Safety and Long-Term Immunogenicity of BNT162b2 Vaccine in Individuals with Down Syndrome
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Local and Systemic Adverse Events after First and Second BNT162b2 Dose
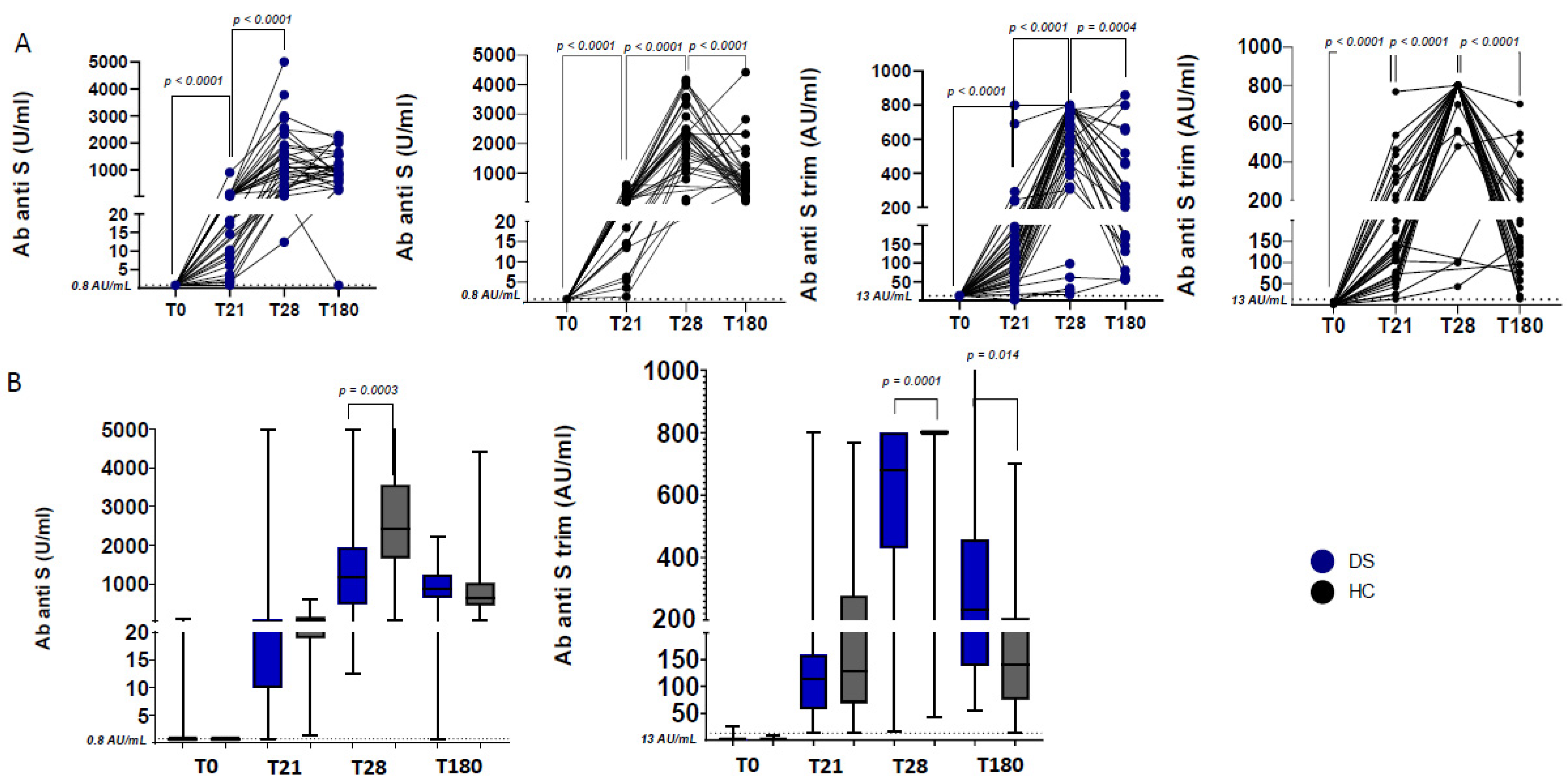
3.2. Humoral Responses after First and Second BNT162b2 Dose
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antonarakis, S.E.; Skotko, B.G.; Rafii, M.S.; Strydom, A.; Pape, S.E.; Bianchi, D.W.; Sherman, S.L.; Reeves, R.H. Down syndrome. Nat. Rev. Dis. Prim. 2020, 6, 9. [Google Scholar] [CrossRef]
- Hüls, A.; Costa, A.C.S.; Dierssen, M.; Bakshfg, R.A.; Bargagnah, S.; Baumer, N.T.; Brandão, A.C.; Carfi, A.; Carmona-Iragui, M.; Chicoinen, B.A.; et al. Medical vulnerability of individuals with Down syndrome to severe COVID-19—Data from the Trisomy 21 Research Society and the UK ISARIC4C survey. EClinicalMedicine 2021, 33, 100769. [Google Scholar] [CrossRef] [PubMed]
- Emes, D.; Hüls, A.; Baumer, N.; Dierssen, M.; Puri, S.; Russell, L.; Sherman, S.L.; Strydom, A.; Bargagna, S.; Brandão, A.C.; et al. COVID-19 in Children with Down Syndrome: Data from the Trisomy 21 Research Society Survey. J. Clin. Med. 2021, 10, 5125. [Google Scholar] [CrossRef] [PubMed]
- Llouz, T.; Biragyn, A.; Iulita, M.F.; Flores-Aguilar, L.; Dierssen, M.; Toma, I.D.; Antonarakis, S.E.; Yu, E.; Herault, Y.; Potier, M.; et al. Immune Dysregulation and the Increased Risk of Complications and Mortality Following Respiratory Tract Infections in Adults With Down Syndrome. Front. Immunol. 2021, 12, 621440. [Google Scholar] [CrossRef]
- Carsetti, R.; Valentini, D.; Marcellini, V.; Scarsella, M.; Marasco, E.; Giustini, F.; Bartuli, A.; Villani, A.; Ugazio, A.G. Reduced numbers of switched memory B cells with high terminal differentiation potential in Down syndrome. Eur. J. Immunol. 2015, 45, 903–914. [Google Scholar] [CrossRef] [Green Version]
- Kusters, M.A.; Manders, N.C.; Jong, B.A.D.; Hout, R.W.V.; Rijkers, G.T.; Vries, E.D. Functionality of the pneumococcal antibody response in Down syndrome subjects. Vaccine 2013, 31, 6261–6265. [Google Scholar] [CrossRef]
- Kusters, M.A.; Bok, V.L.; Bolz, W.E.; Huijskens, E.G.; Peeters, M.F.; Vries, E.D. Influenza A/H1N1 vaccination response is inadequate in down syndrome children when the latest cut-off values are used. Pediatr. Infect. Dis. J. 2012, 31, 1284–1285. [Google Scholar] [CrossRef]
- Valentini, D.; Marcellini, V.; Bianchi, S.; Villani, A.; Facchini, M.; Donatelli, I.; Castrucci, M.R.; Marasco, E.; Farroni, C.; Carsetti, R. Generation of switched memory B cells in response to vaccination in Down syndrome children and their siblings. Vaccine 2015, 33, 6689–6696. [Google Scholar] [CrossRef] [Green Version]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Cotugno, N.; Pighi, C.; Morrocchi, E.; Ruggiero, A.; Amodio, D.; Medri, C.; Colagrossi, L.; Russo, C.; Di Cesare, S.; Santilli, V.; et al. BNT162B2 mRNA COVID-19 Vaccine in Heart and Lung Transplanted Young Adults: Is an Alternative SARS-CoV-2 Immune Response Surveillance Needed? Transplantation 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 2021, 325, 2204–2206. [Google Scholar] [CrossRef] [PubMed]
- Amodio, D.; Ruggiero, A.; Sgrulletti, M.; Pighi, C.; Cotugno, N.; Medri, C.; Morrocchi, E.; Colagrossi, L.; Russo, C.; Zaffina, S.; et al. Humoral and Cellular Response Following Vaccination with the BNT162b2 mRNA COVID-19 Vaccine in Patients Affected by Primary Immunodeficiencies. Front. Immunol. 2021, 12, 727850. [Google Scholar] [CrossRef] [PubMed]
- Hagin, D.; Freund, T.; Navon, M.; Halperin, T.; Adir, D.; Marom, R.; Levi, I.; Benor, S.; Alcalay, Y.; Freund, N.T. Immunogenicity of Pfizer-BioNTech COVID-19 Vaccine in Patients With Inborn Errors of Immunity. J. Allergy Clin. Immunol. 2021, 148, 739–749. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Zaffina, S.; Alteri, C.; Ruggiero, A.; Cotugno, N.; Vinci, M.R.; Camisa, V.; Santoro, A.P.; Brugaletta, R.; Deriu, G.; Mortari, E.P.; et al. Induction of Immune Response after SARS-CoV-2 mRNA BNT162b2 Vaccination in Healthcare Workers. J. Virus Erad. 2021, 7, 100046. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Henry, B.M.; Pighi, L.; De Nitto, S.; Gianfilippi, G.; Lippi, G. The pronounced decline of anti-SARS-CoV-2 spike trimeric IgG and RBD IgG in baseline seronegative individuals six months after BNT162b2 vaccination is consistent with the need for vaccine boosters. Clin. Chem. Lab. Med. 2021, 60, e29–e31. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning immune humoral response to BNT162b2 COVID-19 vaccine over 6 months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef]
- Kusters, M.A.; Zijde, J.-V.D.; Tol, M.J.V.; Bolz, W.E.; Bok, L.A.; Visser, M.; Vries, E.D. Impaired avidity maturation after tetanus toxoid booster in children with Down syndrome. Pediatr. Infect. Dis. J. 2011, 30, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Verstegen, R.H.; Driessen, G.J.; Bartol, S.J.; van Noesel, C.J.; Boon, L.; Burg, M.V.D.; Dongen, J.J.M.V.; Vries, E.; Zelm, M.C.V. Defective B-cell memory in patients with Down syndrome. J. Allergy Clin. Immunol. 2014, 134, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Illouz, T.; Biragyn, A.; Frenkel-Morgenstern, M.; Weissberg, O.; Gorohovski, A.; Merzon, E.; Green, I.; Iulita, F.; Flores-Aguilar, L.; Dierssen, M.; et al. Specific Susceptibility to COVID-19 in Adults with Down Syndrome. Neuromol. Med. 2021, 23, 561–571. [Google Scholar] [CrossRef] [PubMed]

| Demographic and Clinical Characteristics | DS n = 40 | HC n = 36 | p Values |
|---|---|---|---|
| Females, n (%) | 17 (42.5%) | 23 (63.9%) | n.s. |
| Age, mean (±SD) | 17.90 (±4.59) | 47.4 (±12.2) | <0.0001 |
| Ethnicity, n (%) | Black 1 (2.5%) East Asian 2 (5%) Latin American 4 (10%) White 33 (82.5%) | n.a. | n.a. |
| Type of trisomy, n (%) | Full/standard 37 (92.5%) Mosaic 0 (0%) Translocation 0 (0%) Don’t known 3 (7.5%) | n.a. | n.a. |
| Comorbidities, n (%) | Congenital heart disease 24 (60%) Recurrent respiratory infections 5 (12.5%) Thyroid disease 21 (52.5%) Celiac disease 3 (7.5%) Obstructive sleep apnea 15 (37.5%) Obesity 9 (2.5%) Hypertension 1 (2.5%) Chronic liver disease 3 (7.5%) Chronic lung disease 2 (5%) GERD 6 (15%) Allergies 2 (5%) Psychiatric disease 5 (12.5%) | n.a. | n.a. |
| Level of intellectual disability, n (%) | Mild 12 (30%) Moderate 15 (37.5%) Severe/profound 5 (12.5%) Don’t known 8 (20%) | n.a. | n.a. |
| n = 40 1st Dose | n = 40 2nd Dose | |
|---|---|---|
| Systemic adverse events | 5 (12.5%) | 6 (15%) |
| Fever | 2/5 | 4/6 |
| Wheezing | 1/5 | 0 |
| Muscle pain | 1/5 | 1/6 |
| Fatigue | 1/5 | 2/6 |
| Headache | 1/5 | 1/6 |
| Chills | 1/5 | 0 |
| Dizziness | 1/5 | 0 |
| Cough | 1/5 | 1/6 |
| Local adverse events | 10 (25%) | 10 (25%) |
| Pain at the site of injection | 10/10 | 10/10 |
| Redness at the site of injection | 1/10 | 2/10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentini, D.; Cotugno, N.; Scoppola, V.; Di Camillo, C.; Colagrossi, L.; Manno, E.C.; Perno, C.F.; Russo, C.; Palma, P.; Rossi, P.; et al. Safety and Long-Term Immunogenicity of BNT162b2 Vaccine in Individuals with Down Syndrome. J. Clin. Med. 2022, 11, 694. https://doi.org/10.3390/jcm11030694
Valentini D, Cotugno N, Scoppola V, Di Camillo C, Colagrossi L, Manno EC, Perno CF, Russo C, Palma P, Rossi P, et al. Safety and Long-Term Immunogenicity of BNT162b2 Vaccine in Individuals with Down Syndrome. Journal of Clinical Medicine. 2022; 11(3):694. https://doi.org/10.3390/jcm11030694
Chicago/Turabian StyleValentini, Diletta, Nicola Cotugno, Vittorio Scoppola, Chiara Di Camillo, Luna Colagrossi, Emma Concetta Manno, Carlo Federico Perno, Cristina Russo, Paolo Palma, Paolo Rossi, and et al. 2022. "Safety and Long-Term Immunogenicity of BNT162b2 Vaccine in Individuals with Down Syndrome" Journal of Clinical Medicine 11, no. 3: 694. https://doi.org/10.3390/jcm11030694
APA StyleValentini, D., Cotugno, N., Scoppola, V., Di Camillo, C., Colagrossi, L., Manno, E. C., Perno, C. F., Russo, C., Palma, P., Rossi, P., & Villani, A. (2022). Safety and Long-Term Immunogenicity of BNT162b2 Vaccine in Individuals with Down Syndrome. Journal of Clinical Medicine, 11(3), 694. https://doi.org/10.3390/jcm11030694






