Long-Term Outcome of Corneal and Anterior Chamber Angle Parameters after Combined Laser Iridotomy and Iridoplasty Using Dual Scheimpflug Analyzer: 1 Year Results
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Laser Peripheral Iridotomy and Peripheral Iridoplasty
2.3. Dual Scheimpflug Analyzer Imaging
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, H.K.; Kee, C. Population-based glaucoma prevalence studies in Asians. Surv. Ophthalmol. 2014, 59, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Congdon, N.; Wang, F.; Tielsch, J.M. Issues in the epidemiology and population-based screening of primary angle-closure glaucoma. Surv. Ophthalmol. 1992, 36, 411–423. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Jung, H.R. Clarifying the nomenclature for primary angle-closure glaucoma. Surv. Ophthalmol. 1997, 42, 125–136. [Google Scholar] [CrossRef]
- Lowe, R.F. Clinical types of primary angle closure glaucoma. Aust. N. Z. J. Ophthalmol. 1988, 16, 245–250. [Google Scholar] [CrossRef]
- He, M.; Foster, P.J.; Johnson, G.J.; Khaw, P.T. Angle-closure glaucoma in East Asian and European people. Different diseases? Eye 2006, 20, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Nongpiur, M.E.; Ku, J.Y.; Aung, T. Angle closure glaucoma: A mechanistic review. Curr. Opin. Ophthalmol. 2011, 22, 96–101. [Google Scholar] [CrossRef]
- Ritch, R.; Lowe, R.F. Angle-closure glaucoma: Mechanisms and epidemiology. In The Glaucomas, 2nd ed.; Ritch, R., Shields, M.B., Krupin, T., Eds.; Mosby: St. Louis, MO, USA, 1996; pp. 801–819. [Google Scholar]
- Nolan, W.P.; Foster, P.J.; Devereux, J.G.; Uranchimeg, D.; Johnson, G.J.; Baasanhu, J. YAG laser iridotomy treatment for pri-mary angle closure in east Asian eyes. Br. J. Ophthalmol. 2000, 84, 1255–1259. [Google Scholar] [CrossRef]
- Saw, S.-M.; Gazzard, G.; Friedman, D.S. Interventions for angle-closure glaucoma: An evidence-based update. Ophthalmology 2003, 110, 1869–1879. [Google Scholar] [CrossRef]
- Azuara-Blanco, A.; Burr, J.; Ramsay, C.; Cooper, D.; Foster, P.J.; Friedman, D.S.; Scotland, G.; Javanbakht, M.; Cochrane, C.; Norrie, J.; et al. Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): A randomised controlled trial. Lancet 2016, 388, 1389–1397. [Google Scholar] [CrossRef]
- Alsagoff, Z.; Aung, T.; Ang, L.P.; Chew, P.T. Long-term clinical course of primary angle-closure glaucoma in an Asian population. Ophthalmology 2000, 107, 2300–2304. [Google Scholar] [CrossRef]
- He, M.; Friedman, D.S.; Ge, J.; Huang, W.; Jin, C.; Lee, P.S.; Khaw, P.T.; Foster, P.J. Laser peripheral iridotomy in primary an-gle-closure suspects: Biometric and gonioscopic outcomes: The Liwan Eye Study. Ophthalmology 2007, 114, 494–500. [Google Scholar] [CrossRef]
- Lee, K.S.; Sung, K.R.; Kang, S.Y.; Cho, J.W.; Kim, D.Y.; Kook, M.S. Residual anterior chamber angle closure in narrow-angle eyes following laser peripheral iridotomy: Anterior segment optical coherence tomography quantitative study. Jpn. J. Ophthalmol. 2011, 55, 213–219. [Google Scholar] [CrossRef]
- Cho, H.K.; Ahn, D.; Kee, C. Evaluation of circumferential angle closure using iridotrabecular contact index after laser iridot-omy by swept-source optical coherence tomography. Acta Ophthalmol. 2017, 95, e190–e196. [Google Scholar] [CrossRef] [PubMed]
- Aung, T.; Ang, L.P.; Chan, S.-P.; Chew, P.T. Acute primary angle-closure: Long-term intraocular pressure outcome in Asian eyes. Am. J. Ophthalmol. 2001, 131, 7–12. [Google Scholar] [CrossRef]
- Ang, L.P.; Aung, T.; Chew, P.T. Acute primary angle closure in an asian population: Long-term outcome of the fellow eye after prophylactic laser peripheral iridotomy. Ophthalmology 2000, 107, 2092–2096. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, Y.Y. Progression of Peripheral Anterior Synechiae After Laser Iridotomy. Am. J. Ophthalmol. 2005, 140, 1125–1127. [Google Scholar] [CrossRef]
- Cho, H.K.; Kee, C.; Yang, H.; Huh, H.D.; Kim, S.J.; Park, Y.M.; Park, J.M. Comparison of circumferential peripheral angle clo-sure using iridotrabecular contact index after laser iridotomy versus combined laser iridotomy and iridoplasty. Acta Ophthalmol. 2017, 95, e539–e547. [Google Scholar] [CrossRef]
- Fukuda, R.; Usui, T.; Tomidokoro, A.; Mishima, K.; Matagi, N.; Miyai, T.; Amano, S.; Araie, M. Noninvasive Observations of Peripheral Angle in Eyes After Penetrating Keratoplasty Using Anterior Segment Fourier-Domain Optical Coherence Tomography. Cornea 2012, 31, 259–263. [Google Scholar] [CrossRef]
- Liu, J.; A Belyea, D.; Lamba, T. Peripheral laser iridoplasty opens angle in plateau iris by thinning the cross-sectional tissues. Clin. Ophthalmol. 2013, 7, 1895–1897. [Google Scholar] [CrossRef][Green Version]
- Chew, P.T.K.; Yeo, L.M.W. Argon laser iridoplasty in chronic angle closure glaucoma. Int. Ophthalmol. 1995, 19, 67–70. [Google Scholar] [CrossRef]
- Ritch, R.; Tham, C.Y.C.; Lam, D.S. Argon Laser Peripheral Iridoplasty (ALPI): An Update. Surv. Ophthalmol. 2007, 52, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, S.; Amano, S.; Honda, N.; Mimura, T.; Usui, T.; Araie, M. Effect of trabeculectomy on ocular and corneal higher order aberrations. Jpn. J. Ophthalmol. 2011, 55, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Hayashi, H.; Oshika, T.; Hayashi, F. Fourier Analysis of Irregular Astigmatism after Trabeculectomy. Ophthalmic Surg. Lasers Imaging Retin. 2000, 31, 94–99. [Google Scholar] [CrossRef]
- Dada, T.; Konkal, V.; Tandon, R.; Singh, R.; Sihota, R. Corneal topographic response to intraocular pressure reduction in pa-tients with vernal keratoconjunctivitis and steroid-induced glaucoma. Eye 2007, 21, 158–163. [Google Scholar] [CrossRef][Green Version]
- Lawless, M.A.; Hodge, C. Wavefront’s role in corneal refractive surgery. Clin. Exp. Ophthalmol. 2005, 33, 199–209. [Google Scholar] [CrossRef]
- Wang, L.; Shirayama, M.; Koch, D.D. Repeatability of corneal power and wavefront aberration measurements with a du-al-Scheimpflug Placido corneal topographer. J. Cataract Refract. Surg. 2010, 36, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Foster, P.J.; Buhrmann, R.; Quigley, H.A.; Johnson, G.J. The definition and classification of glaucoma in prevalence surveys. Br. J. Ophthalmol. 2002, 86, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Montés-Micó, R. Role of the tear film in the optical quality of the human eye. J. Cataract Refract. Surg. 2007, 33, 1631–1635. [Google Scholar] [CrossRef]
- Jiang, Y.; Chang, D.S.; Zhu, H.; Khawaja, A.P.; Aung, T.; Huang, S.; Chen, Q.; Munoz, B.; Grossi, C.M.; He, M.; et al. Longitu-dinal changes of angle configuration in primary angle-closure suspects: The Zhongshan Angle-Closure Prevention Trial. Ophthalmology 2014, 121, 1699–1705. [Google Scholar] [CrossRef]
- Lee, K.S.; Sung, K.R.; Shon, K.; Sun, J.H.; Lee, J.R. Longitudinal Changes in Anterior Segment Parameters After Laser Peripheral Iridotomy Assessed by Anterior Segment Optical Coherence Tomography. Investig. Opthalmology Vis. Sci. 2013, 54, 3166–3170. [Google Scholar] [CrossRef]
- Kwon, J.; Sung, K.R.; Han, S. Long-term Changes in Anterior Segment Characteristics of Eyes With Different Primary An-gle-Closure Mechanisms. Am. J. Ophthalmol. 2018, 191, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.S.; Baskaran, M.; Friedman, D.S.; Xu, Y.; Wong, H.-T.; Lavanya, R.; Chew, P.T.; Foster, P.J.; Aung, T. Effect of prophylactic laser iridotomy on corneal endothelial cell density over 3 years in primary angle closure suspects. Br. J. Ophthalmol. 2013, 97, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Iida, M.; Sakisaka, T.; Minami, K.; Miyata, K. Effect of laser peripheral iridotomy using argon and neodymium-YAG lasers on corneal endothelial cell density: 7-year longitudinal evaluation. Jpn. J. Ophthalmol. 2018, 62, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Zhang, J.; Jiang, Y.; Huang, S.; Aung, T.; Foster, P.J.; Friedman, D.; He, M. Long-term effect of YAG laser iridotomy on corneal endothelium in primary angle closure suspects: A 72-month randomised controlled study. Br. J. Ophthalmol. 2021, 105, 348–353. [Google Scholar] [CrossRef]
- Muller, L.; Reeves, G.M.; Leong, J.C.; Wells, A.P. How safe is diode laser peripheral iridoplasty for the corneal endothelium? Clin. Exp. Ophthalmol. 2016, 44, 735–737. [Google Scholar] [CrossRef]
- Read, S.A.; Buehren, T.; Collins, M.J. Influence of accommodation on the anterior and posterior cornea. J. Cataract Refract. Surg. 2007, 33, 1877–1885. [Google Scholar] [CrossRef]
- Siso-Fuertes, I.; Dominguez-Vicent, A.; del Aguila-Carrasco, A.; Ferrer-Blasco, T.; Montes-Mico, R. Corneal changes with ac-commodation using dual Scheimpflug photography. J. Cataract Refract. Surg. 2015, 41, 981–989. [Google Scholar] [CrossRef]
- Kim, S.J.; Cho, H.-K.; Park, Y.M.; Han, Y.S.; Park, J.M. Corneal topography and angle parameters after laser iridotomy combined with iridoplasty assessed by dual Scheimpflug analyzer. Int. Ophthalmol. 2020, 40, 447–457. [Google Scholar] [CrossRef]
- Koh, S.; Maeda, N.; Ikeda, C.; Takai, Y.; Fujimoto, H.; Oie, Y.; Soma, T.; Tsujikawa, M.; Nishida, K. Effect of Instillation of Eyedrops for Dry Eye on Optical Quality. Investig. Opthalmology Vis. Sci. 2013, 54, 4927–4933. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, J.M.; Cho, H.K.; Kim, S.J.; Huh, H.D.; Park, Y.M. Influence of Sodium Hyaluronate Concentration on Corneal Aberrations in Soft Contact Lens Wearers. Korean J. Ophthalmol. 2018, 32, 89–94. [Google Scholar] [CrossRef][Green Version]
- Ramakrishnan, R.; Mitra, A.; Kader, M.A.; Das, S. To Study the Efficacy of Laser Peripheral Iridoplasty in the Treatment of Eyes With Primary Angle Closure and Plateau Iris Syndrome, Unresponsive to Laser Peripheral Iridotomy, Using Anterior-Segment OCT as a Tool. J. Glaucoma 2016, 25, 440–446. [Google Scholar] [CrossRef] [PubMed]
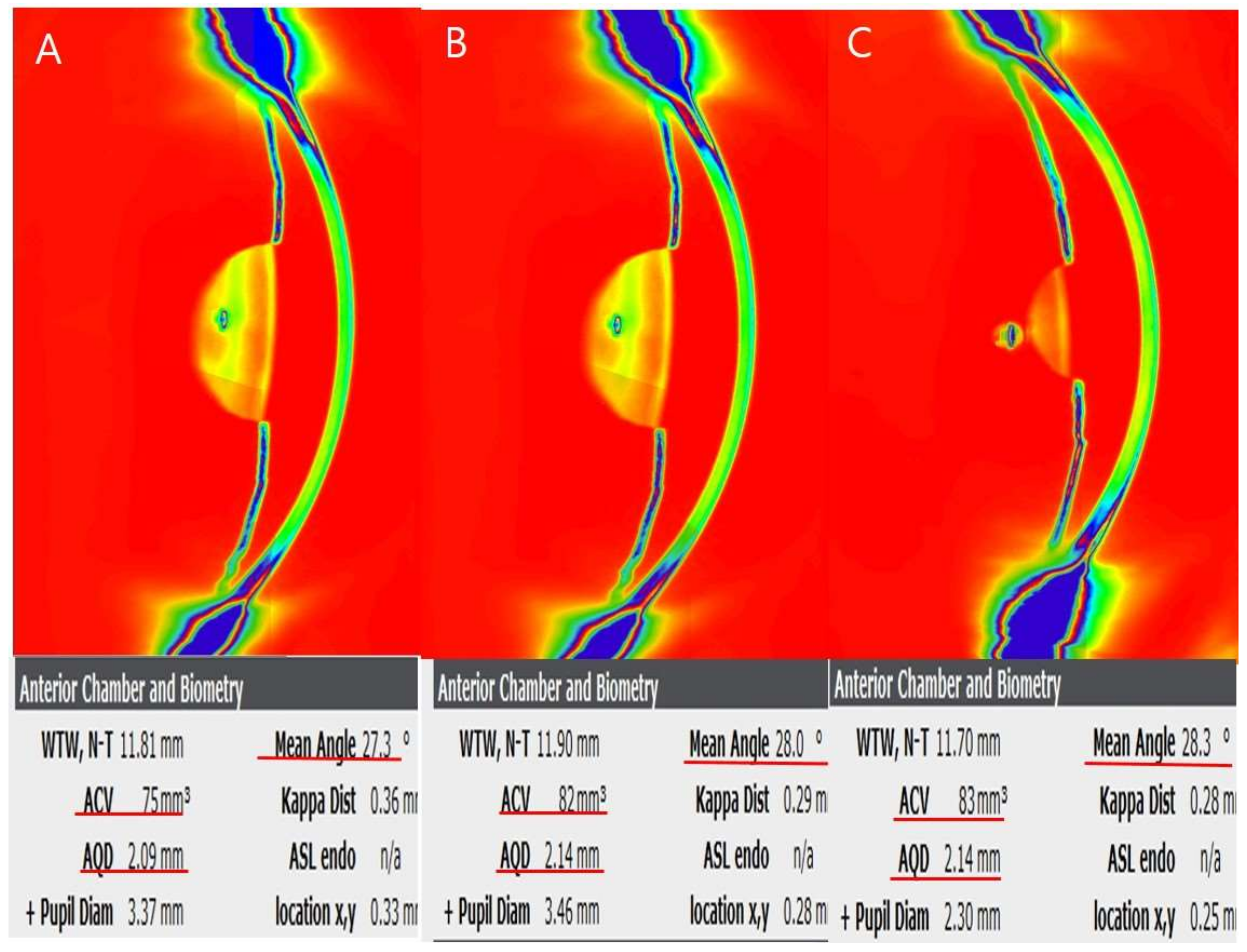
| Baseline Characteristics | Values |
|---|---|
| Number of subjects | 58 eyes (58 patients) |
| Mean age | 62.43 ± 7.38 years |
| Gender (Male: Female) | 15 eyes: 43 eyes |
| Diagnosis | |
| PACS | 27 eyes |
| PAC | 7 eyes |
| PACG | 24 eyes |
| Ocular factors | |
| SE | 0.78 ± 1.55 D |
| CCT | 553.48 ± 37 μm |
| WTW N-T | 11.62 ± 0.32 mm |
| WTW S-I | 11.59 ± 0.32 mm |
| Parameters | Before Laser | After 1 Week | p Values | After 1 Year | p Values |
|---|---|---|---|---|---|
| IOP (mmHg) | 15.97 ± 4.20 | 13.77 ± 2.30 | <0.0001 † | 13.73 ± 2.63 | <0.0001 * |
| Central ACD (mm) | 2.09 ± 0.25 | 2.12 ± 0.23 | 0.001 † | 2.10 ± 0.23 | 0.018 * |
| ACV (mm3) | 80.72 ± 12.34 | 85.36 ± 14.57 | <0.0001 † | 83.00 ± 12.17 | 0.013 * |
| AC angle S (degree) | 24.70 ± 3.64 | 26.48 ± 4.96 | 0.004 † | 26.60 ± 5.61 | 0.003 * |
| AC angle I (degree) | 29.57 ± 6.84 | 30.67 ± 5.53 | 0.11 † | 29.84 ± 4.84 | 0.579 * |
| AC angle N (degree) | 25.64 ± 3.13 | 26.42 ± 3.13 | 0.011 † | 26.59 ± 3.88 | 0.011 * |
| AC angle T (degree) | 26.29 ± 3.89 | 26.98 ± 3.70 | 0.058 † | 27.07 ± 4.17 | 0.038 * |
| Corneal Aberrations | Before Laser | After 1 Week | p Values | After 1 Year | p Values |
|---|---|---|---|---|---|
| Total corneal aberration (μm) | 1.09 ± 0.45 | 1.18 ± 0.66 | 0.285 † | 1.05 ± 0.33 | 0.562 * |
| Vertical Coma (μm) | −0.06 ± 0.28 | −0.06 ± 0.35 | 0.847 † | −0.04 ± 0.27 | 0.522 * |
| Horizontal Coma (μm) | 0.04 ± 0.36 | 0.02 ± 0.38 | 0.431 † | 0.03 ± 0.33 | 0.827 * |
| Total Coma (μm) | −0.02 ± 0.43 | −0.04 ± 0.50 | 0.553 † | −0.01 ± 0.42 | 0.627 * |
| Vertical Trefoil (μm) | −0.03 ± 0.32 | −0.01 ± 0.34 | 0.691 † | −0.03 ± 0.22 | 0.894 * |
| Oblique Trefoil (μm) | −0.08 ± 0.23 | −0.07 ± 0.24 | 0.729 † | −0.07 ± 0.22 | 0.788 * |
| Total Trefoil (μm) | −0.12 ± 0.45 | −0.09 ± 0.39 | 0.598 † | −0.10 ± 0.32 | 0.761 * |
| SE (D) | 0.78 ± 1.55 | 0.76 ± 1.84 | 0.601 † | 0.75 ± 1.88 | 0.87 * |
| Parameters | Before Laser | After 1 Week | p Values | After 1 Year | p Values |
|---|---|---|---|---|---|
| CCT (μm) | 553.48 ± 37.00 | 555.42 ± 38.30 | 0.76 † | 552.53 ± 36.45 | 0.293 * |
| Pachymetry Central (μm) | 564.00 ± 37.21 | 566.01 ± 37.93 | 0.727 † | 563.36 ± 36.63 | 0.346 * |
| Pachymetry Mid (μm) | 614.83 ± 36.58 | 616.98 ± 39.24 | 0.679 † | 614.66 ± 36.69 | 0.49 * |
| Pachymetry Pph (μm) | 696.89 ± 49.30 | 701.36 ± 50.96 | 0.543 † | 689.54 ± 43.35 | 0.167 * |
| Corneal volume (mm3) | 32.17 ± 1.92 | 32.30 ± 2.06 | 0.606 † | 32.12 ± 1.92 | 0.342 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.-k.; Choae, W. Long-Term Outcome of Corneal and Anterior Chamber Angle Parameters after Combined Laser Iridotomy and Iridoplasty Using Dual Scheimpflug Analyzer: 1 Year Results. J. Clin. Med. 2022, 11, 813. https://doi.org/10.3390/jcm11030813
Cho H-k, Choae W. Long-Term Outcome of Corneal and Anterior Chamber Angle Parameters after Combined Laser Iridotomy and Iridoplasty Using Dual Scheimpflug Analyzer: 1 Year Results. Journal of Clinical Medicine. 2022; 11(3):813. https://doi.org/10.3390/jcm11030813
Chicago/Turabian StyleCho, Hyun-kyung, and Wooseok Choae. 2022. "Long-Term Outcome of Corneal and Anterior Chamber Angle Parameters after Combined Laser Iridotomy and Iridoplasty Using Dual Scheimpflug Analyzer: 1 Year Results" Journal of Clinical Medicine 11, no. 3: 813. https://doi.org/10.3390/jcm11030813
APA StyleCho, H.-k., & Choae, W. (2022). Long-Term Outcome of Corneal and Anterior Chamber Angle Parameters after Combined Laser Iridotomy and Iridoplasty Using Dual Scheimpflug Analyzer: 1 Year Results. Journal of Clinical Medicine, 11(3), 813. https://doi.org/10.3390/jcm11030813






