Correlation of Clinical Fibrillar Layer Detection and Corneal Thickness in Advanced Fuchs Endothelial Corneal Dystrophy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Clinical Examination and Imaging
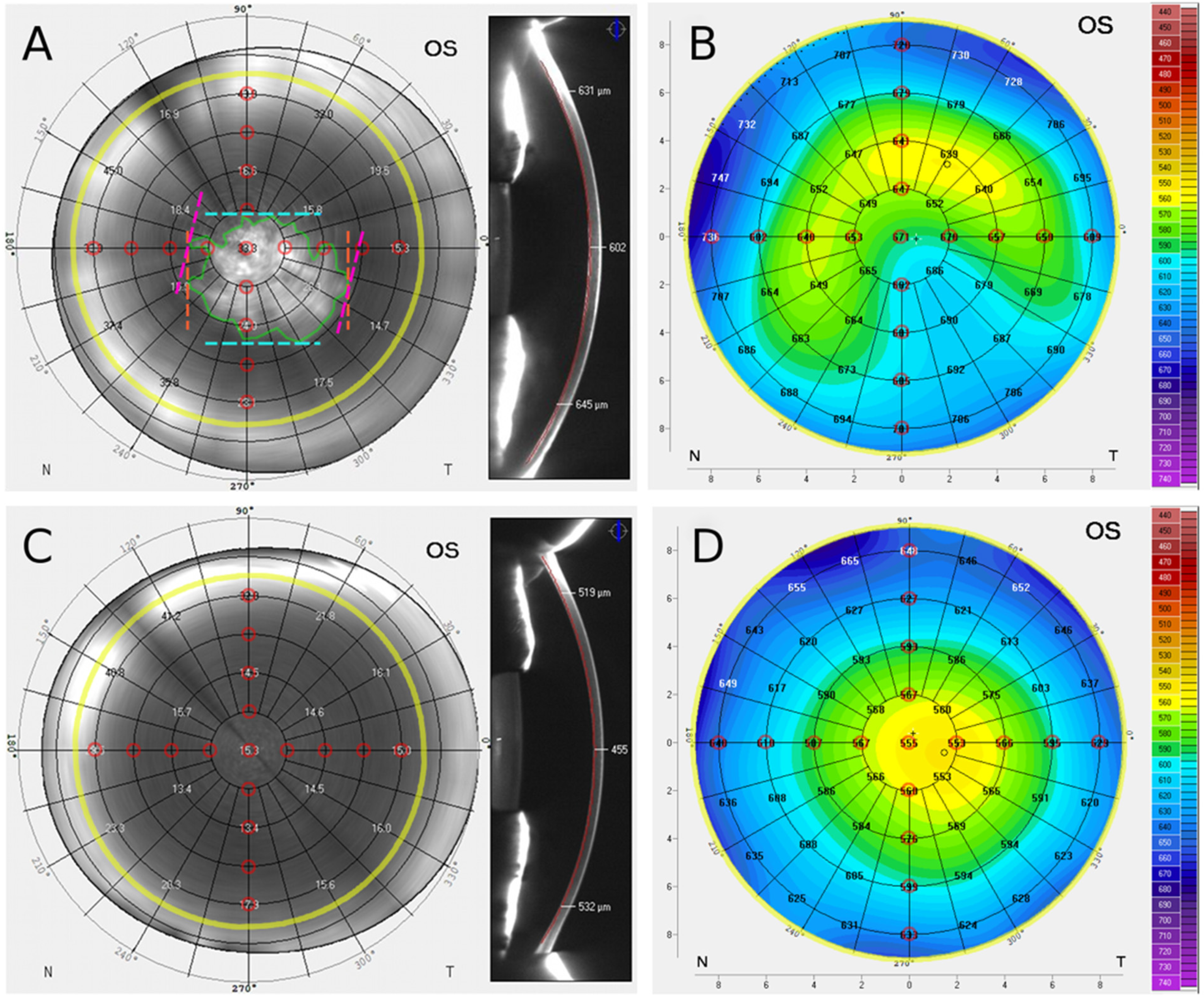
2.2.1. Scheimpflug Imaging
2.2.2. Scheimpflug Pachymetry Data
2.2.3. Comparison of Fibrillar Layer-Positive and -Negative Eyes
2.2.4. Correlation Analysis
2.3. Statistics
3. Results
3.1. En Face Scheimpflug Backscatter Data Analysis
3.2. Comparative Analysis of Focal Backscatter in FL-Positive and FL-Negative Eyes
3.3. Pachymetry Data Analysis
3.4. Comparative Analysis of Pachymetry in FL-Positive vs. FL-Negative Eyes
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthaei, M.; Hribek, A.; Clahsen, T.; Bachmann, B.; Cursiefen, C.; Jun, A.S. Fuchs Endothelial corneal dystrophy: Clinical, genetic, pathophysiologic, and therapeutic aspects. Annu. Rev. Vis. Sci. 2019, 5, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Loreck, N.; Adler, W.; Siebelmann, S.; Rokohl, A.C.; Heindl, L.M.; Cursiefen, C.; Matthaei, M. Morning myopic shift and glare in advanced fuchs endothelial corneal dystrophy. Am. J. Ophthalmol. 2020, 213, 69–75. [Google Scholar] [CrossRef]
- Wacker, K.; Grewing, V.; Fritz, M.; Bohringer, D.; Reinhard, T. Morphological and optical determinants of visual disability in fuchs endothelial corneal dystrophy. Cornea 2020, 39, 726–731. [Google Scholar] [CrossRef]
- Wacker, K.; McLaren, J.W.; Amin, S.R.; Baratz, K.H.; Patel, S.V. Corneal High-order aberrations and backscatter in fuchs’ endothelial corneal dystrophy. Ophthalmology 2015, 122, 1645–1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wacker, K.; McLaren, J.W.; Patel, S.V. Optical and anatomic changes in fuchs endothelial dystrophy corneas. In Current Treatment Options for Fuchs Endothelial Dystrophy; Cursiefen, C., Jun, A.S., Eds.; Springer: Cham, Switzerland, 2017; pp. 51–71. [Google Scholar]
- Fuchs, E. Dystrophia epithelialis corneae. Albr. Græfe. Arch. Ophthalmol. 1910, 76, 478–508. [Google Scholar] [CrossRef] [Green Version]
- Krachmer, J.H.; Purcell, J.J., Jr.; Young, C.W.; Bucher, K.D. Corneal endothelial dystrophy. A study of 64 families. Arch. Ophthalmol. 1978, 96, 2036–2039. [Google Scholar] [CrossRef]
- Hribek, A.; Clahsen, T.; Horstmann, J.; Siebelmann, S.; Loreck, N.; Heindl, L.M.; Matthaei, M. Fibrillar layer as a marker for areas of pronounced corneal endothelial cell loss in advanced fuchs endothelial corneal dystrophy. Am. J. Ophthalmol. 2020, 222, 292–301. [Google Scholar] [CrossRef]
- Hribek, A.; Mestanoglu, M.; Clahsen, T.; Reinking, N.; Frentzen, F.; Howaldt, A.; Matthaei, M. Scheimpflug backscatter imaging of the fibrillar layer in fuchs endothelial corneal dystrophy. Am. J. Ophthalmol. 2021, 235, 63–70. [Google Scholar] [CrossRef]
- Louttit, M.D.; Kopplin, L.J.; Igo, R.P., Jr.; Fondran, J.R.; Tagliaferri, A.; Bardenstein, D.; FECD Genetics Multi-Center Study Group. A multicenter study to map genes for Fuchs endothelial corneal dystrophy: Baseline characteristics and heritability. Cornea 2012, 31, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Fritz, M.; Grewing, V.; Maier, P.; Lapp, T.; Böhringer, D.; Reinhard, T.; Wacker, K. Diurnal variation in corneal edema in fuchs endothelial corneal dystrophy. Am. J. Ophthalmol. 2019, 207, 351–355. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Cardona, A. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Schaub, F.; Enders, P.; Bluhm, C.; Bachmann, B.O.; Cursiefen, C.; Heindl, L.M. Two-year course of corneal densitometry after descemet membrane endothelial keratoplasty. Am. J. Ophthalmol. 2017, 175, 60–67. [Google Scholar] [CrossRef]
- Hos, D.; Matthaei, M.; Bock, F.; Maruyama, K.; Notara, M.; Clahsen, T.; Cursiefen, C. Immune reactions after modern lamellar (DALK, DSAEK, DMEK) versus conventional penetrating corneal transplantation. Prog. Retin. Eye Res. 2019, 73, 100768. [Google Scholar] [CrossRef]
- Kinoshita, S.; Koizumi, N.; Ueno, M.; Okumura, N.; Imai, K.; Tanaka, H.; Hamuro, J. Injection of cultured cells with a ROCK inhibitor for bullous keratopathy. N. Engl. J. Med. 2018, 378, 995–1003. [Google Scholar] [CrossRef]
- Colby, K. Descemet stripping only for fuchs endothelial corneal dystrophy: Will it become the gold standard? Cornea 2021, 41, 269–271. [Google Scholar] [CrossRef]
- Kinoshita, S.; Colby, K.A.; Kruse, F.E. A Close Look at the clinical efficacy of rho-associated protein kinase inhibitor eye drops for fuchs endothelial corneal dystrophy. Cornea 2021, 40, 1225–1228. [Google Scholar] [CrossRef]
- Price, M.O.; Price, F.W., Jr. Randomized, double-masked, pilot study of netarsudil 0.02% ophthalmic solution for treatment of corneal edema in fuchs dystrophy. Am. J. Ophthalmol. 2021, 227, 100–105. [Google Scholar] [CrossRef]
- Lam, F.C.; Baydoun, L.; Dirisamer, M.; Lie, J.; Dapena, I.; Melles, G.R.J. Hemi-Descemet membrane endothelial keratoplasty transplantation: A potential method for increasing the pool of endothelial graft tissue. JAMA Ophthalmol. 2014, 132, 1469–1473. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.V. Imaging fuchs endothelial corneal dystrophy in clinical practice and clinical trials. Cornea 2021, 40, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, H.; Maeda, N.; Soma, T.; Oie, Y.; Koh, S.; Tsujikawa, M.; Nishida, K. Quantitative regional differences in corneal endothelial abnormalities in the central and peripheral zones in Fuchs’ endothelial corneal dystrophy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5090–5098. [Google Scholar] [CrossRef] [PubMed]
- Soh, Y.Q.; Peh, G.S.L.; Naso, S.L.; Kocaba, V.; Mehta, J.S. Automated clinical assessment of corneal guttae in fuchs endothelial corneal dystrophy. Am. J. Ophthalmol. 2021, 221, 260–272. [Google Scholar] [CrossRef]
- Brunette, I.; Sherknies, D.; Terry, M.A.; Chagnon, M.; Bourges, J.L.; Meunier, J. 3-D characterization of the corneal shape in Fuchs dystrophy and pseudophakic keratopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 206–214. [Google Scholar] [CrossRef] [Green Version]
- Bourne, W.M.; Johnson, D.H.; Campbell, R.J. The ultrastructure of Descemet’s membrane. III. Fuchs’ dystrophy. Arch. Ophthalmol. 1982, 100, 1952–1955. [Google Scholar] [CrossRef]
- Eleiwa, T.K.; Cook, J.C.; Elsawy, A.S.; Roongpoovapatr, V.; Volante, V.; Yoo, S.; Abou Shousha, M. Diagnostic performance of three-dimensional endothelium/descemet membrane complex thickness maps in active corneal graft rejection. Am. J. Ophthalmol. 2020, 210, 48–58. [Google Scholar] [CrossRef]
- Shousha, M.A.; Perez, V.L.; Wang, J.; Ide, T.; Jiao, S.; Chen, Q.; Yoo, S.H. Use of ultra-high-resolution optical coherence tomography to detect in vivo characteristics of Descemet’s membrane in Fuchs’ dystrophy. Ophthalmology 2010, 117, 1220–1227. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.V.; Hodge, D.O.; Treichel, E.J.; Spiegel, M.R.; Baratz, K.H. Predicting the Prognosis of Fuchs Endothelial Corneal Dystrophy by Using Scheimpflug Tomography. Ophthalmology 2020, 127, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Repp, D.J.; Hodge, D.O.; Baratz, K.H.; McLaren, J.W.; Patel, S.V. Fuchs’ endothelial corneal dystrophy: Subjective grading versus objective grading based on the central-to-peripheral thickness ratio. Ophthalmology 2013, 120, 687–694. [Google Scholar] [CrossRef] [Green Version]
- Zander, D.; Grewing, V.; Glatz, A.; Lapp, T.; Maier, P.C.; Reinhard, T.; Wacker, K. Predicting edema resolution after descemet membrane endothelial keratoplasty for fuchs dystrophy using scheimpflug tomography. JAMA Ophthalmol. 2021, 139, 423–430. [Google Scholar] [CrossRef]
- Alnawaiseh, M.; Zumhagen, L.; Wirths, G.; Eveslage, M.; Eter, N.; Rosentreter, A. Corneal densitometry, central corneal thickness, and corneal central-to-peripheral thickness ratio in patients with fuchs endothelial dystrophy. Cornea 2016, 35, 358–362. [Google Scholar] [CrossRef]
- Ni Dhubhghaill, S.; Rozema, J.J.; Jongenelen, S.; Ruiz Hidalgo, I.; Zakaria, N.; Tassignon, M.J. Normative values for corneal densitometry analysis by Scheimpflug optical assessment. Investig. Ophthalmol. Vis. Sci. 2014, 55, 162–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| FL Positive | FL Negative | Total | p-Value | |
|---|---|---|---|---|
| Patients, n (%) | 71 (74) | 25 (26) | 96 (100) | - |
| FECD Krachmer Grade 5 | 12 (16.9%) | 13 (52%) | 25 (26%) | <0.01 |
| FECD Krachmer Grade 6 | 59 (83.1%) | 12 (48%) | 71 (74%) | |
| Age (years), mean ± standard deviation | 67.2 ± 9.5 | 69.2 ± 9.2 | 67.7 ± 9.4 | 0.37 |
| Female, n (%) | 39 (54.9%) | 13 (52%) | 52 (54.2%) | 0.80 |
| Pseudophakic, n (%) | 24 (33.8%) | 5 (20.0%) | 29 (30.2%) | 0.20 |
| Time of Scheimpflug imaging: AM, n (%) | 22 (31%) | 8 (32%) | 30 (31.3%) | 0.93 |
| Distance | Position | FL Positive | FL Negative | p-Value |
|---|---|---|---|---|
| (n = 71) | (n = 25) | |||
| ACB (GSU) | mean | 31.7 ± 7.7 | 23.1 ± 4.8 | p < 0.001 |
| PCB1mm (GSU) | mean | 27.9 ± 6.4 | 20.9 ± 3.5 | p < 0.001 |
| superior | 24.4 ± 8.2 | 20.2 ± 4.0 | p = 0.001 | |
| nasal | 25.4 ± 7.6 | 20.6 ± 3.7 | p < 0.001 | |
| inferior | 30.0 ± 7.9 | 20.9 ± 4.4 | p < 0.001 | |
| temporal | 31.4 ± 9.1 | 21.8 ± 3.4 | p < 0.001 | |
| PCB2mm (GSU) | mean | 23.4 ± 5.7 | 19.2 ± 3.7 | p = 0.001 |
| superior | 22.0 ± 5.8 | 20.0 ± 5.1 | p = 0.124 | |
| nasal | 21.7 ± 6.5 | 20.1 ± 5.2 | p = 0.284 | |
| inferior | 23.9 ± 7.5 | 17.8 ± 3.5 | p < 0.001 | |
| temporal | 25.9 ± 8.0 | 19.0 ± 3.3 | p < 0.001 | |
| PCB3mm (GSU) | mean 1 | 22.8 ± 5.5 | 20.8 ± 4.9 | p = 0.127 |
| superior 2 | 28.3 ± 10.7 | 25.4 ± 8.4 | p = 0.235 | |
| nasal | 23.2 ± 6.0 | 23.5 ± 9.5 | p = 0.862 | |
| inferior | 19.4 ± 5.2 | 17.6 ± 2.9 | p = 0.040 | |
| temporal | 20.0 ± 5.2 | 18.0 ± 3.9 | p = 0.048 | |
| PCB4mm (GSU) | mean 3 | 27.4 ± 6.6 | 28.6 ± 10.1 | p = 0.579 |
| superior 4 | 40.4 ± 15.1 | 38.6 ± 23.6 | p = 0.672 | |
| nasal | 34.1 ± 10.4 | 36.4 ± 12.4 | p = 0.374 | |
| inferior | 20.6 ± 5.0 | 21.7 ± 5.4 | p = 0.341 | |
| temporal | 19.9 ± 5.1 | 19.0 ± 4.2 | p = 0.430 |
| Distance | Position | FL Positive | FL Negative | p-Value |
|---|---|---|---|---|
| (n = 71) | (n = 25) | |||
| ACT (µm) | mean | 614.0 ± 51.6 | 575.2 ± 46.2 | p = 0.001 |
| PCT1mm (µm) | mean | 615.7 ± 50.0 | 580.2 ± 44.2 | p = 0.002 |
| superior | 612.9 ± 51,0 | 583.9 ± 39.1 | p = 0.011 | |
| nasal | 610.5 ± 49 | 581.8 ± 42.6 | p = 0.011 | |
| inferior | 619.4 ± 50.6 | 579.0 ± 50.5 | p = 0.001 | |
| temporal | 618.8 ± 54.0 | 576.2 ± 47.8 | p = 0.001 | |
| PCT2mm (µm) | mean | 625.2 ± 47.6 | 599.1 ± 40.9 | p = 0.017 |
| superior | 625.9 ± 48.2 | 609.9 ± 38.1 | p = 0.135 | |
| nasal | 620.9 ± 46.6 | 602.7 ± 39,5 | p = 0.084 | |
| inferior | 627.4 ± 49.0 | 594.5 ± 46.5 | p = 0.004 | |
| temporal | 626.5 ± 54.6 | 589.2 ± 47.7 | p = 0.003 | |
| PCT3mm (µm) | mean | 651.0 ± 46.3 | 635.0 ±39.9 | p = 0.128 |
| superior | 659.8 ± 47.5 | 654.7 ± 42.4 | p = 0.635 | |
| nasal | 654.3 ± 46.6 | 643.2 ± 41.2 | p = 0.294 | |
| inferior | 645.7 ± 49.8 | 624.1 ± 41.8 | p = 0.055 | |
| temporal | 644.4 ± 53.5 | 618.2 ± 44.1 | p = 0.031 | |
| PCT4mm (µm) | mean | 695.1 ± 51.8 | 686.0 ± 43.2 | p = 0.435 |
| superior | 712.9 ± 56.5 | 713.8 ± 53.8 | p = 0.939 | |
| nasal | 704.7 ± 53.7 | 696.6 ± 48.8 | p = 0.507 | |
| inferior | 685.6 ± 61.2 | 672.6 ± 45.7 | p = 0.333 | |
| temporal | 677.1 ± 56.1 | 661.0 ± 39.2 | p = 0.189 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özer, O.; Mestanoglu, M.; Howaldt, A.; Clahsen, T.; Schiller, P.; Siebelmann, S.; Reinking, N.; Cursiefen, C.; Bachmann, B.; Matthaei, M. Correlation of Clinical Fibrillar Layer Detection and Corneal Thickness in Advanced Fuchs Endothelial Corneal Dystrophy. J. Clin. Med. 2022, 11, 2815. https://doi.org/10.3390/jcm11102815
Özer O, Mestanoglu M, Howaldt A, Clahsen T, Schiller P, Siebelmann S, Reinking N, Cursiefen C, Bachmann B, Matthaei M. Correlation of Clinical Fibrillar Layer Detection and Corneal Thickness in Advanced Fuchs Endothelial Corneal Dystrophy. Journal of Clinical Medicine. 2022; 11(10):2815. https://doi.org/10.3390/jcm11102815
Chicago/Turabian StyleÖzer, Orlando, Mert Mestanoglu, Antonia Howaldt, Thomas Clahsen, Petra Schiller, Sebastian Siebelmann, Niklas Reinking, Claus Cursiefen, Björn Bachmann, and Mario Matthaei. 2022. "Correlation of Clinical Fibrillar Layer Detection and Corneal Thickness in Advanced Fuchs Endothelial Corneal Dystrophy" Journal of Clinical Medicine 11, no. 10: 2815. https://doi.org/10.3390/jcm11102815
APA StyleÖzer, O., Mestanoglu, M., Howaldt, A., Clahsen, T., Schiller, P., Siebelmann, S., Reinking, N., Cursiefen, C., Bachmann, B., & Matthaei, M. (2022). Correlation of Clinical Fibrillar Layer Detection and Corneal Thickness in Advanced Fuchs Endothelial Corneal Dystrophy. Journal of Clinical Medicine, 11(10), 2815. https://doi.org/10.3390/jcm11102815






