The Feasibility of Surface Electromyography in Monitoring Orbicularis Oculi Recovery after Anterior Approach Levator Aponeurosis Advancement
Abstract
1. Introduction
The Anatomical Aspects of Cross-Talk Limitation
2. Materials and Methods
2.1. Participants
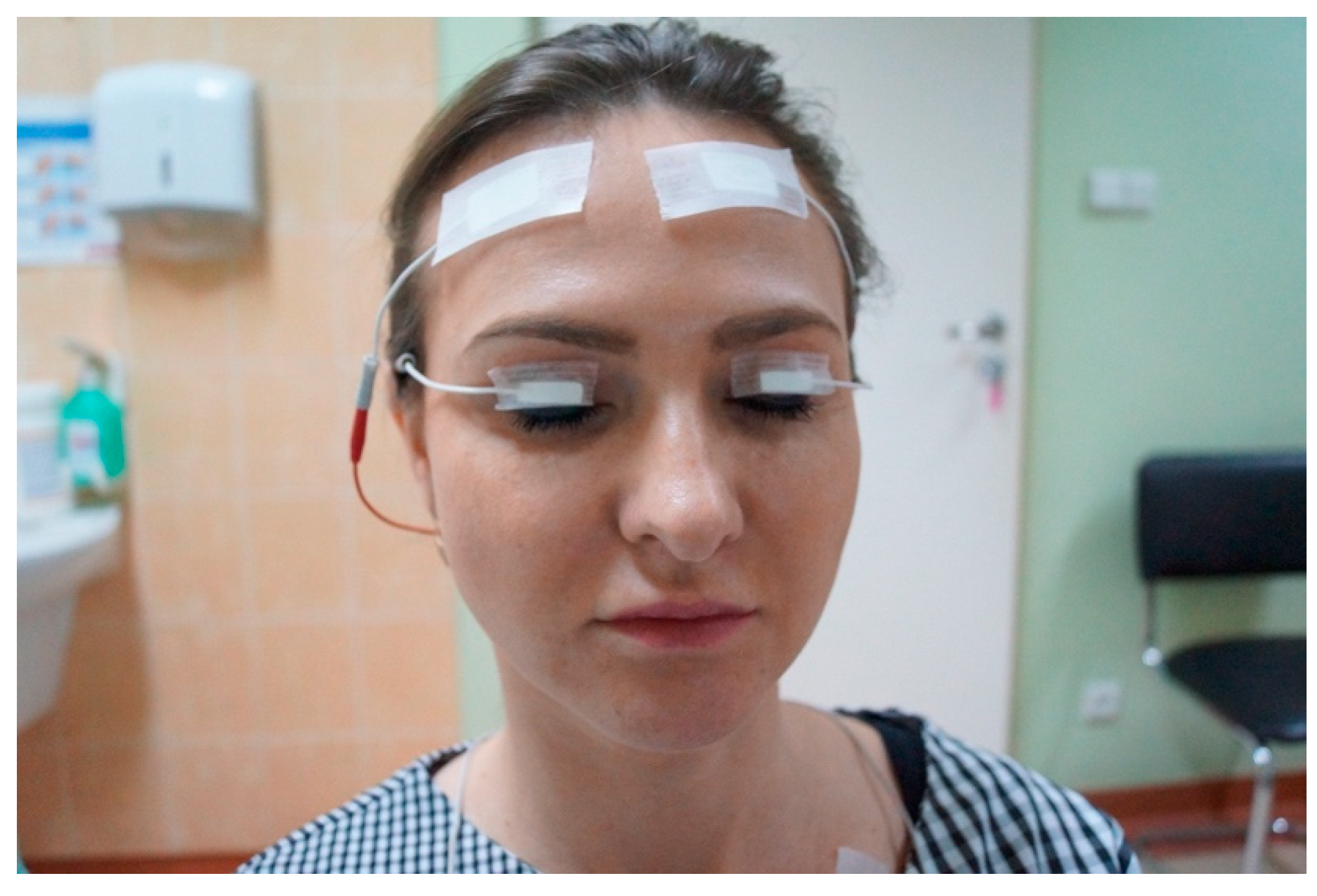
2.2. Study Implementation
2.3. Ptosis Repair
2.4. Statistical Analysis
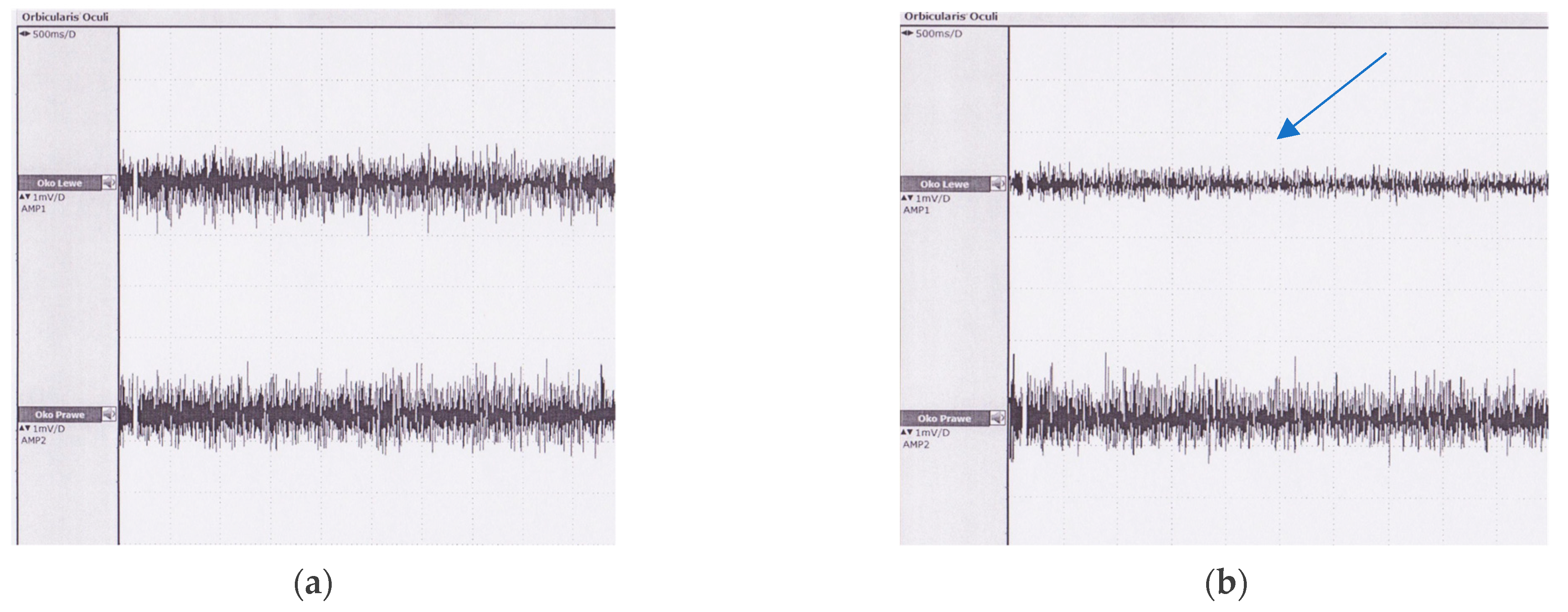
3. Results
4. Discussion
4.1. Study Implementation
4.2. Comparison between Controls and Patients
4.3. Patients before and after Surgery
4.4. Advantages and Limitations
- The electrodes attached to the upper eyelid may disconnect during forced eyelid closure since the electrodes are secured with adhesive tape. This problem could be resolved by introducing new materials used for electrode construction, such as soft carbons [17].
- The electrode can be attached exclusively to the undamaged skin. This limits the utility of the procedure within the first week post-op.
- We encountered some difficulties in correctly placing the electrodes in patients with dermatochalasis. Excessive drooping of the skin may be a contraindication in some cases.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, L.T.; Quickert, M.H.; Wobig, J.L. The Cure of Ptosis by Aponeurotic Repair. Arch. Ophthalmol. 1975, 93, 629–634. [Google Scholar] [CrossRef]
- Fasanella, R.M. Surgery for minimal ptosis: The Fasanella-Servat operation. Trans. Ophthalmol. Soc. UK 1973, 93, 425–438. [Google Scholar] [PubMed]
- Anderson, R.L.; Dixon, R.S. Aponeurotic Ptosis Surgery. Arch. Ophthalmol. 1979, 97, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, A.; Cavallari, P.; Frigeni, M.; Pedrocchi, A.; Sarasola, A.; Ferrante, S. Surface Electromyographic Mapping of the Orbicularis Oculi Muscle for Real-Time Blink Detection. JAMA Facial Plast. Surg. 2014, 16, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, T.; Kawashima, N.; Tsunekuni, T.; Imagawa, K.; Miyasaka, M. A blinking periorbital prosthesis using surface electromyographic signals of the orbicularis oculi muscle. Eur. J. Plast. Surg. 2015, 38, 371–376. [Google Scholar] [CrossRef][Green Version]
- Ryu, H.-M.; Lee, S.-J.; Park, E.-J.; Kim, S.-G.; Kim, K.H.; Choi, Y.M.; Kim, J.U.; Song, B.Y.; Kim, C.H.; Yoon, H.-M.; et al. Study on the Validity of Surface Electromyography as Assessment Tools for Facial Nerve Palsy. J. Pharmacopunct. 2018, 21, 258–267. [Google Scholar] [CrossRef]
- Kim, B.-H.; Kim, K.H.; Kim, L.-H.; Kim, J.-U.; Yook, T.-H. Difference between Right and Left Facial Surface Electromyography in Healthy People. Evid.-Based Complement. Altern. Med. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dutton, J. Atlas of Clinical and Surgical Orbital Anatomy, 2nd ed.; Sauders: Philadelphia, PA, USA, 2011. [Google Scholar]
- Pessa, J.; Rohrich, R.J. Facial Topography: Clinical Anatomy of the Face, 1st ed.; Quality Medical Publishing, Inc.: St. Louis, MO, USA, 2012. [Google Scholar]
- Drost, G.; Stegeman, D.F.; van Engelen, B.G.; Zwarts, M.J. Clinical applications of high-density surface EMG: A systematic review. J. Electromyogr. Kinesiol. 2006, 16, 586–602. [Google Scholar] [CrossRef] [PubMed]
- Merletti, R.; Bottin, A.; Cescon, C.; Farina, D.; Gazzoni, M.; Martina, S.; Mesin, L.; Pozzo, M.; Rainoldi, A.; Enck, P. Multichannel Surface EMG for the Non-Invasive Assessment of the Anal Sphincter Muscle. Digestion 2004, 69, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Golabchi, F.N.; Sapienza, S.; Severini, G.; Reaston, P.; Tomecek, F.; Demarchi, D.; Reaston, M.; Bonato, P. Assessing aberrant muscle activity patterns via the analysis of surface EMG data collected during a functional evaluation. BMC Musculoskelet. Disord. 2019, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, P. Analysis of surface EMG baseline for detection of hidden muscle activity. J. Neural Eng. 2014, 11, 016011. [Google Scholar] [CrossRef] [PubMed]
- Cram Kasman, G.S., Jr. Introduction to Surface Electromyography; Aspen Publishers: Gaithersburg, MD, USA, 1998. [Google Scholar]
- Winter, D.; Fuglevand, A.; Archer, S. Crosstalk in surface electromyography: Theoretical and practical estimates. J. Electromyogr. Kinesiol. 1994, 4, 15–26. [Google Scholar] [CrossRef]
- Hwang, K. Surgical anatomy of the upper eyelid relating to upper blepharoplasty or blepharoptosis surgery. Anat. Cell Biol. 2013, 46, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Bareket, L.; Inzelberg, L.; Rand, D.; David-Pur, M.; Rabinovich, D.; Brandes, B.; Hanein, Y. Temporary-tattoo for long-term high fidelity biopotential recordings. Sci. Rep. 2016, 6, 25727. [Google Scholar] [CrossRef] [PubMed]

| Variables | Controls (n = 39) | Patients (n = 29) | |||
|---|---|---|---|---|---|
| Gender (F/M) | 26/13 | 24/5 | |||
| Mean ± SD | Min–Max | Mean ± SD | Min–Max | p-Value | |
| Age (years) | 67.28 ± 12.8 | 39–87 | 67.16 ± 83 | 45–83 | 0.96 |
| Age F (years) | 66.85 ± 12.54 | 40–87 | 65.81 ± 83 | 45–83 | 0.78 |
| Age M (years) | 68.15 ± 13.79 | 39–86 | 70.4 ± 78 | 64–78 | 0.73 |
| Body height (cm) | 168 ± 33.19 | 154–189 | 166 ± 183 | 156–183 | 0.89 |
| Variables | Controls (n = 39) | Patients (n = 29) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Min–Max | Mean ± SD | Min–Max | p-Value | |||||
| RMS Max | RMS Relax | RMS Max | RMS Relax | RMS Max | RMS Relax | RMS Max | RMS Relax | ||
| RMS | 150.1 ± 84.65 | 10.21 ± 6.82 | 14.7–321.3 | 3.2–35.3 | 160.37 ± 74.52 | 11.84 ± 5.18 | 64.8–239.3 | 64.8–25.7 | 0.85 |
| Right eye | 149.44 ± 85.91 | 10.51 ± 4.5 | 14.7–312 | 3.3–21.7 | 160.38 ± 85.55 | 13.06 ± 5.72 | 45–305.3 | 45–25.7 | 0.73 |
| Left eye | 150.77 ± 83.38 | 9.92 ± 8.5 | 64.6–255.7 | 4.1–35.3 | 148.18 ± 87.98 | 10.62 ± 4.23 | 55.4–243.7 | 55.4–16.7 | 0.94 |
| Male | 163.8 ± 88.15 | 7.45 ± 3.08 | 87.1–249.3 | 17.1–35.3 | 141.08 ± 76.11 | 13,12 ± 3.33 | 93.3–189.3 | 93.3–18.9 | 0.57 |
| Female | 143.25 ± 82.34 | 11.6 ± 7.97 | 14.7–321.3 | 3.2–35.3 | 162.25 ± 88.4 | 11.65 ± 5.37 | 45–239.3 | 45–25.7 | 0.57 |
| Right eye male | 171.67 ± 92.79 | 11.45 ± 3.67 | 96–249.3 | 3.3–17.1 | 145.86 ± 71.62 | 15.23 ± 2.98 | 93.3–189 | 93.3–18.9 | 0.4 |
| Right eye female | 138.33 ± 81.4 | 6.27 ± 4.79 | 14.7–209.7 | 6–21.7 | 164.64 ± 86.92 | 12.74 ± 5.96 | 71.9–239.3 | 71.9–25.7 | 0.69 |
| Left eye male | 155.94 ± 82.94 | 6.27 ± 2.17 | 87.1–249 | 4.1–9.7 | 136.3 ± 75.02 | 11 ± 2.1 | 93.8–189.3 | 93.8–13 | 0.21 |
| Left eye female | 148.18 ± 83.2 | 11.75 ± 10.11 | 64.6–321.3 | 3.2–35.3 | 159.85 ± 89.82 | 10.56 ± 4.46 | 45–305.3 | 45–16.7 | 0.7 |
| Mean ± SD | Min–Max | p-Value | ||||
|---|---|---|---|---|---|---|
| RMS Max | RMS Relax | RMS Max | RMS Relax | RMS Max | RMS Relax | |
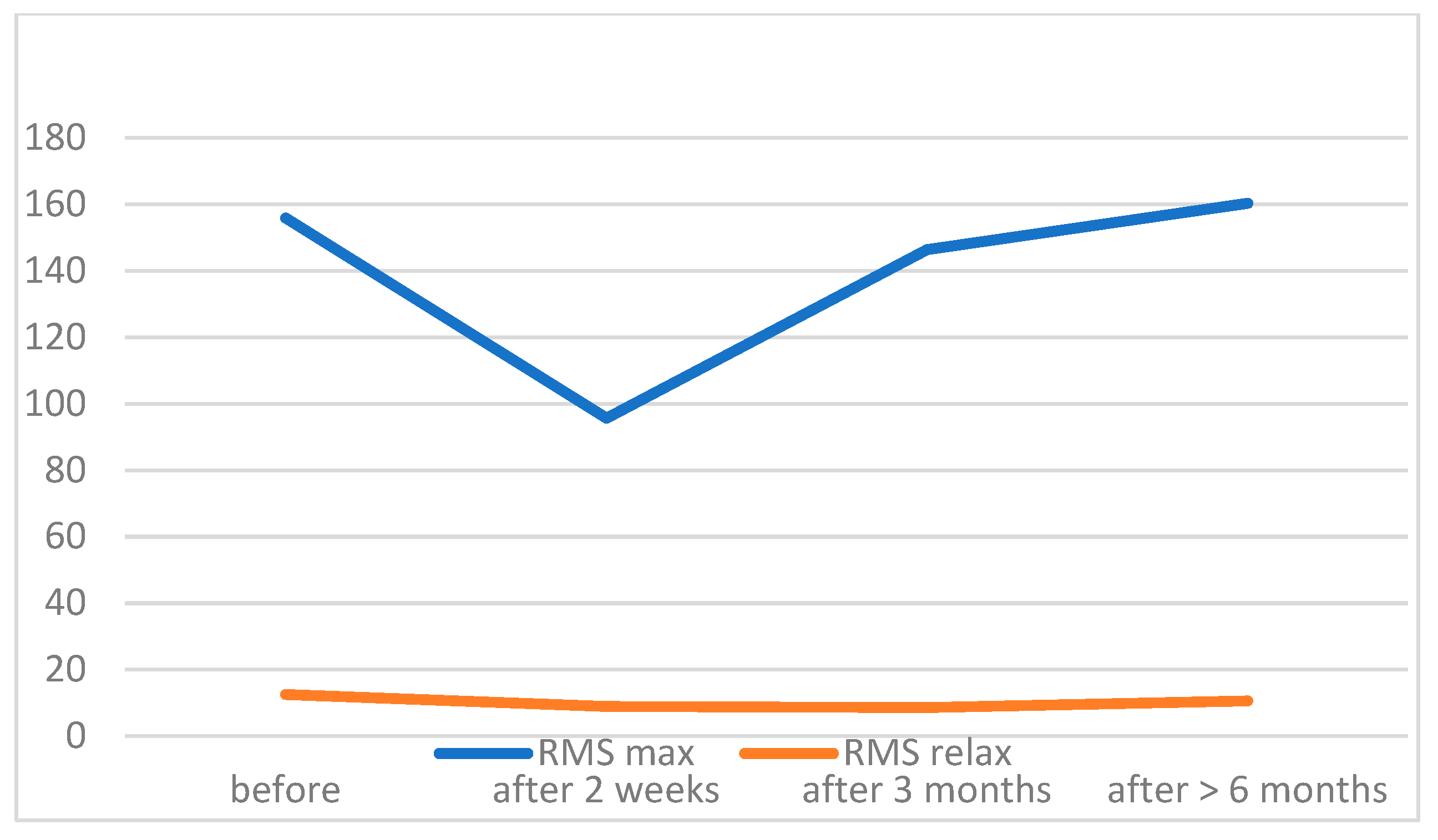
| before | 160.37 ± 74.52 | 11.84 ± 5.18 | 64.8–239.3 | 4.7–25.7 | N.A. * | N.A. * |
| 2 weeks | 125.2 ± 54.04 | 9.8 ± 4.45 | 8–266 | 5–19.9 | <0.00001 | 0.0158 |
| 3 months | 154.68 ± 46.63 | 10.38 ± 4.88 | 55.4–213 | 4.9–22.5 | 0.00362 | 0.01314 |
| 6 months | 161.2 ± 27.99 | 12.19 ± 4.45 | 111–210.3 | 4.2–18.9 | 0.08364 | 0.97606 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krajewska-Węglewicz, L.; Banach, M.; Filipiak, E.; Sempińska-Szewczyk, J.; Skopiński, P.; Dorobek, M. The Feasibility of Surface Electromyography in Monitoring Orbicularis Oculi Recovery after Anterior Approach Levator Aponeurosis Advancement. J. Clin. Med. 2022, 11, 731. https://doi.org/10.3390/jcm11030731
Krajewska-Węglewicz L, Banach M, Filipiak E, Sempińska-Szewczyk J, Skopiński P, Dorobek M. The Feasibility of Surface Electromyography in Monitoring Orbicularis Oculi Recovery after Anterior Approach Levator Aponeurosis Advancement. Journal of Clinical Medicine. 2022; 11(3):731. https://doi.org/10.3390/jcm11030731
Chicago/Turabian StyleKrajewska-Węglewicz, Larysa, Marta Banach, Ewa Filipiak, Joanna Sempińska-Szewczyk, Piotr Skopiński, and Małgorzata Dorobek. 2022. "The Feasibility of Surface Electromyography in Monitoring Orbicularis Oculi Recovery after Anterior Approach Levator Aponeurosis Advancement" Journal of Clinical Medicine 11, no. 3: 731. https://doi.org/10.3390/jcm11030731
APA StyleKrajewska-Węglewicz, L., Banach, M., Filipiak, E., Sempińska-Szewczyk, J., Skopiński, P., & Dorobek, M. (2022). The Feasibility of Surface Electromyography in Monitoring Orbicularis Oculi Recovery after Anterior Approach Levator Aponeurosis Advancement. Journal of Clinical Medicine, 11(3), 731. https://doi.org/10.3390/jcm11030731





