Usefulness of Dermoscopy in Localized Scleroderma (LoS, Morphea) Diagnosis and Assessment-Monocentric Cross-Sectional Study
Abstract
:1. Introduction
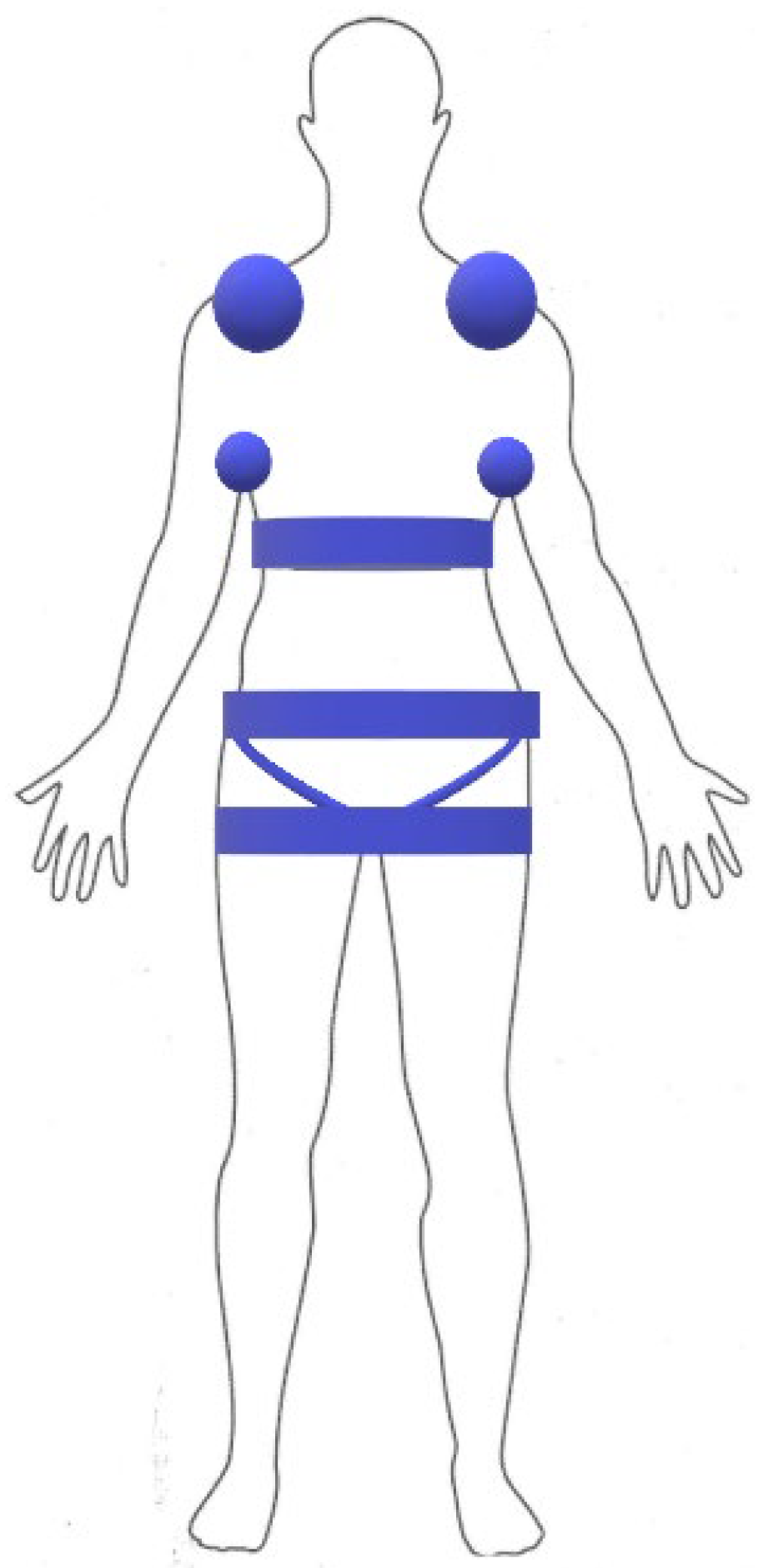
2. Materials and Methods
Statistical Analysis
- -
- Mann–Whitney U test—to test the significance of differences in the expression of dermoscopic features within the observed area between inactive and active lesions and between the absence and presence of pressure.
- -
- Spearman’s rank correlation coefficient significance test—to test the correlation between disease activity index and tissue damage index and expression of features within the observed field.
3. Results
3.1. Characteristics of the Study Population
3.2. Clinical Evaluation of Skin Involvement
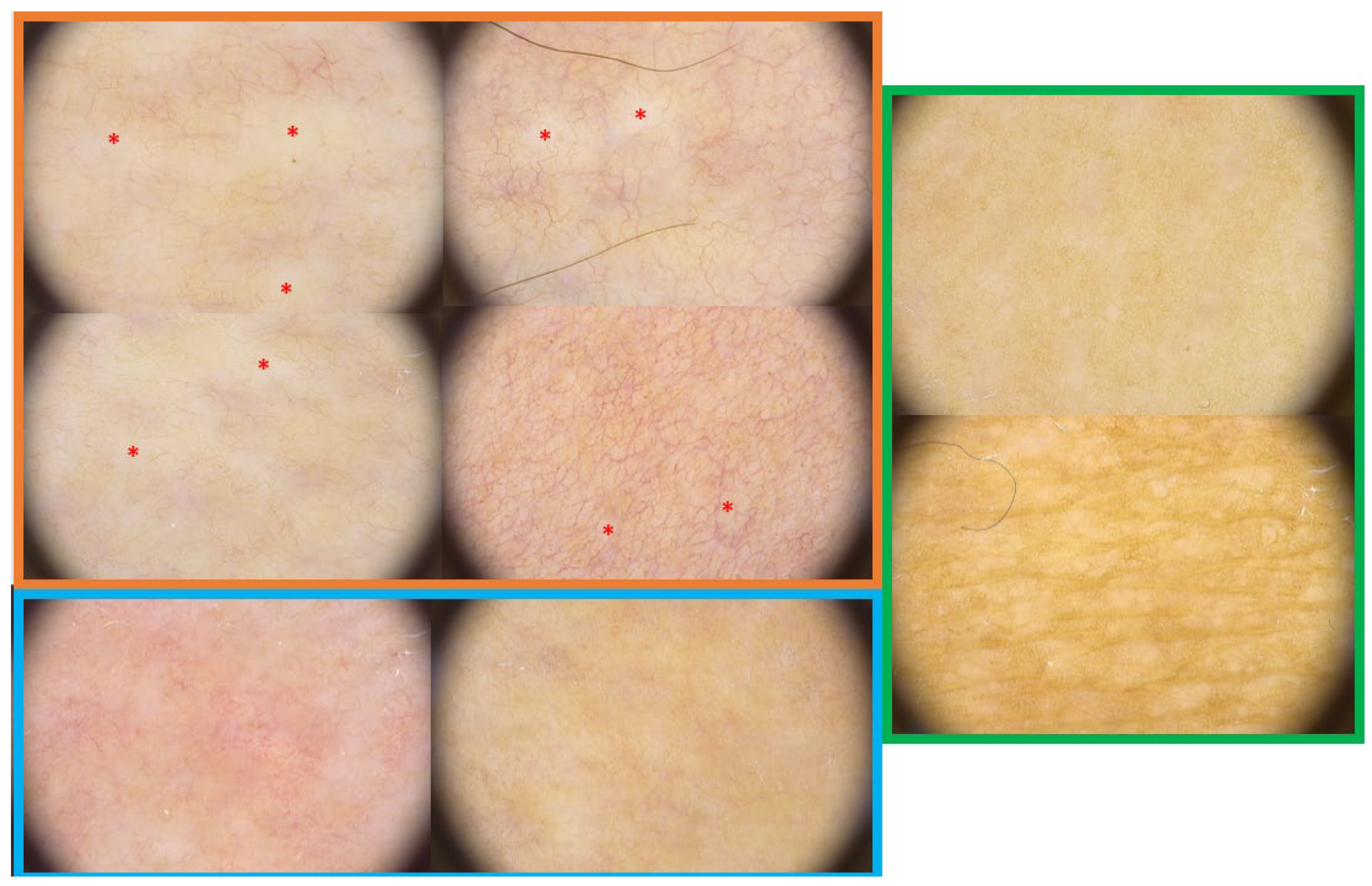
3.3. Dermoscopic Findings
3.4. Correlation of Dermoscopic Findings with Lesion Activity, mLoSSI, mLoSDI, and the Presence of Pressure
3.4.1. The Dermoscopic Findings vs. Lesion Activity
3.4.2. The Dermoscopic Findings vs. mLoSSI Value
3.4.3. The Dermoscopic Findings vs. LoSDI Value
3.4.4. The Dermoscopic Findings vs. Pressure Location
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krasowska, D.; Rudnicka, L.; Dańczak-Pazdrowska, A.; Chodorowska, G.; Woźniacka, A.; Lis-Święty, A.; Czuwara, J.; Maj, J.; Majewski, S.; Sysa-Jędrzejowska, A.; et al. Localized scleroderma (morphea). Diagnostic and therapeutic recommendations of the Polish Dermatological Society. Dermatol. Rev. 2019, 106, 333–353. [Google Scholar] [CrossRef]
- Abbas, L.; Joseph, A.; Kunzler, E.; Jacobe, H.T. Morphea: Progress to date and the road ahead. Ann. Transl. Med. 2021, 9, 437. [Google Scholar] [CrossRef] [PubMed]
- Eckes, B.; Wang, F.; Moinzadeh, P.; Hunzelmann, N.; Krieg, T. Pathophysiological Mechanisms in Sclerosing Skin Diseases. Front. Med. 2017, 4, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Errichetti, E.; Stinco, G. Morphea. In Atlas of Pediatric Dermatoscopy; Micali, G., Lacarrubba, F., Stinco, G., Argenziano, G., Neri, I., Eds.; Springer: Cham, Switzerland, 2018; pp. 115–119. [Google Scholar] [CrossRef]
- Knobler, R.; Moinzadeh, P.; Hunzelmann, N.; Kreuter, A.; Cozzio, A.; Mouthon, L.; Cutolo, M.; Rongioletti, F.; Denton, C.; Rudnicka, L.; et al. European Dermatology Forum S1-guideline on the diagnosis and treatment of sclerosing diseases of the skin, Part 1: Localized scleroderma, systemic sclerosis and overlap syndromes. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1401–1424. [Google Scholar] [CrossRef] [PubMed]
- Szczęch, J.; Samotij, D.; Jaworecka, K.; Tobiasz, A.; Reich, A. Quality of Life in Patients with Morphea: A Cross-Sectional Study and a Review of the Current Literature. BioMed Res. Int. 2020, 2020, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narbutt, J.; Hołdrowicz, A.; Lesiak, A. Morphea—Selected local treatment methods and their effectiveness. Reum. 2017, 55, 305–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arkachaisri, T.; Vilaiyuk, S.; Torok, K.S.; Medsger, T.A., Jr. Development and initial validation of the Localized Scleroderma Skin Damage Index and Physician Global Assessment of disease Damage: A proof-of-concept study. Rheumatology 2010, 49, 373–381. [Google Scholar] [CrossRef] [Green Version]
- Skrzypek-Salamon, A.; Lis-Święty, A.; Ranosz-Janicka, I.; Brzezińska-Wcisło, L. Localized Scleroderma Cutaneous Assessment Tool (LoSCAT) adapted for use in adult patients: Report from an initial validation study. Heal. Qual. Life Outcomes 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Florez-Pollack, S.; Kunzler, E.; Jacobe, H.T. Morphea: Current concepts. Clin. Dermatol. 2018, 36, 475–486. [Google Scholar] [CrossRef]
- Errichetti, E.; Lallas, A.; Apalla, Z.; Di Stefani, A.; Stinco, G. Dermoscopy of Morphea and Cutaneous Lichen Sclerosus: Clinicopathological Correlation Study and Comparative Analysis. Dermatology 2017, 233, 462–470. [Google Scholar] [CrossRef]
- Chiu, Y.E.; Abban, C.Y.; Konicke, K.; Segura, A.; Sokumbi, O. Histopathologic Spectrum of Morphea. Am. J. Dermatopathol. 2021, 43, 1–8. [Google Scholar] [CrossRef]
- Sgouros, D.; Apalla, Z.; Ioannides, D.; Katoulis, A.; Rigopoulos, D.; Sotiriou, E.; Stratigos, A.; Vakirlis, E.; Lallas, A. Dermoscopy of Common Inflammatory Disorders. Dermatol. Clin. 2018, 36, 359–368. [Google Scholar] [CrossRef]
- Errichetti, E. Dermoscopy in Monitoring and Predicting Therapeutic Response in General Dermatology (Non-Tumoral Dermatoses): An Up-To-Date Overview. Dermatol. Ther. 2020, 10, 1199–1214. [Google Scholar] [CrossRef]
- Campione, E.; Paternò, E.J.; Diluvio, L.; Orlandi, A.; Bianchi, L.; Chimenti, S. Localized morphea treated with imiquimod 5% and dermoscopic assessment of effectiveness. J. Dermatol. Treat. 2009, 20, 10–13. [Google Scholar] [CrossRef]
- Shim, W.-H.; Jwa, S.-W.; Song, M.; Kim, H.-S.; Ko, H.-C.; Kim, M.-B.; Kim, B.-S. Diagnostic usefulness of dermatoscopy in differentiating lichen sclerous et atrophicus from morphea. J. Am. Acad. Dermatol. 2012, 66, 690–691. [Google Scholar] [CrossRef]
- Nóbrega, M.M.; Cabral, F.; Corrêa, M.C.; Barcaui, C.B.; Bressan, A.L.; Gripp, A.C. Lichen sclerosus associated with localized scleroderma: Dermoscopy contribution. An. Bras. Dermatol. 2016, 91, 534–536. [Google Scholar] [CrossRef] [Green Version]
- Toader, M.P.; Esanu, I.M.; Taranu, T.; Toader, S.V. Utility of polarized dermoscopy in the diagnosis of cutaneous lupus erythematosus and morphea. In Proceedings of the E-Health and Bioengineering Conference (EHB), Sinaia, Romania, 22–24 June 2017; pp. 583–586. [Google Scholar] [CrossRef]
- Saceda-Corralo, D.; Tosti, A. Trichoscopic Features of Linear Morphea on the Scalp. Ski. Appendage Disord. 2017, 4, 31–33. [Google Scholar] [CrossRef]
- Peña-Romero, A.G.; Garcia-Romero, M.T. Diagnosis and management of linear scleroderma in children. Curr. Opin. Pediatr. 2019, 31, 482–490. [Google Scholar] [CrossRef]
- Bhat, Y.J.; Akhtar, S.; Hassan, I. Dermoscopy of Morphea. Indian Dermatol. Online J. 2019, 10, 92–93. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Hao, J.-C.; Liu, J.; Liu, Y.-H.; Jin, H.-Z. Dermoscopic features of morphea and extragenital lichen sclerosus in Chinese patients. Chin. Med. J. 2020, 133, 2109–2111. [Google Scholar] [CrossRef]
- Grabell, D.; Hsieh, C.; Andrew, R.; Martires, K.; Kim, A.; Vasquez, R.; Jacobe, H. The role of skin trauma in the distribution of morphea lesions: A cross-sectional survey of the Morphea in Adults and Children cohort IV. J. Am. Acad. Dermatol. 2014, 71, 493–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mertens, J.S.; Seyger, M.M.B.; Thurlings, R.M.; Radstake, T.R.D.J.; De Jong, E.M.G.J. Morphea and Eosinophilic Fasciitis: An Update. Am. J. Clin. Dermatol. 2017, 18, 491–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almuqati, R.R.; Hariri, J.; Abduljabbar, M. Histopathological Coexistence of Extragenital Lichen Sclerosus and Morphea in a Single Lesion. Cureus 2020, 12, 12215. [Google Scholar] [CrossRef]
- Beergouder, S.L.; Rajput, C.D.; Koti, V.R. Miscellaneous Conditions. In Dermoscopy—Histopathology Correlation; Ankad, B.S., Mukherjee, S.S., Nikam, B.P., Eds.; Springer: Singapore, 2021; pp. 405–406. [Google Scholar] [CrossRef]
- Errichetti, E. Dermoscopy of Inflammatory Dermatoses (Inflammoscopy): An Up-to-Date Overview. Dermatol. Pr. Concept. 2019, 9, 169–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| LoS Differential Diagnosis | |
|---|---|
| LoS Phase | Skin Diseases |
| Early lesions: inflammatory and sclerotic | Mycosis fungoides (early lesions) Lichen sclerosus (early lesions) Stasis dermatitis Radiation dermatitis Vascular malformations in children Necrobiosis lipoidica diabeticorum |
| Predominantly fibrotic lesions | Systemic sclerosis Cutaneous sclerosis at the injection site Nephrogenic systemic fibrosis Drug-induced scleroderma-like lesions (bleomycin, taxanes) Keloid Carcinoma en cuirasse Skin metastasis |
| Late lesions: atrophic | Mycosis fungoides (advanced lesions) Lichen sclerosus (advanced lesions) Acrodermatitis chronica atrophicans Vitiligo Lichen planus planopilaris (late-stage lesions) |
| Location of Lesions | n | % |
|---|---|---|
| Trunk | 94 | 58.0 |
| Upper limb | 43 | 26.5 |
| Lower limb | 24 | 14.8 |
| Head | 1 | 0.6 |
| Total | 162 | 100.0 |
| Compression Localization | n | % |
| No | 112 | 69.1 |
| Yes | 50 | 30.9 |
| Total | 162 | 100.0 |
| Dermoscopic Findings | 0 | 1 | 2 | 3 | 4 | Dermoscopic Findings | n | Mean | Standard Deviation | Median | Min. | Max. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Erythematous areas | 142 (87.7) | 17 (10.5) | 3 (1.9) | 0 (0.0) | 0 (0.0) | Erythematous areas | 162 | 0.14 | 0.40 | 0 | 0 | 2 |
| Linear branching vessels | 69 (42.6) | 18 (11.1) | 36 (22.2) | 36 (22.2) | 3 (1.9) | Linear branching vessels | 162 | 1.30 | 1.28 | 1 | 0 | 4 |
| Linear irregular vessels | 109 (67.3) | 28 (17.3) | 24 (14.8) | 1 (0.6) | 0 (0.0) | Linear irregular vessels | 162 | 0.49 | 0.77 | 0 | 0 | 3 |
| Dotted vessels | 73 (45.1) | 33 (20.4) | 29 (17.9) | 19 (11.7) | 8 (4.9) | Dotted vessels | 162 | 1.11 | 1.24 | 1 | 0 | 4 |
| Large purple vessels | 143 (88.3) | 19 (11.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | Large purple vessels | 162 | 0.12 | 0.32 | 0 | 0 | 1 |
| White clouds | 14 (8.6) | 61 (37.7) | 36 (22.2) | 40 (24.7) | 11 (6.8) | White clouds | 162 | 1.83 | 1.10 | 2 | 0 | 4 |
| Crystalline structures | 158 (97.5) | 3 (1.9) | 1 (0.6) | 0 (0.0) | 0 (0.0) | Crystalline structures | 162 | 0.03 | 0.21 | 0 | 0 | 2 |
| Structureless brownish areas | 26 (16.0) | 38 (23.5) | 52 (32.1) | 40 (24.7) | 6 (3.7) | Structureless brownish areas | 162 | 1.77 | 1.11 | 2 | 0 | 4 |
| Reticular brownish areas | 140 (86.4) | 1 (0.6) | 8 (4.9) | 9 (5.6) | 4 (2.5) | Reticular brownish areas | 162 | 0.37 | 0.98 | 0 | 0 | 4 |
| Brownish dots | 135 (83.3) | 19 (11.7) | 8 (4.9) | 0 (0.0) | 0 (0.0) | Brownish dots | 162 | 0.22 | 0.52 | 0 | 0 | 2 |
| Dermoscopic Findings | Lesion Activity | Z | p | |||
|---|---|---|---|---|---|---|
| Inactive n = 31 | Active n = 131 | |||||
| Mean ± Standard Deviation | Median (Min.-Max.) | Mean ± Standard Deviation | Median (Min.-Max.) | |||
| Erythematous areas | 0.23 ± 0.50 | 0 (0–2) | 0.12 ± 0.37 | 0 (0–2) | 1.32 | 0.1877 |
| Linear branching vessels | 1.00 ± 1.29 | 0 (0–4) | 1.37 ± 1.27 | 1 (0–4) | −1.47 | 0.1427 |
| Linear irregular vessels | 0.52 ± 0.81 | 0 (0–3) | 0.48 ± 0.76 | 0 (0–2) | 0.25 | 0.8052 |
| Dotted vessels | 0.65 ± 1.02 | 0 (0–3) | 1.22 ± 1.27 | 1 (0–4) | −2.42 | 0.0153 |
| Large purple vessels | 0.13 ± 0.34 | 0 (0–1) | 0.11 ± 0.32 | 0 (0–1) | 0.22 | 0.8247 |
| White clouds | 1.42 ± 1.34 | 1 (0–4) | 1.93 ± 1.02 | 2 (0–4) | −2.46 | 0.0138 |
| Crystalline structures | 0.06 ± 0.36 | 0 (0–2) | 0.02 ± 0.15 | 0 (0–1) | 0.32 | 0.7514 |
| Structureless brownish areas | 1.94 ± 1.36 | 2 (0–4) | 1.73 ± 1.04 | 2 (0–3) | 0.58 | 0.5625 |
| Reticular brownish areas | 0.61 ± 1.43 | 0 (0–4) | 0.31 ± 0.84 | 0 (0–3) | 0.73 | 0.4656 |
| Brownish dots | 0.32 ± 0.60 | 0 (0–2) | 0.19 ± 0.50 | 0 (0–2) | 1.47 | 0.1409 |
| Variables | n | Rs | p |
|---|---|---|---|
| mLoSSI & erythematous areas | 162 | 0.058 | 0.4661 |
| mLoSSI & linear branching vessels | 162 | 0.246 | 0.0016 |
| mLoSSI & linear irregular vessels | 162 | −0.099 | 0.2118 |
| mLoSSI & dotted vessels | 162 | 0.001 | 0.9867 |
| mLoSSI & large purple vessels | 162 | 0.022 | 0.7806 |
| mLoSSI & white clouds | 162 | 0.535 | <0.0001 |
| mLoSSI & crystalline structures | 162 | 0.041 | 0.6085 |
| mLoSSI & structureless brownish areas | 162 | −0.141 | 0.0734 |
| mLoSSI & reticular brownish areas | 162 | −0.179 | 0.0230 |
| mLoSSI & brownish dots | 162 | −0.124 | 0.1153 |
| Variables | n | Rs | p |
|---|---|---|---|
| LoSDI & erythematous areas | 162 | −0.011 | 0.8944 |
| LoSDI & linear branching vessels | 162 | −0.166 | 0.0344 |
| LoSDI & linear irregular vessels | 162 | 0.076 | 0.3337 |
| LoSDI & dotted vessels | 162 | −0.001 | 0.9940 |
| LoSDI & large purple vessels | 162 | 0.065 | 0.4079 |
| LoSDI & white clouds | 162 | −0.286 | 0.0002 |
| LoSDI & crystalline structures | 162 | 0.038 | 0.6315 |
| LoSDI & structureless brownish areas | 162 | 0.264 | 0.0007 |
| LoSDI & reticular brownish areas | 162 | 0.117 | 0.1384 |
| LoSDI & brownish dots | 162 | 0.153 | 0.0513 |
| Dermoscopic Findings | Pressure Location | Z | p | |||
|---|---|---|---|---|---|---|
| No n = 112 | Yes n = 50 | |||||
| Mean ± Standard Deviation | Median (Min.-Max.) | Mean ± Standard Deviation | Median (Min.-Max.) | |||
| Erythematous areas | 0.08 ± 0.30 | 0 (0–2) | 0.28 ± 0.54 | 0 (0–2) | −3.01 | 0.0026 |
| Linear branching vessels | 1.10 ± 1.16 | 1 (0–3) | 1.74 ± 1.41 | 2 (0–4) | −2.81 | 0.0050 |
| Linear irregular vessels | 0.53 ± 0.75 | 0 (0–2) | 0.40 ± 0.81 | 0 (0–3) | 1.53 | 0.1252 |
| Dotted vessels | 1.32 ± 1.30 | 1 (0–4) | 0.64 ± 0.94 | 0 (0–3) | 3.15 | 0.0016 |
| Large purple vessels | 0.12 ± 0.32 | 0 (0–1) | 0.12 ± 0.33 | 0 (0–1) | −0.07 | 0.9455 |
| White clouds | 1.78 ± 1.05 | 1 (0–4) | 1.96 ± 1.23 | 2 (0–4) | −1.09 | 0.2754 |
| Crystalline structures | 0.00 ± 0.00 | 0 (0–0) | 0.10 ± 0.36 | 0 (0–2) | −3.01 | 0.0026 |
| Structureless brownish areas | 1.81 ± 1.13 | 2 (0–4) | 1.66 ± 1.06 | 2 (0–4) | 1.14 | 0.2535 |
| Reticular brownish areas | 0.38 ± 1.01 | 0 (0–4) | 0.36 ± 0.94 | 0 (0–4) | −0.04 | 0.9684 |
| Brownish dots | 0.21 ± 0.53 | 0 (0–2) | 0.22 ± 0.51 | 0 (0–2) | −0.25 | 0.8033 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczepanik-Kułak, P.; Michalak-Stoma, A.; Krasowska, D. Usefulness of Dermoscopy in Localized Scleroderma (LoS, Morphea) Diagnosis and Assessment-Monocentric Cross-Sectional Study. J. Clin. Med. 2022, 11, 764. https://doi.org/10.3390/jcm11030764
Szczepanik-Kułak P, Michalak-Stoma A, Krasowska D. Usefulness of Dermoscopy in Localized Scleroderma (LoS, Morphea) Diagnosis and Assessment-Monocentric Cross-Sectional Study. Journal of Clinical Medicine. 2022; 11(3):764. https://doi.org/10.3390/jcm11030764
Chicago/Turabian StyleSzczepanik-Kułak, Paulina, Anna Michalak-Stoma, and Dorota Krasowska. 2022. "Usefulness of Dermoscopy in Localized Scleroderma (LoS, Morphea) Diagnosis and Assessment-Monocentric Cross-Sectional Study" Journal of Clinical Medicine 11, no. 3: 764. https://doi.org/10.3390/jcm11030764
APA StyleSzczepanik-Kułak, P., Michalak-Stoma, A., & Krasowska, D. (2022). Usefulness of Dermoscopy in Localized Scleroderma (LoS, Morphea) Diagnosis and Assessment-Monocentric Cross-Sectional Study. Journal of Clinical Medicine, 11(3), 764. https://doi.org/10.3390/jcm11030764







