 ± 131.9 | 254.4 ± 121.2 | 0.135 2 |
| Fixations per sec. eTMT-A | 3.2 ± 0.5 b | 2.7 ± 0.5 a | 3.3 ± 0.4 b | 3.3 ± 0.4 b | 3.4 ± 0.5 b | 3.2 ± 0.5 | <0.001 1 |
| Fixations per sec. eTMT-B | 3.1 ± 0.4 | 2.9 ± 0.3 a | 3.3 ± 0.4 b | 3.2 ± 0.3 | 3.7 ± 1.3 b | 3.4 ± 0.9 | <0.001 2 |
| Errors per fixation eTMT-A | 0.007 ± 0.007 | 0.015 ± 0.018 | 0.006 ± 0.008 | 0.012 ± 0.013 | 0.003 ± 0.007 | 0.007 ± 0.01 | <0.05 2 |
| Errors per fixation eTMT-B | 0.030 ± 0.018 | 0.037 ± 0.023 | 0.024 ± 0.015 | 0.032 ± 0.011a | 0.021 ± 0.014 | 0.027 ± 0.017 | <0.05 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmitz-Peiffer, H.; Aust, E.; Linse, K.; Rueger, W.; Joos, M.; Löhle, M.; Storch, A.; Hermann, A. Motor-Independent Cognitive Testing in Motor Degenerative Diseases. J. Clin. Med. 2022, 11, 814. https://doi.org/10.3390/jcm11030814
Schmitz-Peiffer H, Aust E, Linse K, Rueger W, Joos M, Löhle M, Storch A, Hermann A. Motor-Independent Cognitive Testing in Motor Degenerative Diseases. Journal of Clinical Medicine. 2022; 11(3):814. https://doi.org/10.3390/jcm11030814
Chicago/Turabian StyleSchmitz-Peiffer, Henning, Elisa Aust, Katharina Linse, Wolfgang Rueger, Markus Joos, Matthias Löhle, Alexander Storch, and Andreas Hermann. 2022. "Motor-Independent Cognitive Testing in Motor Degenerative Diseases" Journal of Clinical Medicine 11, no. 3: 814. https://doi.org/10.3390/jcm11030814
APA StyleSchmitz-Peiffer, H., Aust, E., Linse, K., Rueger, W., Joos, M., Löhle, M., Storch, A., & Hermann, A. (2022). Motor-Independent Cognitive Testing in Motor Degenerative Diseases. Journal of Clinical Medicine, 11(3), 814. https://doi.org/10.3390/jcm11030814






