Elevated Levels of Growth/Differentiation Factor-15 in the Aqueous Humor and Serum of Glaucoma Patients
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Elevated Levels of GDF15 in Both AH and Serum Samples of POAG Patients
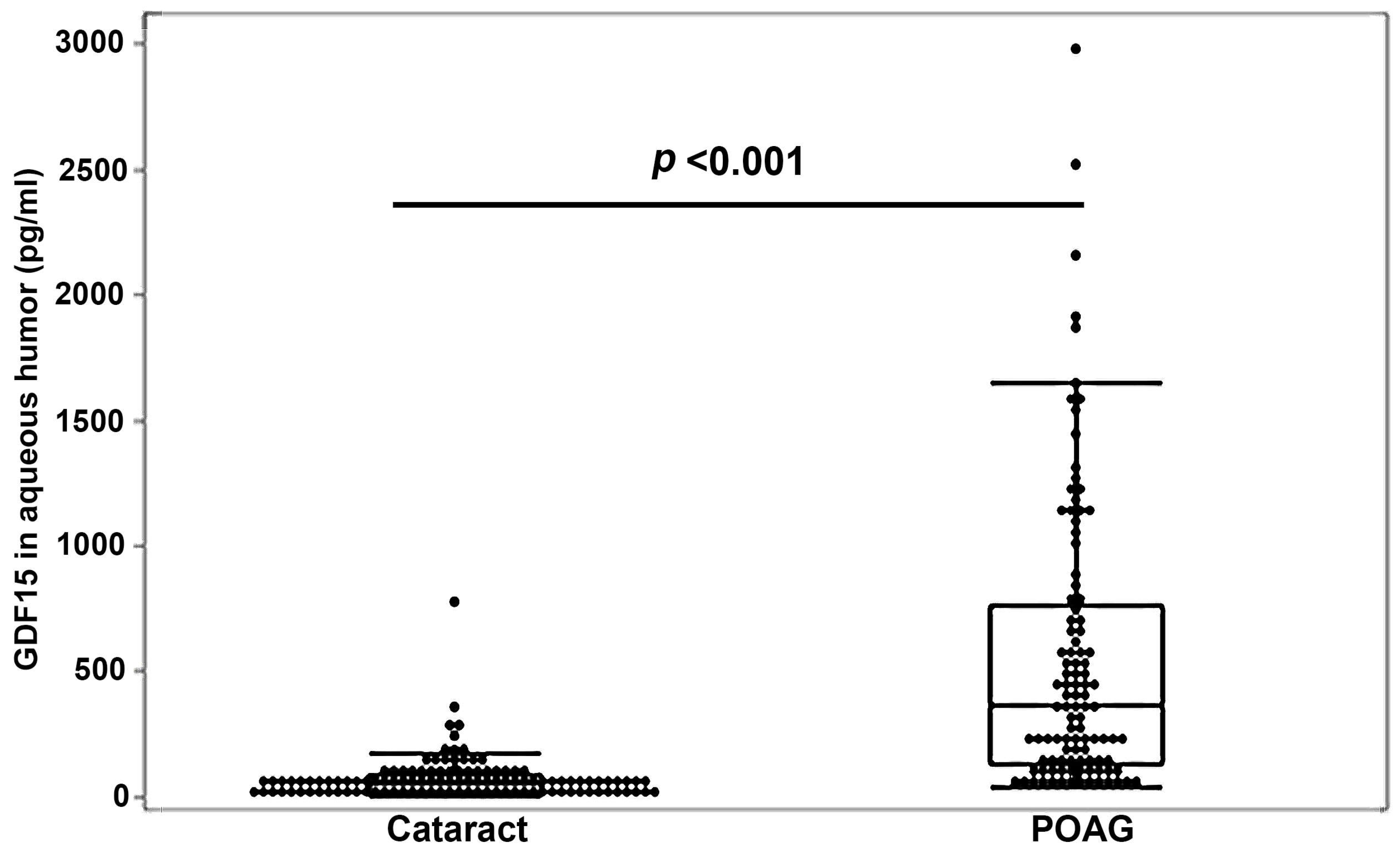
3.2. Robust Elevation of GDF15 Levels in the Aqueous Humor of a Large Cohort of POAG Patients
3.3. Aqueous Humor GDF15 Levels, IOP, and Age Relationships in POAG Patients
3.4. Relationship between AH GDF15 and POAG Disease Severity
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allison, K.; Patel, D.; Alabi, O. Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future. Cureus 2020, 12, e11686. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Leung, C.K.; Crowston, J.G.; Medeiros, F.A.; Friedman, D.S.; Wiggs, J.L.; Martin, K.R. Primary open-angle glaucoma. Nat. Rev. Dis. Primers 2016, 2, 16067. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, Y.H.; Fingert, J.H.; Kuehn, M.H.; Alward, W.L. Primary open-angle glaucoma. N. Engl. J. Med. 2009, 360, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamer, W.D.; Acott, T.S. Current understanding of conventional outflow dysfunction in glaucoma. Curr. Opin. Ophthalmol. 2012, 23, 135–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, P.V.; Pattabiraman, P.P.; Kopczynski, C. Role of the Rho GTPase/Rho kinase signaling pathway in pathogenesis and treatment of glaucoma: Bench to bedside research. Exp. Eye Res. 2017, 158, 23–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, L.T.Y.; Osterwald, A.; Ruf, I.; Hunziker, D.; Mattei, P.; Challa, P.; Vann, R.; Ullmer, C.; Rao, P.V. Role of the autotaxin-lysophosphatidic acid axis in glaucoma, aqueous humor drainage and fibrogenic activity. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165560. [Google Scholar] [CrossRef]
- Muralidharan, A.R.; Maddala, R.; Skiba, N.P.; Rao, P.V. Growth Differentiation Factor-15-Induced Contractile Activity and Extracellular Matrix Production in Human Trabecular Meshwork Cells. Invest. Ophthalmol. Vis. Sci. 2016, 57, 6482–6495. [Google Scholar] [CrossRef] [Green Version]
- Mullican, S.E.; Rangwala, S.M. Uniting GDF15 and GFRAL: Therapeutic Opportunities in Obesity and Beyond. Trends Endocrinol. Metab. 2018, 29, 560–570. [Google Scholar] [CrossRef]
- Breit, S.N.; Brown, D.A.; Tsai, V.W. The GDF15-GFRAL Pathway in Health and Metabolic Disease: Friend or Foe? Annu. Rev. Physiol. 2021, 83, 127–151. [Google Scholar] [CrossRef] [PubMed]
- Basisty, N.; Kale, A.; Jeon, O.H.; Kuehnemann, C.; Payne, T.; Rao, C.; Holtz, A.; Shah, S.; Sharma, V.; Ferrucci, L.; et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020, 18, e3000599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baek, S.J.; Eling, T. Growth differentiation factor 15 (GDF15): A survival protein with therapeutic potential in metabolic diseases. Pharmacol. Ther. 2019, 198, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Desmedt, S.; Desmedt, V.; De Vos, L.; Delanghe, J.R.; Speeckaert, R.; Speeckaert, M.M. Growth differentiation factor 15: A novel biomarker with high clinical potential. Crit. Rev. Clin. Lab. Sci. 2019, 56, 333–350. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.N.; Johnen, H.; Cook, A.D.; Tsai, V.W.; Mohammad, M.G.; Kuffner, T.; Zhang, H.P.; Marquis, C.P.; Jiang, L.; Lockwood, G.; et al. The TGF-beta superfamily cytokine, MIC-1/GDF15: A pleotrophic cytokine with roles in inflammation, cancer and metabolism. Growth Factors 2011, 29, 187–195. [Google Scholar] [CrossRef]
- Ban, N.; Siegfried, C.J.; Lin, J.B.; Shui, Y.B.; Sein, J.; Pita-Thomas, W.; Sene, A.; Santeford, A.; Gordon, M.; Lamb, R.; et al. GDF15 is elevated in mice following retinal ganglion cell death and in glaucoma patients. JCI Insight 2017, 2, e91455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.B.; Sheybani, A.; Santeford, A.; De Maria, A.; Apte, R.S. Increased Aqueous Humor GDF15 Is Associated with Worse Visual Field Loss in Pseudoexfoliative Glaucoma Patients. Transl. Vis. Sci. Technol. 2020, 9, 16. [Google Scholar] [CrossRef]
- Hubens, W.H.G.; Kievit, M.T.; Berendschot, T.; de Coo, I.F.M.; Smeets, H.J.M.; Webers, C.A.B.; Gorgels, T. Plasma GDF-15 concentration is not elevated in open-angle glaucoma. PLoS ONE 2021, 16, e0252630. [Google Scholar]
- Bourouki, E.; Oikonomou, E.; Moschos, M.; Siasos, G.; Siasou, G.; Gouliopoulos, N.; Deftereos, S.; Miliou, A.; Zacharia, E.; Tousoulis, D. Pseudoexfoliative Glaucoma, Endothelial Dysfunction, and Arterial Stiffness: The Role of Circulating Apoptotic Endothelial Microparticles. J. Glaucoma 2019, 28, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.H.; Wang, A.; Hilliard, B.K.; Carvalho, F.; Rosen, C.E.; Ahasic, A.M.; Herzog, E.L.; Kang, I.; Pisani, M.A.; Yu, S.; et al. GDF15 Is an Inflammation-Induced Central Mediator of Tissue Tolerance. Cell 2019, 178, 1231–1244 e11. [Google Scholar] [CrossRef]
- Yang, L.; Chang, C.C.; Sun, Z.; Madsen, D.; Zhu, H.; Padkjaer, S.B.; Wu, X.; Huang, T.; Hultman, K.; Paulsen, S.J.; et al. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat. Med. 2017, 23, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Mullican, S.E.; Lin-Schmidt, X.; Chin, C.N.; Chavez, J.A.; Furman, J.L.; Armstrong, A.A.; Beck, S.C.; South, V.J.; Dinh, T.Q.; Cash-Mason, T.D.; et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat. Med. 2017, 23, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.Y.; Crawley, S.; Chen, M.; Ayupova, D.A.; Lindhout, D.A.; Higbee, J.; Kutach, A.; Joo, W.; Gao, Z.; Fu, D.; et al. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature 2017, 550, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.C.; Sun, C.; Cameron, E.G.; Madaan, A.; Wu, S.; Xia, X.; Zhang, X.; Tenerelli, K.; Nahmou, M.; Knasel, C.M.; et al. Opposing Effects of Growth and Differentiation Factors in Cell-Fate Specification. Curr. Biol. 2019, 29, 1963–1975 e5. [Google Scholar] [CrossRef] [PubMed]
- Iwata, Y.; Inagaki, S.; Morozumi, W.; Nakamura, S.; Hara, H.; Shimazawa, M. Treatment with GDF15, a TGFbeta superfamily protein, induces protective effect on retinal ganglion cells. Exp. Eye Res. 2021, 202, 108338. [Google Scholar] [CrossRef]
- Lind, L.; Wallentin, L.; Kempf, T.; Tapken, H.; Quint, A.; Lindahl, B.; Olofsson, S.; Venge, P.; Larsson, A.; Hulthe, J.; et al. Growth-differentiation factor-15 is an independent marker of cardiovascular dysfunction and disease in the elderly: Results from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) Study. Eur. Heart J. 2009, 30, 2346–2353. [Google Scholar] [CrossRef] [Green Version]
- Kalayci, M.; Cetinkaya, E.; Erol, M.K. Prevalence of primary open-angle glaucoma in a Somalia population. Int. Ophthalmol. 2021, 41, 581–586. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahman, N.; Foster, P.J.; Haque, Z.; Zaman, A.U.; Dineen, B.; Johnson, G.J. The prevalence of glaucoma in Bangladesh: A population based survey in Dhaka division. Br. J. Ophthalmol. 2004, 88, 1493–1497. [Google Scholar] [CrossRef]



| Gender | Cataract | POAG | p-Value * | ||
|---|---|---|---|---|---|
| GDF-15 (AH) | Female | n | 64 | 53 | |
| Mean (SD) | 60.41 (65.33) | 543.55 (664.30) | |||
| Min, Median, Max | 0.3, 39.0, 357.1 | 36.1, 246.0, 2978.0 | <0.001 | ||
| GDF-15 (AH) | Male | n | 53 | 52 | |
| Mean (SD) | 80.07 (108.36) | 556.95 (478.73) | |||
| Min, Median, Max | 1.5, 52.0, 776.0 | 48.0, 438.6, 1868.2 | <0.001 |
| Variable | Race | Statistic | Cataract | POAG | p-Value * |
|---|---|---|---|---|---|
| GDF-15 (AH) | African | n | 16 | 53 | |
| American | Mean (SD) | 54.90 (43.12) | 515.41 (561.68) | ||
| Min, Median, Max | 2.0, 49.0, 146.0 | 44.7, 225.0, 2156.0 | <0.001 | ||
| Caucasian | n | 94 | 50 | ||
| Mean (SD) | 74.26 (95.43) | 571.55 (591.09) | |||
| Min, Median, Max | 0.3, 51.0, 776.0 | 36.1, 419.5, 2978.0 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maddala, R.; Ho, L.T.Y.; Karnam, S.; Navarro, I.; Osterwald, A.; Stinnett, S.S.; Ullmer, C.; Vann, R.R.; Challa, P.; Rao, P.V. Elevated Levels of Growth/Differentiation Factor-15 in the Aqueous Humor and Serum of Glaucoma Patients. J. Clin. Med. 2022, 11, 744. https://doi.org/10.3390/jcm11030744
Maddala R, Ho LTY, Karnam S, Navarro I, Osterwald A, Stinnett SS, Ullmer C, Vann RR, Challa P, Rao PV. Elevated Levels of Growth/Differentiation Factor-15 in the Aqueous Humor and Serum of Glaucoma Patients. Journal of Clinical Medicine. 2022; 11(3):744. https://doi.org/10.3390/jcm11030744
Chicago/Turabian StyleMaddala, Rupalatha, Leona T. Y. Ho, Shruthi Karnam, Iris Navarro, Anja Osterwald, Sandra S. Stinnett, Christoph Ullmer, Robin R. Vann, Pratap Challa, and Ponugoti V. Rao. 2022. "Elevated Levels of Growth/Differentiation Factor-15 in the Aqueous Humor and Serum of Glaucoma Patients" Journal of Clinical Medicine 11, no. 3: 744. https://doi.org/10.3390/jcm11030744
APA StyleMaddala, R., Ho, L. T. Y., Karnam, S., Navarro, I., Osterwald, A., Stinnett, S. S., Ullmer, C., Vann, R. R., Challa, P., & Rao, P. V. (2022). Elevated Levels of Growth/Differentiation Factor-15 in the Aqueous Humor and Serum of Glaucoma Patients. Journal of Clinical Medicine, 11(3), 744. https://doi.org/10.3390/jcm11030744






