The TSH/Thyroid Hormones Axis and Breast Cancer
Abstract
1. Introduction
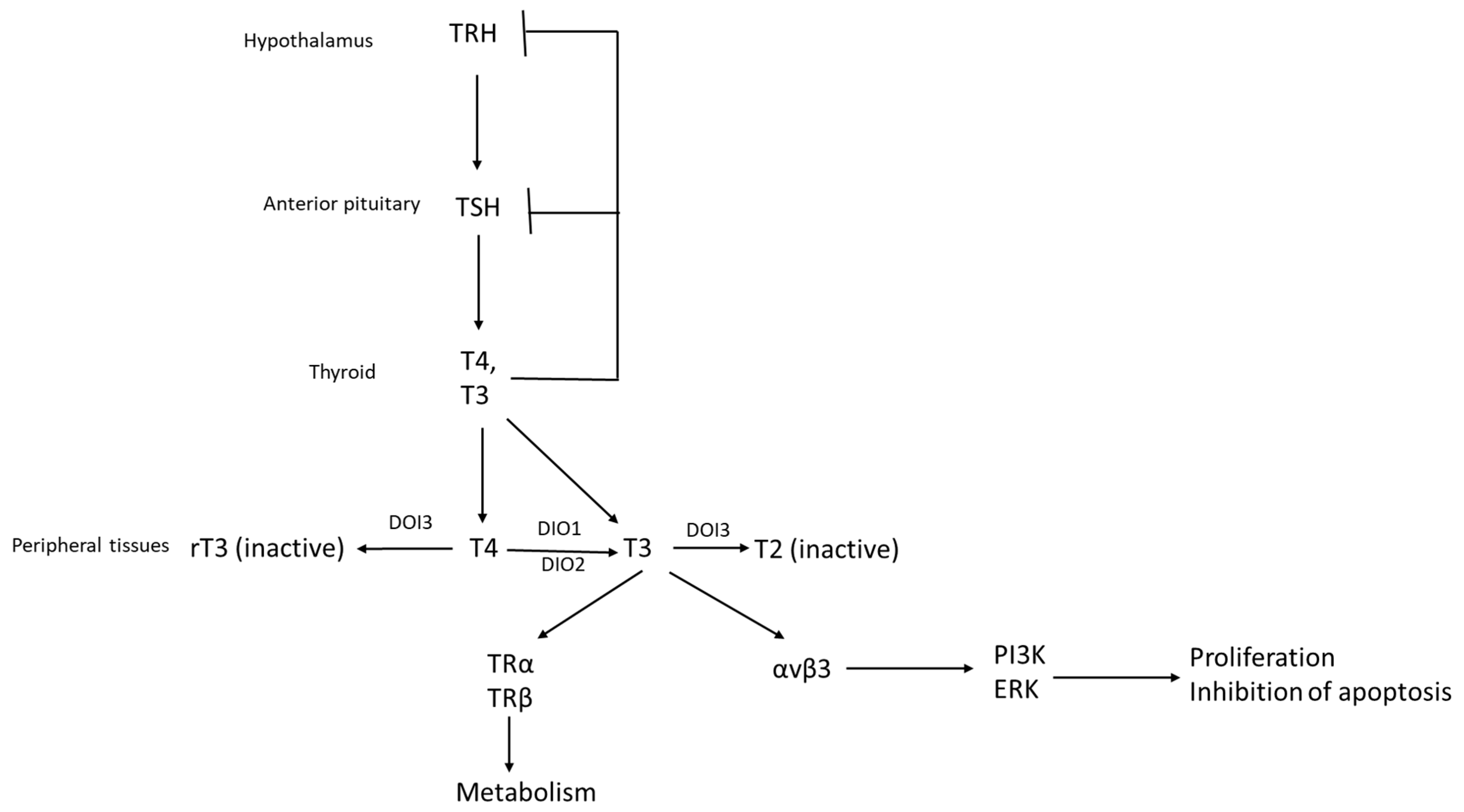
2. The Hypothalamic–Pituitary–Thyroid Axis and Systemic Effects Related to Cancer Pathogenesis
3. Expression and Actions of Thyroid Hormone Receptors and Other Proteins of the Axis on Breast Cancer Cells In Vitro and In Vivo
4. Expression of TRs, TSHR and Related Proteins in Human Breast Cancers and Prognosis According to Subtype
| Study | Number of Patients | Type of Cancer | Receptor Sub-Type | Findings |
|---|---|---|---|---|
| Alyusuf et al. [54] | 46 patients with cancer and 100 controls with benign lesions and normal tissues | All | TRα1 | Decreased expression in cancer compared to normal mammary tissue and benign lesions. |
| Charalampoudis et al. [55] | 41 patients (tumors and normal tissues) | All (23 ER-positive, 11 HER2-positive) | TRα | Decreased expression in cancer compared to normal mammary tissue. Partial loss in larger and high-grade tumors. |
| Conde et al. [56] | 52 patients with invasive cancers, 20 patients with in situ cancers and 12 controls with benign lesions | All | TRα, TRβ | TRα located in nuclei of normal cells and the cytoplasm of pathologic lesions. The reverse is true for TRβ except for invasive cancers, which showed a similar cytoplasmic localization to TRα. |
| Heublein et al. [57] | 86 sporadic and 38 BRCA1-associated breast cancer patients | All | TRα, TRβ | TRβ is a good prognostic factor in BRCA1-associated cancers and TRα is an adverse prognostic factor. |
| Ditsch et al. [58] | 82 patients | All | TRα1, TRα2, TRβ1, TRβ2 | Both receptor isotypes are expressed in subsets of breast cancers. |
| Jerzak et al. [59] | 130 patient | All (95 ER-positive, 17 HER2 positive) | TRα1, TRα2 | TRα1 and TRα2 positivity (Allred score above 6) was observed in 74% and 40% of cases. |
| Jerzak et al. [62] | 796 patients | All (616 ER-positive, 219 HER2-positive) | TRβ1 | High expression of TRβ1 is associated with better cancer-specific survival. |
| Gu et al. [63] | 227 patients | Triple-negative breast cancers | TRβ | High expression of TRβ1 is associated with better disease-free survival |
5. Circulating Thyroid Hormones and Breast Cancer Prognosis
6. Therapeutic Perspective
7. Conclusions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.M.; Smith, K.L.; Stearns, V. Management of hormone receptor-positive, HER2-negative early breast cancer. Semin. Oncol. 2020, 47, 187–200. [Google Scholar] [CrossRef]
- Bronte, G.; Rocca, A.; Ravaioli, S.; Puccetti, M.; Tumedei, M.M.; Scarpi, E.; Andreis, D.; Maltoni, R.; Sarti, S.; Cecconetto, L.; et al. Androgen receptor in advanced breast cancer: Is it useful to predict the efficacy of anti-estrogen therapy? BMC Cancer 2018, 18, 348. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J. Function of the vitamin D endocrine system in mammary gland and breast cancer. Mol. Cell Endocrinol. 2017, 453, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Voutsadakis, I.A. Vitamin D receptor (VDR) and metabolizing enzymes CYP27B1 and CYP24A1 in breast cancer. Mol. Biol. Rep. 2020, 47, 9821–9830. [Google Scholar] [CrossRef]
- Voutsadakis, I.A. Vitamin D baseline levels at diagnosis of breast cancer: A systematic review and meta-analysis. Hematol. Oncol. Stem Cell Ther. 2021, 14, 16–26. [Google Scholar] [CrossRef]
- Shahid, M.A.; Ashraf, M.A.; Sharma, S. Physiology, thyroid hormone. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Gauthier, B.R.; Sola-García, A.; Cáliz-Molina, M.Á.; Lorenzo, P.I.; Cobo-Vuilleumier, N.; Capilla-González, V.; Martin-Montalvo, A. Thyroid hormones in diabetes, cancer, and aging. Aging Cell 2020, 19, e13260. [Google Scholar] [CrossRef]
- Beck-Peccoz, P.; Rodari, G.; Giavoli, C.; Lania, A. Central hypothyroidism—A neglected thyroid disorder. Nat. Rev. Endocrinol. 2017, 13, 588–598. [Google Scholar] [CrossRef]
- Brent, G.A. Mechanisms of thyroid hormone action. J. Clin. Investig. 2012, 122, 3035–3043. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Yeh, C.T.; Lin, K.H. Molecular functions of thyroid hormone signaling in regulation of cancer progression and anti-apoptosis. Int. J. Mol. Sci. 2019, 20, 4986. [Google Scholar] [CrossRef] [PubMed]
- Ayers, S.; Switnicki, M.P.; Angajala, A.; Lammel, J.; Arumanayagam, A.S.; Webb, P. Genome-wide binding patterns of thyroid hormone receptor beta. PLoS ONE 2014, 9, e81186. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Y.; Leonard, J.L.; Davis, P.J. Molecular aspects of thyroid hormone actions. Endocr. Rev. 2010, 31, 139–170. [Google Scholar] [CrossRef] [PubMed]
- Nappi, A.; De Stefano, M.A.; Dentice, M.; Salvatore, D. Deiodinases and cancer. Endocrinology 2021, 162, bqab016. [Google Scholar] [CrossRef]
- Mariotti, S.; Beck-Peccoz, P. Physiology of the hypothalamic-pituitary-thyroid axis. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Grossman, A., Hershman, J.M., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Kim, M.I. Hypothyroidism in older adults. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Plateroti, M.; Kress, E.; Mori, J.I.; Samarut, J. Thyroid hormone receptor alpha1 directly controls transcription of the beta-catenin gene in intestinal epithelial cells. Mol. Cell Biol. 2006, 26, 3204–3214. [Google Scholar] [CrossRef]
- Kress, E.; Skah, S.; Sirakov, M.; Nadjar, J.; Gadot, N.; Scoazec, J.Y.; Samarut, J.; Plateroti, M. Cooperation between the thyroid hormone receptor TRalpha1 and the WNT pathway in the induction of intestinal tumorigenesis. Gastroenterology 2010, 138, 1863–1874. [Google Scholar] [CrossRef]
- Krashin, E.; Piekiełko-Witkowska, A.; Ellis, M.; Ashur-Fabian, O. Thyroid hormones and cancer: A comprehensive review of preclinical and clinical studies. Front. Endocrinol. 2019, 10, 59. [Google Scholar] [CrossRef]
- Khan, S.R.; Chaker, L.; Ruiter, R.; Aerts, J.G.; Hofman, A.; Dehghan, A.; Franco, O.H.; Stricker, B.H.; Peeters, R.P. Thyroid function and cancer risk: The Rotterdam study. J. Clin. Endocrinol. Metab. 2016, 101, 5030–5036. [Google Scholar] [CrossRef]
- Saraiva, P.P.; Figueiredo, N.B.; Padovani, C.R.; Brentani, M.M.; Nogueira, C.R. Profile of thyroid hormones in breast cancer patients. Braz. J. Med. Biol. Res. 2005, 38, 761–765. [Google Scholar] [CrossRef]
- Bach, L.; Kostev, K.; Schiffmann, L.; Kalder, M. Association between thyroid gland diseases and breast cancer: A case-control study. Breast Cancer Res. Treat. 2020, 182, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Ditsch, N.; Liebhardt, S.; Von Koch, F.; Lenhard, M.; Vogeser, M.; Spitzweg, C.; Gallwas, J.; Toth, B. Thyroid function in breast cancer patients. Anticancer Res. 2010, 30, 1713–1717. [Google Scholar] [PubMed]
- Tosovic, A.; Bondeson, A.G.; Bondeson, L.; Ericsson, U.B.; Malm, J.; Manjer, J. Prospectively measured triiodothyronine levels are positively associated with breast cancer risk in postmenopausal women. Breast Cancer Res. 2010, 12, R33. [Google Scholar] [CrossRef] [PubMed]
- Tosovic, A.; Bondeson, A.G.; Bondeson, L.; Ericsson, U.B.; Manjer, J. T3 levels in relation to prognostic factors in breast cancer: A population-based prospective cohort study. BMC Cancer 2014, 14, 536. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Q.; Band, S.; Kesavadas, M.; Madak Erdogan, Z. Obesity and postmenopausal hormone receptor-positive breast cancer: Epidemiology and mechanisms. Endocrinology 2021, 162, bqab195. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Yamamura, Y.; Kau, S.W.; Bevers, T.; Strom, S.; Patangan, M.; Hsu, L.; Krishnamurthy, S.; Theriault, R.L.; Hortobagyi, G.N. Thyroid hormone and breast carcinoma. Primary hypothyroidism is associated with a reduced incidence of primary breast carcinoma. Cancer 2005, 103, 1122–1128. [Google Scholar] [CrossRef]
- Dinda, S.; Sanchez, A.; Moudgil, V. Estrogen-like effects of thyroid hormone on the regulation of tumor suppressor proteins, p53 and retinoblastoma, in breast cancer cells. Oncogene 2002, 21, 761–768. [Google Scholar] [CrossRef]
- Hall, L.C.; Salazar, E.P.; Kane, S.R.; Liu, N. Effects of thyroid hormones on human breast cancer cell proliferation. J. Steroid Biochem. Mol. Biol. 2008, 109, 57–66. [Google Scholar] [CrossRef]
- Shao, Z.M.; Sheikh, M.S.; Rishi, A.K.; Dawson, M.I.; Li, X.S.; Wilber, J.F.; Feng, P.; Fontana, J.A. Thyroid hormone enhancement of estradiol stimulation of breast carcinoma proliferation. Exp. Cell Res. 1995, 218, 1–8. [Google Scholar] [CrossRef]
- Zyla, L.E.; Cano, R.; Gómez, S.; Escudero, A.; Rey, L.; Santiano, F.E.; Bruna, F.A.; Creydt, V.P.; Carón, R.W.; Fontana, C.L. Effects of thyroxine on apoptosis and proliferation of mammary tumors. Mol. Cell Endocrinol. 2021, 538, 111454. [Google Scholar] [CrossRef]
- Nogueira, C.R.; Brentani, M.M. Triiodothyronine mimics the effects of estrogen in breast cancer cell lines. J. Steroid Biochem. Mol. Biol. 1996, 59, 271–279. [Google Scholar] [CrossRef]
- Sar, P.; Peter, R.; Rath, B.; Das Mohapatra, A.; Mishra, S.K. 3, 3′5 Triiodo L thyronine induces apoptosis in human breast cancer MCF-7 cells, repressing SMP30 expression through negative thyroid response elements. PLoS ONE 2011, 6, e20861. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Iglesias, O.; Garcia-Silva, S.; Tenbaum, S.P.; Regadera, J.; Larcher, F.; Paramio, J.M.; Vennström, B.; Aranda, A. Thyroid hormone receptor beta1 acts as a potent suppressor of tumor invasiveness and metastasis. Cancer Res. 2009, 69, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Bolf, E.L.; Gillis, N.E.; Davidson, C.D.; Cozzens, L.M.; Kogut, S.; Tomczak, J.A.; Frietze, S.; Carr, F.E. Common tumor-suppressive signaling of thyroid hormone receptor beta in breast and thyroid cancer cells. Mol. Carcinog. 2021, 60, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Iglesias, O.; Olmeda, D.; Alonso-Merino, E.; Gómez-Rey, S.; González-López, A.M.; Luengo, E.; Soengas, M.S.; Palacios, J.; Regadera, J.; Aranda, A. The nuclear corepressor 1 and the thyroid hormone receptor β suppress breast tumor lymphangiogenesis. Oncotarget 2016, 7, 78971–78984. [Google Scholar] [CrossRef]
- Guigon, C.J.; Kim, D.W.; Willingham, M.C.; Cheng, S.Y. Mutation of thyroid hormone receptor-β in mice predisposes to the development of mammary tumors. Oncogene 2011, 30, 3381–3390. [Google Scholar] [CrossRef]
- Guigon, C.J.; Kim, D.W.; Zhu, X.; Zhao, L.; Cheng, S.Y. Tumor suppressor action of liganded thyroid hormone receptor beta by direct repression of beta-catenin gene expression. Endocrinology 2010, 151, 5528–5536. [Google Scholar] [CrossRef]
- Park, J.W.; Zhao, L.; Willingham, M.C.; Cheng, S.Y. Loss of tyrosine phosphorylation at Y406 abrogates the tumor suppressor functions of the thyroid hormone receptor β. Mol. Carcinog. 2017, 56, 489–498. [Google Scholar] [CrossRef]
- Gasparini, G.; Brooks, P.C.; Biganzoli, E.; Vermeulen, P.B.; Bonoldi, E.; Dirix, L.Y.; Ranieri, G.; Miceli, R.; Cheresh, D.A. Vascular integrin alpha(v)beta3: A new prognostic indicator in breast cancer. Clin. Cancer Res. 1998, 4, 2625–2634. [Google Scholar]
- Beer, A.J.; Niemeyer, M.; Carlsen, J.; Sarbia, M.; Nährig, J.; Watzlowik, P.; Wester, H.J.; Harbeck, N.; Schwaiger, M. Patterns of alphavbeta3 expression in primary and metastatic human breast cancer as shown by 18F-Galacto-RGD PET. J. Nucl. Med. 2008, 49, 255–259. [Google Scholar] [CrossRef]
- Naber, H.P.; Wiercinska, E.; Pardali, E.; van Laar, T.; Nirmala, E.; Sundqvist, A.; van Dam, H.; van der Horst, G.; van der Pluijm, G.; Heckmann, B.; et al. BMP-7 inhibits TGF-β-induced invasion of breast cancer cells through inhibition of integrin β(3) expression. Cell Oncol. 2012, 35, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Takayama, S.; Ishii, S.; Ikeda, T.; Masamura, S.; Doi, M.; Kitajima, M. The relationship between bone metastasis from human breast cancer and integrin alpha(v)beta3 expression. Anticancer Res. 2005, 25, 79–83. [Google Scholar] [PubMed]
- Pécheur, I.; Peyruchaud, O.; Serre, C.M.; Guglielmi, J.; Voland, C.; Bourre, F.; Margue, C.; Cohen-Solal, M.; Buffet, A.; Kieffer, N.; et al. Integrin alpha(v)beta3 expression confers on tumor cells a greater propensity to metastasize to bone. FASEB J. 2002, 16, 1266–1268. [Google Scholar] [CrossRef] [PubMed]
- Flamini, M.I.; Uzair, I.D.; Pennacchio, G.E.; Neira, F.J.; Mondaca, J.M.; Cuello-Carrión, F.D.; Jahn, G.A.; Simoncini, T.; Sanchez, A.M. Thyroid hormone controls breast cancer cell movement via integrin αV/β3/SRC/FAK/PI3-kinases. Horm. Cancer 2017, 8, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yao, J.F.; Deng, X.F.; Zheng, X.D.; Jia, M.; Wang, Y.Q.; Huang, Y.; Zhu, J.H. 14, 15-EET induces breast cancer cell EMT and cisplatin resistance by up-regulating integrin αvβ3 and activating FAK/PI3K/AKT signaling. J. Exp. Clin. Cancer Res. 2018, 37, 23. [Google Scholar] [CrossRef]
- Kim, W.G.; Cheng, S.Y. Thyroid hormone receptors and cancer. Biochim. Biophys. Acta 2013, 1830, 3928–3936. [Google Scholar] [CrossRef]
- Hill, B.S.; Sarnella, A.; Capasso, D.; Comegna, D.; Del Gatto, A.; Gramanzini, M.; Albanese, S.; Saviano, M.; Zaccaro, L.; Zannetti, A. Therapeutic potential of a novel αvβ₃ antagonist to hamper the aggressiveness of mesenchymal triple negative breast cancer sub-type. Cancers 2019, 11, 139. [Google Scholar] [CrossRef]
- Glinskii, A.B.; Glinsky, G.V.; Lin, H.Y.; Tang, H.Y.; Sun, M.; Davis, F.B.; Luidens, M.K.; Mousa, S.A.; Hercbergs, A.H.; Davis, P.J. Modification of survival pathway gene expression in human breast cancer cells by tetraiodothyroacetic acid (tetrac). Cell Cycle 2009, 8, 3562–3570. [Google Scholar] [CrossRef]
- Sirakov, M.; Skah, S.; Nadjar, J.; Plateroti, M. Thyroid hormone’s action on progenitor/stem cell biology: New challenge for a classic hormone? Biochim. Biophys. Acta 2013, 1830, 3917–3927. [Google Scholar] [CrossRef]
- Kress, E.; Rezza, A.; Nadjar, J.; Samarut, J.; Plateroti, M. The frizzled-related sFRP2 gene is a target of thyroid hormone receptor alpha1 and activates beta-catenin signaling in mouse intestine. J. Biol. Chem 2009, 284, 1234–1241. [Google Scholar] [CrossRef]
- López-Fontana, C.M.; Sasso, C.V.; Maselli, M.E.; Santiano, F.E.; Semino, S.N.; Cuello Carrión, F.D.; Jahn, G.A.; Carón, R.W. Experimental hypothyroidism increases apoptosis in dimethylbenzanthracene-induced mammary tumors. Oncol. Rep. 2013, 30, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Sterle, H.A.; Hildebrandt, X.; Álvarez, M.V.; Paulazo, M.A.; Gutierrez, L.M.; Klecha, A.J.; Cayrol, F.; Díaz Flaqué, M.C.; Rosemblit, C.; Barreiro Arcos, M.L.; et al. Thyroid status regulates the tumor microenvironment delineating breast cancer fate. Endocr. Relat. Cancer 2021, 28, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Alyusuf, R.H.; Matouq, J.A.; Taha, S.; Wazir, J.F. The pattern of expression and role of triiodothyronine (T3) receptors and type I 5'-deiodinase in breast carcinomas, benign breast diseases, lactational change, and normal breast epithelium. Appl. Immunohistochem. Mol. Morphol. 2014, 22, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Charalampoudis, P.; Agrogiannis, G.; Kontzoglou, K.; Kouraklis, G.; Sotiropoulos, G.C. Thyroid hormone receptor alpha (TRa) tissue expression in ductal invasive breast cancer: A study combining quantitative immunohistochemistry with digital slide image analysis. Eur. J. Surg. Oncol. 2017, 43, 1428–1432. [Google Scholar] [CrossRef]
- Conde, I.; Paniagua, R.; Zamora, J.; Blánquez, M.J.; Fraile, B.; Ruiz, A.; Arenas, M.I. Influence of thyroid hormone receptors on breast cancer cell proliferation. Ann. Oncol. 2006, 17, 60–64. [Google Scholar] [CrossRef]
- Heublein, S.; Mayr, D.; Meindl, A.; Angele, M.; Gallwas, J.; Jeschke, U.; Ditsch, N. Thyroid Hormone Receptors Predict Prognosis in BRCA1 Associated Breast Cancer in Opposing Ways. PLoS ONE 2015, 10, e0127072. [Google Scholar] [CrossRef]
- Ditsch, N.; Toth, B.; Himsl, I.; Lenhard, M.; Ochsenkühn, R.; Friese, K.; Mayr, D.; Jeschke, U. Thyroid hormone receptor (TR)alpha and TRbeta expression in breast cancer. Histol. Histopathol. 2013, 28, 227–237. [Google Scholar] [CrossRef]
- Jerzak, K.J.; Cockburn, J.; Pond, G.R.; Pritchard, K.I.; Narod, S.A.; Dhesy-Thind, S.K.; Bane, A. Thyroid hormone receptor α in breast cancer: Prognostic and therapeutic implications. Breast Cancer Res. Treat. 2015, 149, 293–301. [Google Scholar] [CrossRef]
- Conde, S.J.; Luvizotto, R.A.; Síbio, M.T.; Katayama, M.L.; Brentani, M.M.; Nogueira, C.R. Tamoxifen inhibits transforming growth factor-alpha gene expression in human breast carcinoma samples treated with triiodothyronine. J. Endocrinol. Investig. 2008, 31, 1047–1051. [Google Scholar] [CrossRef]
- Conde, S.J.; Luvizotto Rde, A.; de Síbio, M.T.; Nogueira, C.R. Thyroid hormone status interferes with estrogen target gene expression in breast cancer samples in menopausal women. Int. Sch. Res. Not. 2014, 2014, 317398. [Google Scholar] [CrossRef]
- Jerzak, K.J.; Cockburn, J.G.; Dhesy-Thind, S.K.; Pond, G.R.; Pritchard, K.I.; Nofech-Mozes, S.; Sun, P.; Narod, S.A.; Bane, A. Thyroid hormone receptor beta-1 expression in early breast cancer: A validation study. Breast Cancer Res. Treat. 2018, 171, 709–717. [Google Scholar] [CrossRef]
- Gu, G.; Gelsomino, L.; Covington, K.R.; Beyer, A.R.; Wang, J.; Rechoum, Y.; Huffman, K.; Carstens, R.; Andò, S.; Fuqua, S.A. Targeting thyroid hormone receptor beta in triple-negative breast cancer. Breast Cancer Res. Treat. 2015, 150, 535–545. [Google Scholar] [CrossRef]
- Sloan, E.K.; Pouliot, N.; Stanley, K.L.; Chia, J.; Moseley, J.M.; Hards, D.K.; Anderson, R.L. Tumor-specific expression of alphavbeta3 integrin promotes spontaneous metastasis of breast cancer to bone. Breast Cancer Res. 2006, 8, R20. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.Z.; Micocci, K.C.; Natoli, A.; Redvers, R.P.; Paquet-Fifield, S.; Martin, A.C.; Denoyer, D.; Ling, X.; Kim, S.H.; Tomasin, R.; et al. Tumour but not stromal expression of β3 integrin is essential, and is required early, for spontaneous dissemination of bone-metastatic breast cancer. J. Pathol. 2015, 235, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.D.; Yeh, C.T. The molecular function and clinical role of thyroid stimulating hormone receptor in cancer cells. Cells 2020, 9, 1730. [Google Scholar] [CrossRef] [PubMed]
- Biondi, B.; Cooper, D.S. Thyroid hormone suppression therapy. Endocrinol. Metab. Clin. 2019, 48, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Ellerhorst, J.A.; Sendi-Naderi, A.; Johnson, M.K.; Cooke, C.P.; Dang, S.M.; Diwan, A.H. Human melanoma cells express functional receptors for thyroid-stimulating hormone. Endocr. Relat. Cancer 2006, 13, 1269–1277. [Google Scholar] [CrossRef]
- Gyftaki, R.; Liacos, C.; Politi, E.; Liontos, M.; Saltiki, K.; Papageorgiou, T.; Thomakos, N.; Haidopoulos, D.; Rodolakis, A.; Alevizaki, M.; et al. Differential transcriptional and protein expression of thyroid-stimulating hormone receptor in ovarian carcinomas. Int. J. Gynecol. Cancer 2014, 24, 851–856. [Google Scholar] [CrossRef]
- Shih, Y.L.; Huang, Y.H.; Lin, K.H.; Chu, Y.D.; Yeh, C.T. Identification of functional thyroid stimulating hormone receptor and TSHR gene mutations in hepatocellular carcinoma. Anticancer Res. 2018, 38, 2793–2802. [Google Scholar] [CrossRef]
- Govindaraj, V.; Yaduvanshi, N.S.; Krishnamachar, H.; Rao, A.J. Expression of thyroid-stimulating hormone receptor, octamer-binding transcription factor 4, and intracisternal A particle-promoted polypeptide in human breast cancer tissues. Horm. Mol. Biol. Clin. Investig. 2012, 9, 173–178. [Google Scholar] [CrossRef]
- Goemann, I.M.; Marczyk, V.R.; Recamonde-Mendoza, M.; Wajner, S.M.; Graudenz, M.S.; Maia, A.L. Decreased expression of the thyroid hormone-inactivating enzyme type 3 deiodinase is associated with lower survival rates in breast cancer. Sci. Rep. 2020, 10, 13914. [Google Scholar] [CrossRef] [PubMed]
- Debski, M.G.; Pachucki, J.; Ambroziak, M.; Olszewski, W.; Bar-Andziak, E. Human breast cancer tissue expresses high level of type 1 5′-deiodinase. Thyroid 2007, 17, 3–10. [Google Scholar] [CrossRef] [PubMed]
- García-Solís, P.; Aceves, C. 5’Deiodinase in two breast cancer cell lines: Effect of triiodothyronine, isoproterenol and retinoids. Mol. Cell Endocrinol. 2003, 201, 25–31. [Google Scholar] [CrossRef]
- Zhu, X.; Pan, D.; Wang, N.; Wang, S.; Sun, G. Relationship between selenium in human tissues and breast cancer: A meta-analysis based on case-control studies. Biol. Trace Elem. Res. 2021, 199, 4439–4446. [Google Scholar] [CrossRef] [PubMed]
- Nisman, B.; Allweis, T.M.; Carmon, E.; Kadouri, L.; Maly, B.; Maimon, O.; Meierovich, A.; Peretz, T. Thyroid hormones, silencing mediator for retinoid and thyroid receptors and prognosis in primary breast cancer. Anticancer Res. 2020, 40, 6417–6428. [Google Scholar] [CrossRef]
- Brandt, J.; Borgquist, S.; Almquist, M.; Manjer, J. Thyroid function and survival following breast cancer. Br. J. Surg. 2016, 103, 1649–1657. [Google Scholar] [CrossRef]
- Elgebaly, M.M.; Abdel-Hamed, A.R.; Mesbah, N.M.; Abo-Elmatty, D.M.; Abouzid, A.; Abdelrazek, M.A. Hypothyroidism affect progression and worse outcomes of breast cancer but not ovarian cancer. J. Immunoass. Immunochem. 2021, 1–11. [Google Scholar] [CrossRef]
- Brandt, J.; Borgquist, S.; Manjer, J. Prospectively measured thyroid hormones and thyroid peroxidase antibodies in relation to risk of different breast cancer subgroups: A Malmö diet and cancer study. Cancer Causes Control. 2015, 26, 1093–1104. [Google Scholar] [CrossRef]
- Angelousi, A.; Diamanti-Kandarakis, E.; Zapanti, E.; Nonni, A.; Ktenas, E.; Mantzou, A.; Kontzoglou, K.; Kouraklis, G. Is there an association between thyroid function abnormalities and breast cancer? Arch. Endocrinol. Metab. 2017, 61, 54–61. [Google Scholar] [CrossRef]
- Villa, N.M.; Li, N.; Yeh, M.W.; Hurvitz, S.A.; Dawson, N.A.; Leung, A.M. Serum thyrotropin concentrations are not predictive of aggressive breast cancer biology in euthyroid individuals. Endocr. Pract 2015, 21, 1040–1045. [Google Scholar] [CrossRef]
- Bera, A.; Subramanian, M.; Karaian, J.; Eklund, M.; Radhakrishnan, S.; Gana, N.; Rothwell, S.; Pollard, H.; Hu, H.; Shriver, C.D.; et al. Functional role of vitronectin in breast cancer. PLoS ONE 2020, 15, e0242141. [Google Scholar] [CrossRef] [PubMed]
- Egorova, A.; Selutin, A.; Maretina, M.; Selkov, S.; Baranov, V.; Kiselev, A. Characterization of iRGD-ligand modified arginine-histidine-rich peptides for nucleic acid therapeutics delivery to αvβ3 integrin-expressing cancer cells. Pharmaceuticals 2020, 13, 300. [Google Scholar] [CrossRef] [PubMed]
- Morabia, A.; Szklo, M.; Stewart, W.; Schuman, L.; Thomas, D.B.; Zacur, H.A. Thyroid hormones and duration of ovulatory activity in the etiology of breast cancer. Cancer Epidemiol. Prev. Biomark. 1992, 1, 389–393. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voutsadakis, I.A. The TSH/Thyroid Hormones Axis and Breast Cancer. J. Clin. Med. 2022, 11, 687. https://doi.org/10.3390/jcm11030687
Voutsadakis IA. The TSH/Thyroid Hormones Axis and Breast Cancer. Journal of Clinical Medicine. 2022; 11(3):687. https://doi.org/10.3390/jcm11030687
Chicago/Turabian StyleVoutsadakis, Ioannis A. 2022. "The TSH/Thyroid Hormones Axis and Breast Cancer" Journal of Clinical Medicine 11, no. 3: 687. https://doi.org/10.3390/jcm11030687
APA StyleVoutsadakis, I. A. (2022). The TSH/Thyroid Hormones Axis and Breast Cancer. Journal of Clinical Medicine, 11(3), 687. https://doi.org/10.3390/jcm11030687






