Taurine and Its Derivatives: Analysis of the Inhibitory Effect on Platelet Function and Their Antithrombotic Potential
Abstract
1. Introduction
2. Overview of the Antithrombotic Potential of Taurine
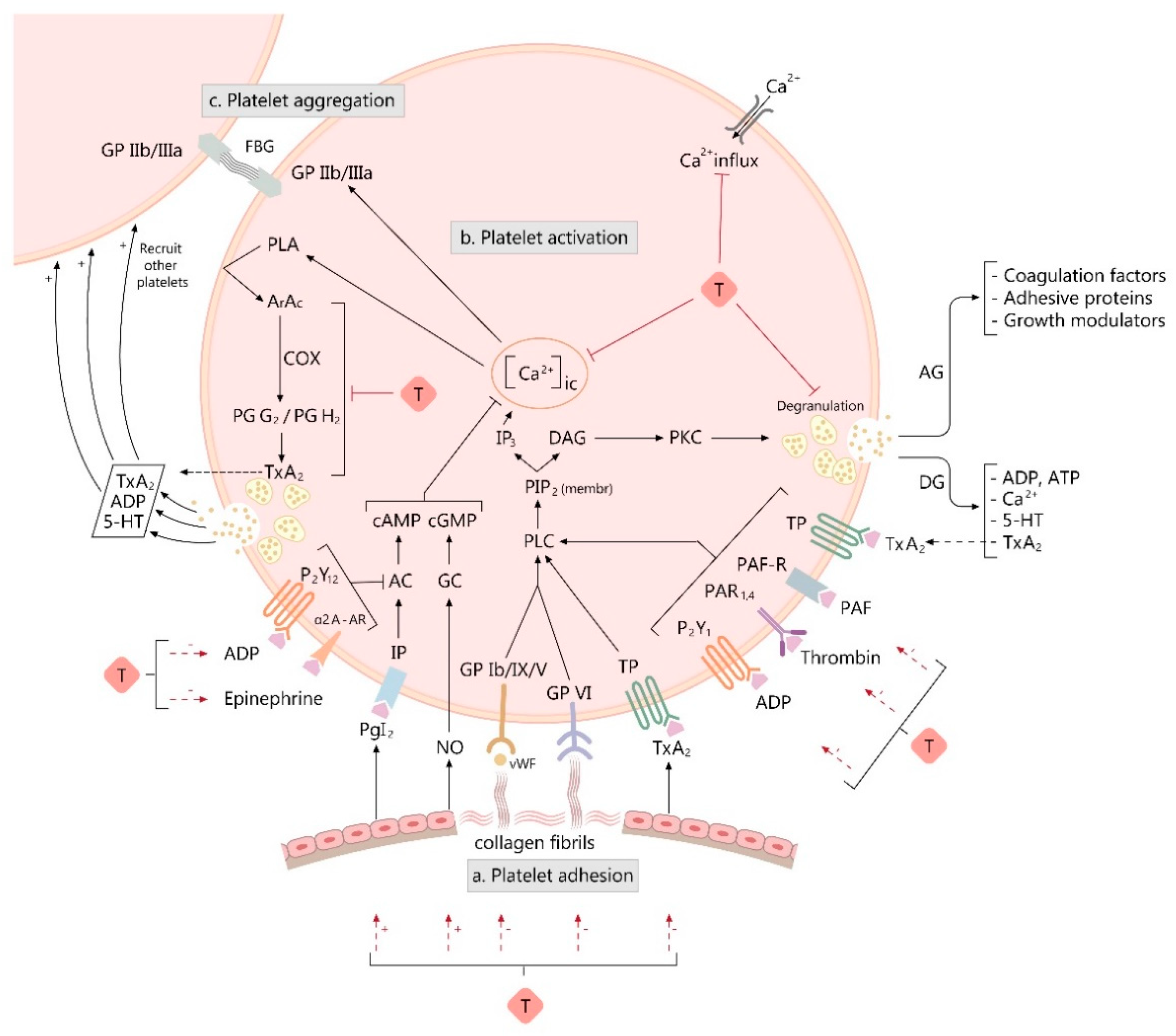
3. Overview of Platelet Function
4. Taurine and Platelet Function
4.1. Taurine Content of the Platelets
4.2. Taurine Influence on Platelet Hemostatic Activity
4.2.1. Evidence from Animal Studies
4.2.2. Evidence from Human Studies
| Reference | Animal Subjects, Sex, Number of Animals per Group, Type of Experiment | Taurine or Related Compounds | Design of the Study (Taurine Dose, Time of Administration) | Platelet Aggregation Variation (Agonist) | Outcome from Other Assays of Platelet Function (Agonist), or from Animal Models of Thrombosis |
|---|---|---|---|---|---|
| Kurachi, M. et al., 1987 [126] | Guinea pigs, in vitro | Taurine | 40 nM, 2 min before adding the agonist | ↓ (PAF) | |
| Hayes, KC. et al., 1989 [114] | Cats, males and females with equal distribution, n = 6, ex vivo | Taurine | 0.5 g T/kg diet (from the time of weaning to the age of 10–24 months) | ↓ (↑PAt by 140% in T-supplemented vs. T-deficient cats, when triggered with collagen) | ↑ of platelet GSH concentration by 53% in T-supplemented vs. T-deficient cats |
| Ji, Y. et al., 1995 [131] | Rats (2k1c), n = 6, ex vivo | Taurine | 30 mg/kg/day, for 9 weeks |
| |
| Huang, HL. et al., 1995 [41] | Rats, n = 6, in vivo | Taurine | 100 mg/kg | ↓ of thrombosis wet weight by a rate of 47.82%, vs. controls | |
| Rats, n = 6, ex vivo | Taurine | 100 mg/kg |
| ↓ platelet TxA2 release (ADP) | |
| Park, IS. et al., 2007 [132] | Rats, n = 10, ex vivo | Taurine | 5% in diet, for 4 weeks |
| |
| Roşca, A. et al., 2013 [133] | Rats, males, n = 10, ex vivo | Taurine | 2% in drinking water, for 3 months | ↓ (ADP, 2.5 µM) | |
| Roşca, A. et al., 2013 [134] | Rats, males, n = 10, ex vivo | Taurine | 2% in drinking water, for 3 months | N outcome for MA measured by TEG | |
| Murina, M.A. et al., 2002 [135] | Mice, in vivo | DT |
|
| |
| Mice, male, ex vivo | DT | 6.8 mg/kg, i.v | ↓ by a rate of 50% (DT vs. C group) (ADP, 10 µM) | ||
| Murina, M.A. et al., 2007 [136] | Rabbits, in vitro | DT | 10 µM | ↓ ISALS by half (ADP, 0.2 µM) | |
| Rabbits, in vitro | DT | 10 millimoles/L | ↓ markedly the impedance measured by whole blood aggregometry (ADP, 10 µM) | ||
| Kaptanoglu, L. et al., 2008 [44] | Rat, n = 10, in vivo | TL | 10 mg, or 20 mg, i.v; Heparin (100 antiXa ICU/2 mL/kg, nadroparin calcium) | ↓ of thrombus weight by a rate of 42 % vs. C (but significantly higher than that in heparin treated group). | |
| Murina, M.A. et al., 2009 [137] | Rabbits, in vitro |
|
|
| |
| Murina, M.A. et al., 2014 [139] | Rabbits, in vitro |
|
|
|
| Reference | Human Subjects, Sex, Number of Individuals per Group, Type of Experiment | Taurine or Related Compounds | Design of the Study (Taurine Dose, Time of Administration) | Platelet Aggregation Variation (Agonist) | Outcome from Other Assays of Platelet Function (Agonist) |
|---|---|---|---|---|---|
| Almazov, V.A. et al., 1985 [140] | Human platelets, n = 10, in vitro | Taurine | 25 nM | ↓ by half (ADP—3.5 µM) | |
| Human platelets, n = 5, in vitro | Taurine | 25 nM | ↑ of platelet Ca, Mg-ATPase activity by 45% | ||
| Hayes, K.C. et al., 1989 [113] | Healthy volunteers, male, n = 5, ex vivo | Taurine | 400 mg/day, for 8 days | ↓ (↑PAt by 25% in T group vs. controls, when triggered with collagen) | ↑ of platelet GSH concentration by 34% |
| Healthy volunteers, male, n = 5, ex vivo | Taurine | 1600 mg/day, for 8 days | ↓ (↑PAt by 72% in T group vs. controls, when triggered with collagen) | ↓ platelet TxB2 release (collagen, 0.93 µg) | |
| Franconi, F. et al., 1994 [145] and Franconi F. et al., 1995 [146] |
| Taurine |
|
| |
| Taurine | 1.5 g/day, for 3 months |
| ||
| Spohr, C. et al., 2005 [118] | Men with predisposition to type II diabetes mellitus, n = 9, ex vivo | Taurine | 1.5 g/day, for two 8-week periods (separated by 2 weeks of washout) | N outcome (TC: 3.86 ± 3.25 µmol/l for T group; 3.86 ± 2.21 µmol/l for placebo group) | |
| Namba, K. et al., 1992 [154] | Human platelets from non-pregnant women, n = 5, 10 experiments, in vitro | Taurine | increasing dose (6.25, 25, or 50 mM) | ↓ with 25.6% to 42.4% (ADP, 0.5–1.5 µM), and with 29.5% to 36.7% (collagen, 0.5–1.25 µg/mL) |
|
| Human platelets from non-pregnant women, n = 5, 5 experiments, in vitro | Taurine | increasing dose (6.25, 25, or 50 mM) |
| ||
| Miglis, M. et al., 2002 [119] | Human platelets, 5 different donors, in vitro | Taurine | increasing dose (5 to 25 mM) | ↓ by 10%, for each T dose (thrombin, 1.0 U/mL) | |
| Human platelets, 5 different donors, in vitro | Taurine | 5 or 25 mM | N outcome for ESC (0.02 mM ADP) | ||
| Human platelets, 5 different donors, in vitro | Taurine | 25 mM | N outcome for MA measured by TEG | ||
| Murina, M.A. et al., 2007 [136] | Platelets from healthy donors, in vitro | Taurine | 10 mM | N outcome (ADP, 10 µM) | |
| Platelets from healthy donors, in vitro | Taurine and NaOCl | 10 mM and 1 mM, respectively | ↓ (↑MI by 1.7 times in the mixed treated vs. NaOCl alone group) (ADP, 10 µM) | ||
| Platelets from healthy donors, in vitro | DT | 0.25 mM | ↓ (↑MI to 40 ± 7) (ADP, 10 µM) |
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Jacobsen, J.G.; Smith, L.H. Biochemistry and physiology of taurine and taurine derivatives. Physiol. Rev. 1968, 48, 424–511. [Google Scholar] [CrossRef] [PubMed]
- Huxtable, R.J. Physiological actions of taurine. Physiol. Rev. 1992, 72, 101–163. [Google Scholar] [CrossRef] [PubMed]
- Lambert, I.H.; Kristensen, D.M.; Holm, J.B.; Mortensen, O.H. Physiological role of taurine—From organism to organelle. Acta Physiol. 2015, 213, 191–212. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.W.; Jong, C.J.; Ramila, K.C.; Azuma, J. Physiological roles of taurine in heart and muscle. J. Biomed. Sci. 2010, 17 (Suppl. 1), S2. [Google Scholar] [CrossRef]
- Xu, Y.J.; Arneja, A.S.; Tappia, P.S.; Dhalla, N.S. The potential health benefits of taurine in cardiovascular disease. Exp. Clin. Cardiol. 2008, 13, 57–65. [Google Scholar]
- Ripps, H.; Shen, W. Review: Taurine: A ”very essential” amino acid. Mol. Vis. 2012, 18, 2673–2686. [Google Scholar]
- Clauson, K.A.; Shields, K.M.; McQueen, C.E.; Persad, N. Safety issues associated with commercially available energy drinks. J. Am. Pharm. Assoc. 2008, 48, e55–e63, quiz e64-7. [Google Scholar] [CrossRef]
- Schaffer, S.W.; Shimada, K.; Jong, C.J.; Ito, T.; Azuma, J.; Takahashi, K. Effect of taurine and potential interactions with caffeine on cardiovascular function. Amino Acids 2014, 46, 1147–1157. [Google Scholar] [CrossRef]
- Collard, J.M.; Sansonetti, P.; Papon, N. Taurine Makes Our Microbiota Stronger. Trends Endocrinol. Metab. 2021, 32, 259–261. [Google Scholar] [CrossRef]
- Rosca, A.E.; Iesanu, M.I.; Zahiu, C.D.M.; Voiculescu, S.E.; Paslaru, A.C.; Zagrean, A.M. Capsaicin and Gut Microbiota in Health and Disease. Molecules 2020, 25, 5681. [Google Scholar] [CrossRef]
- Schaffer, S.; Kim, H.W. Effects and Mechanisms of Taurine as a Therapeutic Agent. Biomol. Ther. 2018, 26, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, J.; Kontny, E. Taurine and inflammatory diseases. Amino Acids 2012, 32, 143. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.W.; Ito, T.; Azuma, J. Clinical significance of taurine. Amino Acids 2014, 46, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, O.P.; Koenig, K.L.; Zeleniuch-Jacquotte, A.; Costa, M.; Chen, Y. The potential protective effects of taurine on coronary heart disease. Atherosclerosis 2010, 208, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Zulli, A. Taurine in cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 57–60. [Google Scholar] [CrossRef]
- Roşca, A.E.; Vlădăreanu, A.-M.; Mititelu, A.; Popescu, B.O.; Badiu, C.; Căruntu, C.; Voiculescu, S.E.; Onisâi, M.; Gologan, Ş.; Mirica, R.; et al. Effects of Exogenous Androgens on Platelet Activity and Their Thrombogenic Potential in Supraphysiological Administration: A Literature Review. J. Clin. Med. 2021, 10, 147. [Google Scholar] [CrossRef]
- Militante, J.D.; Lombardini, J.B. Treatment of hypertension with oral taurine: Experimental and clinical studies. Amino Acids 2002, 23, 381–393. [Google Scholar] [CrossRef]
- Abebe, W.; Mozaffari, M.S. Role of taurine in the vasculature: An overview of experimental and human studies. Am. J. Cardiovasc. Dis. 2011, 1, 293–311. [Google Scholar]
- Roşca, A.; Stoian, I.; Badiu, C.; Gaman, L.; Popescu, B.; Iosif, L.; Mirica, R.; Tivig, I.; Stancu, C.; Căruntu, C.; et al. Impact of chronic administration of anabolic androgenic steroids and taurine on blood pressure in rats. Braz. J. Med Biol. Res. 2016, 49, e5116. [Google Scholar] [CrossRef]
- Waldron, M.; Patterson, S.D.; Tallent, J.; Jeffries, O. The Effects of Oral Taurine on Resting Blood Pressure in Humans: A Meta-Analysis. Curr. Hypertens. Rep. 2018, 20, 81. [Google Scholar] [CrossRef]
- Pion, P.D.; Kittleson, M.D.; Thomas, W.P.; Delellis, L.A.; Rogers, Q.R. Response of cats with dilated cardiomyopathy to taurine supplementation. J. Am. Vet. Med Assoc. 1992, 201, 275–284. [Google Scholar] [PubMed]
- Militante, J.D.; Lombardini, J.B.; Schaffer, S.W. The role of taurine in the pathogenesis of the cardiomyopathy of insulin-dependent diabetes mellitus. Cardiovasc. Res. 2000, 46, 393–402. [Google Scholar] [CrossRef]
- Sanderson, S.L. Taurine and carnitine in canine cardiomyopathy. Vet. Clin. N. Am. Small Anim. Pract. 2006, 36, 1325–1343. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Kimura, Y.; Uozumi, Y.; Takai, M.; Muraoka, S.; Matsuda, T.; Ueki, K.; Yoshiyama, M.; Ikawa, M.; Okabe, M.; et al. Taurine depletion caused by knocking out the taurine transporter gene leads to cardiomyopathy with cardiac atrophy. J. Mol. Cell. Cardiol. 2008, 44, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Ontiveros, E.S.; Whelchel, B.D.; Yu, J.; Kaplan, J.L.; Sharpe, A.N.; Fousse, S.L.; Crofton, A.E.; Fascetti, A.J.; Stern, J.A. Development of plasma and whole blood taurine reference ranges and identification of dietary features associated with taurine deficiency and dilated cardiomyopathy in golden retrievers: A prospective, observational study. PLoS ONE 2020, 15, e0233206. [Google Scholar] [CrossRef]
- Ansar, M.; Ranza, E.; Shetty, M.; Paracha, S.A.; Azam, M.; Kern, I.; Iwaszkiewicz, J.; Farooq, O.; Pournaras, C.J.; Malcles, A.; et al. Taurine treatment of retinal degeneration and cardiomyopathy in a consanguineous family with SLC6A6 taurine transporter deficiency. Hum. Mol. Genet. 2020, 29, 618–623. [Google Scholar] [CrossRef]
- Schaffer, S.W.; Jong, C.J.; Ito, T.; Azuma, J. Effect of taurine on ischemia–reperfusion injury. Amino Acids 2012, 46, 21–30. [Google Scholar] [CrossRef]
- Lourenço, R.; Camilo, M.E. Taurine: A conditionally essential amino acid in humans? An overview in health and disease. Nutr. Hosp. 2002, 17, 262–270. [Google Scholar]
- Eby, G.; Halcomb, W.W. Elimination of Cardiac Arrhythmias Using Oral Taurine With L-Arginine With Case Histories: Hypothesis for Nitric Oxide Stabilization of the Sinus Node. Med. Hypotheses 2006, 6, 1200–1204. [Google Scholar] [CrossRef]
- Krylova, I.B.; Bul’On, V.V.; Selina, E.N.; Sapronov, N.S.; Shabanov, P.D. Antiarrhythmic Activity of Taurepar during Ischemic and Reperfusion Damage to Myocardium. Bull. Exp. Biol. Med. 2015, 160, 228–230. [Google Scholar] [CrossRef]
- Yang, Q.; Lv, Q.; Feng, M.; Liu, M.; Feng, Y.; Lin, S.; Yang, J.; Hu, J. Taurine Prevents the Electrical Remodeling in Ach-CaCl2 Induced Atrial Fibrillation in Rats. Adv. Exp. Med. Biol. 2017, 975, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Adameova, A.; Tappia, P.S.; Hatala, R.; Dhalla, N.S. Potential of Sulphur-containing Amino Acids in the Prevention of Catecholamine-induced Arrhythmias. Curr. Med. Chem. 2018, 25, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Azuma, J.; Sawamura, A.; Awata, N. Usefulness of Taurine in Chronic Congestive Heart Failure and Its Prospective Application. Jpn. Circ. J. 1992, 56, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Shao, A.; Hathcock, J.N. Risk assessment for the amino acids taurine, l-glutamine and l-arginine. Regul. Toxicol. Pharmacol. 2008, 50, 376–399. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Roshan, V.D.; Aslani, E.; Stannard, S.R. Taurine supplementation has anti-atherogenic and anti-inflammatory effects before and after incremental exercise in heart failure. Ther. Adv. Cardiovasc. Dis. 2017, 11, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Brøns, C.; Spohr, C.; Storgaard, H.; Dyerberg, J.; Vaag, A.A. Effect of taurine treatment on insulin secretion and action, and on serum lipid levels in overweight men with a genetic predisposition for type II diabetes mellitus. Eur. J. Clin. Nutr. 2004, 58, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Storey, K.; Storey, J.; Brooks, S.; Churchill, T.A.; Brooks, R.J. Hatchling turtles survive freezing during winter hibernation. Proc. Natl. Acad. Sci. USA 1988, 85, 8350–8354. [Google Scholar] [CrossRef]
- Ahmad, N.; Dube, B.; Agarwal, G.P.; Dube, R.K. Comparative studies of blood coagulation in hibernating and non-hibernating frogs (Rana tigrina). Thromb. Haemost. 1979, 42, 959–964. [Google Scholar] [CrossRef]
- Boral, M.C.; Deb, C. Seasonal changes in body fluids and haematology in toad Bufo melanostictus a poikilothermic cold torpor. Proc. Indian Natl. Sci. Acad. 1970, 36, 369–379. [Google Scholar]
- Zain-ul-Abedin, M.; Katorski, B. Increased blood clotting time in a hibernating lizard. Can. J. Physiol. Pharmacol. 1966, 44, 505–507. [Google Scholar] [CrossRef]
- Huang, H.L.; Rao, M.R. Effects of neferine and its combination with taurine on platelet aggregation and experimental thrombosis in rats. Yao Xue Xue Bao 1995, 30, 486–489. [Google Scholar] [PubMed]
- Ding, W.; Li, D.; Zhang, J. Influences of taurine on thrombolysis. Zhonghua Nei Ke Za Zhi 1996, 35, 378–381. (In Chinese) [Google Scholar] [PubMed]
- Murina, M.A.; Fesenko, O.D.; Sergienko, V.I.; Chudina, N.A.; Roshchupkin, D.I. Antithrombotic activity of N,N-dichlorotaurine on mouse model of thrombosis in vivo. Bull Exp. Biol. Med. 2002, 134, 36–38. [Google Scholar] [CrossRef] [PubMed]
- Kaptanoglu, L.; Kucuk, H.F.; Colak, E.; Kurt, N.; Bingul, S.M.; Akyol, H.; Torlak, O.A.; Yazici, F. The effect of taurolidine on experimental thrombus formation. Eur. J. Pharmacol. 2008, 578, 238–241. [Google Scholar] [CrossRef]
- Jeynes, B.J. Combined streptokinase and taurochenodeoxycholate action on experimentally induced atherothromboemboli. Chandler Tube Study. Arch. Pathol. Lab. Med. 1986, 110, 1143–1148. [Google Scholar]
- Jeynes, B.J. Treatment of experimentally induced cerebral atherothromboembolism in an animal model with streptokinase and taurochenodeoxycholate. Artery 1988, 15, 259–271. [Google Scholar]
- Sun, M.; Xu, C. Neuroprotective Mechanism of Taurine due to Up-regulating Calpastatin and Down-regulating Calpain and Caspase-3 during Focal Cerebral Ischemia. Cell. Mol. Neurobiol. 2008, 28, 593–611. [Google Scholar] [CrossRef]
- Sun, M.; Zhao, Y.; Gu, Y.; Xu, C. Anti-inflammatory mechanism of taurine against ischemic stroke is related to down-regulation of PARP and NF-κB. Amino Acids 2012, 42, 1735–1747. [Google Scholar] [CrossRef]
- Guan, W.; Zhao, Y.; Xu, C. A Combined Treatment with Taurine and Intra-arterial Thrombolysis in an Embolic Model of Stroke in Rats: Increased Neuroprotective Efficacy and Extended Therapeutic Time Window. Transl. Stroke Res. 2011, 2, 80–91. [Google Scholar] [CrossRef]
- Sun, M.; Zhao, Y.-M.; Gu, Y.; Xu, C. Therapeutic window of taurine against experimental stroke in rats. Transl. Res. 2012, 160, 223–229. [Google Scholar] [CrossRef]
- Rukan, T.A.; Mksimovich, N.E.; Zimatkin, S.M. Morphofunctional state of vessel endothelium at the early stage of cerebral ischemia-reperfusion and the effect of taurin administration. Eksp. Klin. Farmakol. 2013, 76, 8–10. [Google Scholar] [PubMed]
- Gharibani, P.M.; Modi, J.; Pan, C.; Menzie, J.; Ma, Z.; Chen, P.C.; Tao, R.; Prentice, H.; Wu, J.Y. The mechanism of taurine protection against endoplasmic reticulum stress in an animal stroke model of cerebral artery occlusion and stroke-related conditions in primary neuronal cell culture. Adv. Exp. Med. Biol. 2013, 776, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Gharibani, P.; Modi, J.; Menzie, J.; Alexandrescu, A.; Ma, Z.; Tao, R.; Prentice, H.; Wu, J.Y. Comparison between single and combined post-treatment with S-Methyl-N,N-diethylthiolcarbamate sulfoxide and taurine following transient focal cerebral ischemia in rat brain. Neuroscience 2015, 300, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Xiao, A.Y.; Liu, S.; Wang, M.; Li, G. Taurine Reduces tPA (Tissue-Type Plasminogen Activator)-Induced Hemorrhage and Microvascular Thrombosis After Embolic Stroke in Rat. Stroke 2018, 49, 1708–1718. [Google Scholar] [CrossRef]
- Seizer, P.; Borst, O.; Langer, H.F.; Bültmann, A.; Münch, G.; Herouy, Y.; Stellos, K.; Krämer, B.; Bigalke, B.; Büchele, B.; et al. EMMPRIN (CD147) is a novel receptor for platelet GPVI and mediates platelet rolling via GPVI-EMMPRIN interaction. Thromb. Haemost. 2009, 101, 682–686. [Google Scholar] [CrossRef]
- Seizer, P.; Ungern-Sternberg, S.N.; Schönberger, T.; Borst, O.; Münzer, P.; Schmidt, E.-M.; Mack, A.F.; Heinzmann, D.; Chatterjee, M.; Langer, H.; et al. Extracellular Cyclophilin A Activates Platelets Via EMMPRIN (CD147) and PI3K/Akt Signaling, Which Promotes Platelet Adhesion and Thrombus Formation In Vitro and In Vivo. Arter. Thromb. Vasc. Biol. 2015, 35, 655–663. [Google Scholar] [CrossRef]
- Jin, R.; Xiao, A.Y.; Chen, R.; Granger, D.N.; Li, G. Inhibition of CD147 (Cluster of Differentiation 147) Ameliorates Acute Ischemic Stroke in Mice by Reducing Thromboinflammation. Stroke 2017, 48, 3356–3365. [Google Scholar] [CrossRef]
- Ijiri, Y.; Ikarugi, H.; Tamura, Y.; Ura, M.; Morishita, M.; Hamada, A.; Mori, M.; Mori, H.; Yamori, Y.; Ishii, H.; et al. Antithrombotic effect of taurine in healthy Japanese people may be related to an increased endogenous thrombolytic activity. Thromb. Res. 2013, 131, 158–161. [Google Scholar] [CrossRef]
- Yamori, Y.; Liu, L.; Ikeda, K.; Miura, A.; Mizushima, S.; Miki, T.; Nara, Y.; Who-Cardiovascular, O.B.O.T. Distribution of Twenty-Four Hour Urinary Taurine Excretion and Association with Ischemic Heart Disease Mortality in 24 Populations of 16 Countries: Results from the WHO-CARDIAC Study. Hypertens. Res. 2001, 24, 453–457. [Google Scholar] [CrossRef]
- Yamori, Y.; Liu, L.; Mori, M.; Sagara, M.; Murakami, S.; Nara, Y.; Mizushima, S. Taurine as the Nutritional Factor for the Longevity of the Japanese Revealed by a World-Wide Epidemiological Survey. Adv. Exp. Med. Biol. 2009, 643, 13–25. [Google Scholar] [CrossRef]
- Yamori, Y.; Taguchi, T.; Mori, H.; Mori, M. Low cardiovascular risks in the middle aged males and females excreting greater 24-hour urinary taurine and magnesium in 41 WHO-CARDIAC study populations in the world. J. Biomed. Sci. 2010, 17, S21. [Google Scholar] [CrossRef] [PubMed]
- Yamori, Y.; Taguchi, T.; Hamada, A.; Kunimasa, K.; Mori, H.; Mori, M. Taurine in health and diseases: Consistent evidence from experimental and epidemiological studies. J. Biomed. Sci. 2010, 17, S6. [Google Scholar] [CrossRef] [PubMed]
- Sagara, M.; Murakami, S.; Mizushima, S.; Liu, L.; Mori, M.; Ikeda, K.; Nara, Y.; Yamori, Y. Taurine in 24-h Urine Samples Is Inversely Related to Cardiovascular Risks of Middle Aged Subjects in 50 Populations of the World. Adv. Exp. Med. Biol. 2015, 803, 623–636. [Google Scholar] [CrossRef]
- Yamori, Y.; Sagara, M.; Arai, Y.; Kobayashi, H.; Kishimoto, K.; Matsuno, I.; Mori, H.; Mori, M. Soy and fish as features of the Japanese diet and cardiovascular disease risks. PLoS ONE 2017, 12, e0176039. [Google Scholar] [CrossRef]
- Wu, F.; Koenig, K.L.; Zeleniuch-Jacquotte, A.; Jonas, S.; Afanasyeva, Y.; Wójcik, O.P.; Costa, M.; Chen, Y. Serum Taurine and Stroke Risk in Women: A Prospective, Nested Case-Control Study. PLoS ONE 2016, 11, e0149348. [Google Scholar] [CrossRef] [PubMed]
- Bellentani, S.; Pecorari, M.; Cordonna, P.; Marchegiano, P.; Manenti, F.; Basisio, E.; Defabiani, E.; Galli, G. Taurine increases bile acid poll size and reduces bile saturation index in the hamster. J. Lipid. Res. 1987, 28, 1021–1027. [Google Scholar] [CrossRef]
- Yokogoshi, H.; Mochizuki, H.; Nanami, K.; Hida, Y.; Miyachi, F.; Oda, H. Dietary Taurine Enhances Cholesterol Degradation and Reduces Serum and Liver Cholesterol Concentrations in Rats Fed a High-Cholesterol Diet. J. Nutr. 1999, 129, 1705–1712. [Google Scholar] [CrossRef]
- Murakami, S.; Nara, Y.; Yamori, Y. Taurine accelerates the regression of hypercholesterolemia in stroke-prone spontaneously hypertensive rats. Life Sci. 1996, 58, 1643–1651. [Google Scholar] [CrossRef]
- Lam, N.V.; Chen, W.; Suruga, K.; Nishimura, N.; Goda, T.; Yokogoshi, H. Enhancing effect of taurine on CYP7A1 mRN expression in Hep G2 cells. Amino Acids 2006, 30, 43–48. [Google Scholar] [CrossRef]
- Yanagita, T.; Han, S.Y.; Hu, Y.; Nagao, K.; Kitajima, H.; Murakami, S. Taurine reduces the secretion of apolipoprotein B100 and lipids in HepG2 cells. Lipid. Health Dis. 2008, 7, 38. [Google Scholar] [CrossRef]
- Murakami, S.; Sakurai, T.; Tomoike, H.; Sakono, M.; Nasu, T.; Fukuda, N. Prevention of hypercholesterolemia and atherosclerosis in the hyperlipidemia- and atherosclerosis-prone Japanese (LAP) quail by taurine supplementation. Amino Acids 2010, 38, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.S. Effect of Taurine Supplementation on Plasma Homocysteine Levels of the Middle-Aged Korean Women. Adv. Exp. Med. Biol. 2009, 643, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Zulli, A.; Lau, E.; Wijaya, B.P.P.; Jin, X.; Sutarga, K.; Schwartz, G.D.; Leamont, J.; Wookey, P.J.; Zinellu, A.; Carru, C.; et al. High dietary taurine reduces apoptosis and atherosclerosis in the left main coronary artery. Hypertension 2009, 53, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Gokce, G.; Ozsarlak-Sozer, G.; Oran, I.; Oktay, G.; Ozkal, S.; Kerry, Z. Taurine suppresses oxidative stress-potentiated expression of lectin-like oxidized low-density lipoprotein receptor and restensosis in balloon-injured rabbit iliac artery. Clin. Exp. Pharmacol. Physiol. 2011, 38, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Merzenich, G.; Zeitler, H.; Vetter, H.; Bhonde, R.R. Protective effects of taurine on endothelial cells impaired by high glucose and oxidized low density lipoproteins. Eur. J. Nutr. 2007, 46, 431–438. [Google Scholar] [CrossRef]
- Yoshimura, H.; Nariai, Y.; Etshima, M.; Mitani, T.; Tanigawa, Y. Taurine suppresses platelet-derived growth factor (PDGF) BB-induced PDGF-beta receptor phosphorylation by protein tyrosine phosphatase-mediated dephosphorylation in vascular smooth muscle cells. Biochim. Biophys. Acta 2005, 1745, 350–360. [Google Scholar] [CrossRef]
- Guizoni, D.M.; Vettorazzi, J.F.; Carneiro, E.M.; Davel, A.P. Modulation of endothelium-derived nitric oxide production and activity by taurine and taurine-conjugated bile acids. Nitric Oxide 2020, 94, 48–53. [Google Scholar] [CrossRef]
- Rosca, A.; Stancu, C.S.; Badiu, C.; Popescu, B.O.; Mirica, R.; Căruntu, C.; Gologan, S.; Voiculescu, S.E.; Zagrean, A.-M. Lipid Profile Changes Induced by Chronic Administration of Anabolic Androgenic Steroids and Taurine in Rats. Medicina 2019, 55, 540. [Google Scholar] [CrossRef]
- Chen, W.; Guo, J.-X.; Chang, P. The effect of taurine on cholesterol metabolism. Mol. Nutr. Food Res. 2012, 56, 681–690. [Google Scholar] [CrossRef]
- Murakami, S. Role of taurine in the pathogenesis of obesity. Mol. Nutr. Food Res. 2015, 59, 1353–1363. [Google Scholar] [CrossRef]
- Ito, T.; Schaffer, S.W.; Azuma, J. The potential usefulness of taurine on diabetes mellitus and its complications. Amino Acids 2012, 42, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Roy, A.; Sil, P.C. Mechanism of the Protective Action of Taurine in Toxin and Drug Induced Organ Pathophysiology and Diabetic Complications: A Review. Food Funct. 2012, 3, 1251–1264. [Google Scholar] [CrossRef] [PubMed]
- Imae, M.; Asano, T.; Murakami, S. Potential role of taurine in the prevention of diabetes and metabolic syndrome. Amino Acids 2012, 46, 81–88. [Google Scholar] [CrossRef]
- Sirdah, M.M. Protective and therapeutic effectiveness of taurine in diabetes mellitus: A rationale for antioxidant supplementation. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 55–64. [Google Scholar] [CrossRef]
- Llah, I.U.; Piao, F.; Aadil, R.M.; Suleman, R.; Li, K.; Zhang, M.; Wu, P.; Shahbaz, M.; Ahmed, Z. Ameliorative effects of taurine against diabetes: A review. Amino Acids 2018, 50, 487–502. [Google Scholar] [CrossRef] [PubMed]
- Baron, A.D. Vascular reactivity. Am. J. Cardiol. 1999, 84, 25J–27J. [Google Scholar] [CrossRef]
- O’Connell, B.J.; Genest, J., Jr. High-density lipoproteins and endothelial function. Circulation 2001, 104, 1978–1983. [Google Scholar] [CrossRef]
- Fowler, B. Homocystein—An independent risk factor for cardiovascular and thrombotic diseases. Ther. Umsch. 2005, 62, 641–646. [Google Scholar] [CrossRef]
- Sudano, I.; Roas, S.; Noll, G. Vascular abnormalities in essential hypertension. Curr. Pharm. Des. 2011, 17, 3039–3044. [Google Scholar] [CrossRef]
- Lei, J.; Vodovotz, Y.; Tzeng, E.; Billiar, T.R. Nitric oxide, a protective molecule in the cardiovascular system. Nitric Oxide 2013, 35, 175–185. [Google Scholar] [CrossRef]
- Kim, Y.-W.; West, X.Z.; Byzova, T.V. Inflammation and oxidative stress in angiogenesis and vascular disease. Klin. Wochenschr. 2013, 91, 323–328. [Google Scholar] [CrossRef] [PubMed]
- van der Stoep, M.; Korporaal, S.J.; Van Eck, M. High-density lipoprotein as a modulator of platelet and coagulation responses. Cardiovasc. Res. 2014, 103, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Baszczuk, A.; Kopczyński, Z.; Thielemann, A. Endothelial dysfunction in patients with primary hypertension and hyperhomocysteinemia. Postepy Hig. Med. Dosw. 2014, 68, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.; Luo, X.; Ni, H.; Shi, H.; Liu, L.; Wen, Z.; Gu, X.; Qiao, J.; Li, J. High levels of LDL-C combined with low levels of HDL-C further increase platelet activation in hypercholesterolemic patients. Braz. J. Med Biol. Res. 2015, 48, 167–173. [Google Scholar] [CrossRef][Green Version]
- Aird, W.C. Endothelium and haemostasis. Hamostaseologie 2015, 35, 11–16. [Google Scholar] [CrossRef]
- Fraer, M.; Kilic, F. Serotonin: A different player in hypertension-associated thrombosis. Hypertension 2015, 65, 942–948. [Google Scholar] [CrossRef]
- Azuma, M.; Takahashi, K.; Fukuda, T.; Ohyabu, Y.; Yamamoto, I.; Kim, S.; Iwao, H.; Schaffer, S.W.; Azuma, J. Taurine attenuates hypertrophy induced by angiotensin II in cultured neonatal rat cardiac myocytes. Eur. J. Pharmacol. 2000, 403, 181–188. [Google Scholar] [CrossRef]
- Schaffer, S.; Solodushko, V.; Pastukh, V.; Ricci, C.; Azuma, J. Possible Cause of Taurine-deficient Cardiomyopathy: Potentiation of Angiotensin II Action. J. Cardiovasc. Pharmacol. 2003, 41, 751–759. [Google Scholar] [CrossRef]
- Oudit, G.Y.; Trivieri, M.G.; Khaper, N.; Husain, T.; Wilson, G.J.; Liu, P.; Sole, M.J.; Backx, P.H. Taurine Supplementation Reduces Oxidative Stress and Improves Cardiovascular Function in an Iron-Overload Murine Model. Circulation 2004, 109, 1877–1885. [Google Scholar] [CrossRef]
- Li, C.; Cao, L.; Zeng, Q.; Liu, X.; Zhang, Y.; Dai, T.; Hu, D.; Huang, K.; Wang, Y.; Wang, X.; et al. Taurine May Prevent Diabetic Rats from Developing Cardiomyopathy also by Downregulating Angiotensin II Type2 Receptor Expression. Cardiovasc. Drugs Ther. 2005, 19, 105–112. [Google Scholar] [CrossRef]
- Ito, T.; Schaffer, S.; Azuma, J. The effect of taurine on chronic heart failure: Actions of taurine against catecholamine and angiotensin II. Amino Acids 2014, 46, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Hanahata, Y.; Kine, K.; Murakami, S.; Schaffer, S.W. Tissue Taurine Depletion Induces Profibrotic Pattern of Gene Expression and Causes Aging-Related Cardiac Fibrosis in Heart in Mice. Biol. Pharm. Bull. 2018, 41, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. Practical prevention of cardiac remodeling and atrial fibrillation with full-spectrum antioxidant therapy and ancillary strategies. Med. Hypotheses 2010, 75, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Brass, L. Understanding and Evaluating Platelet Function. Hematology 2010, 2010, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Estevez, B.; Du, X. New Concepts and Mechanisms of Platelet Activation Signaling. Physiology 2017, 32, 162–177. [Google Scholar] [CrossRef]
- Rivera, J.; Lozano, M.L.; Navarro-Núñez, L.; Vicente, V. Platelet receptors and signaling in the dynamics of thrombus formation. Haematologica 2009, 94, 700–711. [Google Scholar] [CrossRef]
- Varga-Szabo, D.; Braun, A.; Nieswandt, B. Calcium signaling in platelets. J. Thromb. Haemost. 2009, 7, 1057–1066. [Google Scholar] [CrossRef]
- McCarty, M.F. Complementary vascular-protective actions of magnesium and taurine: A rationale for magnesium taurate. Med. Hypotheses 1996, 46, 89–100. [Google Scholar] [CrossRef]
- Maupin, B. Blood Platelets in Man and Animals; Pergamon Press: Oxford, UK, 1969; pp. 1–487. ISBN 9781483282978. [Google Scholar] [CrossRef]
- Ahtee, L.; Boullini, D.J.; Paasonen, M.K. Transport of taurine by normal human blood platelets. Br. J. Pharmac. 1974, 52, 245–251. [Google Scholar] [CrossRef]
- Vinton, N.E.; Laidlaw, S.A.; Ament, M.E.; Kopple, J.D. Taurine concentrations in plasma and blood cells of patients undergoing long-term parenteral nutrition. Am. J. Clin. Nutr. 1986, 44, 398–404. [Google Scholar] [CrossRef]
- Laidlaw, S.A.; Sturman, J.A.; Kopple, J.D. Effect of dietary taurine on plasma and blood cell taurine concentrations in cats. J. Nutr. 1987, 117, 1945–1959. [Google Scholar] [CrossRef] [PubMed]
- Hayes, K.; Pronczuk, A.; Addesa, A.; Stephan, Z. Taurine modulates platelet aggregation in cats and humans. Am. J. Clin. Nutr. 1989, 49, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Calpona, P.; Caponetti, A.; Romano, G.; Di Benedetto, A.; Cucinotta, D.; Di Giorgio, R. Taurine and osmoregulation: Platelet taurine content, uptake, and release in type 2 diabetic patients. Metabolism 2001, 50, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Voaden, M.J.; Hussain, A.A.; Chan, I.P. Studies on retinitis pigmentosa in man. I. Taurine and blood platelets. Br. J. Ophthalmol. 1982, 66, 771–775. [Google Scholar] [CrossRef]
- Torres, C.L.; Walker, N.J.; Rogers, Q.R.; Tablin, F. Platelet Taurine Concentration Can Be Predicted from Whole Blood Taurine Concentrations in Dogs. J. Nutr. 2006, 136 (Suppl. 7), 2055S–2057S. [Google Scholar] [CrossRef]
- Otani, F.; Ejiri, K.; Kanemori, H.; Kudo, T.; Sekiba, K. Platelet taurine concentration and uptake in gestosis patients with edema, proteinuria and hypertension. Acta Med. Okayama 1992, 46, 17–22. [Google Scholar] [CrossRef]
- Spohr, C.; Brøns, C.; Winther, K.; Dyerberg, J.; Vaag, A. No effect of taurine on platelet aggregation in men with a predisposition to type 2 diabetes mellitus. Platelets 2005, 16, 301–305. [Google Scholar] [CrossRef]
- Miglis, M.; Wilder, D.; Reid, T.; Bakaltcheva, I. Effect of taurine on platelets and the plasma coagulation system. Platelets 2002, 13, 5–10. [Google Scholar] [CrossRef]
- Weingarten, F. Darstellung von Homologen des Taurins und von Polymethylen-dithioschwefelsauren Salzen und Prüfung ihrer Wirkung auf die Blutgerinnung [The production of homologues of taurine and of polymethylene dithiosulfonic acid salts and examination of their effect on blood coagulation]. Arzneimittelforschung 1954, 4, 344–346. [Google Scholar]
- Krøll, J.; Krøsll, J. Influence of Bile Salts on the Lysis of131I-Labelled Plasma Clots in Human Subcutaneous Tissuein Vivo. Scand. J. Clin. Lab. Investig. 1966, 18, 691–692. [Google Scholar] [CrossRef]
- Baele, G.; Beke, R.; Barbier, F. In vitro inhibition of platelet aggregation by bile salts. Thromb. Haemost. 1980, 44, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Takano, S.; Suzuki, T. Effect of Bile on Aggregation and Morphology of Rabbit Platelets. Tohoku J. Exp. Med. 1980, 131, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Krauss, J.S.; Jonah, M.H. Platelet Dysfunction (Thrombocytopathy) in Extrahepatic Biliary Obstruction. South Med. J. 1982, 75, 506–507. [Google Scholar] [CrossRef] [PubMed]
- Bowen, D.J.; Clemmons, R.M.; Meyer, D.J.; Dorsey-Lee, M.R. Platelet functional changes secondary to hepatocholestasis and elevation of serum bile acids. Thromb. Res. 1988, 52, 649–654. [Google Scholar] [CrossRef]
- Kurachi, M.; Hongoh, K.; Watanabe, A.; Aihara, H. Suppression of bronchial response to platelet activating factor following taurine administration. Adv. Exp. Med. Biol. 1987, 217, 189–198. [Google Scholar] [CrossRef] [PubMed]
- El Tahir, K.E.; Ageel, A.M.; Abu-Jayyab, A.R. Effect of taurine on arterial, uterine and cardiac PGI2 and TXA2 synthesis in the rat. Prostaglandins 1987, 33, 17–24. [Google Scholar] [CrossRef]
- Hofmann, J.; Lösche, W.; Till, U.; Bosia, A.; Arese, P.; Pescarmona, G.P.; Thielmann, K. Effect of decreased GSH level on human platelet functions. Artery 1980, 8, 431–436. [Google Scholar]
- Harpster, N.K. Feline Cardiomyopathy. Vet. Clin. N. Am. 1977, 7, 355–371. [Google Scholar] [CrossRef]
- Welles, E.G.; Boudreaux, M.K.; Tyler, J.W. Platelet, antithrombin, and fibrinolytic activities in taurine-deficient and taurine-replete cats. Am. J. Vet. Res. 1993, 54, 1235–1243. [Google Scholar]
- Ji, Y.; Tao, L.; Xu, H.L.; Rao, M.R. Effects of taurine and enalapril on blood pressure, platelet aggregation and the regression of left ventricular hypertrophy in two-kidney-one-clip renovascular hypertensive rats. Yao Xue Xue Bao 1995, 30, 886–890. [Google Scholar]
- Park, I.S.; Kang, Y.H.; Kang, J.S. Effects of taurine on plasma and liver lipids, erythrocyte ouabain sensitive Na efflux and platelet aggregation in Sprague Dawley rats. Nutr. Res. Pract. 2007, 1, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Rosca, A.; Badiu, C.; Uscatescu, V.; Mirica, R.; Bragam, R.; Pavel, B.; Zagrean, L. Effect of chronic administration of anabolic androgenic steroids and taurine on platelet aggregation in rats. Acta Endocrinol. 2013, 9, 33–38. [Google Scholar] [CrossRef]
- Roşca, A.E.; Badiu, C.; Uscătescu, V.; Stoian, I.; Mirică, R.; Braga, R.I.; Pavel, B.; Zăgrean, L. Influence of chronic administration of anabolic androgenic steroids and taurine on haemostasis profile in rats: A thrombelastographic study. Blood Coagul. Fibrinolysis 2013, 24, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Murina, M.A.; Savel’eva, E.L.; Roshchupkin, D.I. Mechanism of action of biogenic chloramines and hypochlorite on initial aggregation of blood platelets. Biofizika 2006, 51, 299–305. [Google Scholar]
- Murina, M.A.; Roshchupkin, D.I.; Kravchenko, N.N.; Petrova, A.O.; Sergienko, V.I. Antiaggregant effects of biogenic chloramines. Bull. Exp. Biol. Med. 2007, 144, 464–470. [Google Scholar] [CrossRef]
- Murina, M.A.; Roshchupkin, D.I.; Chudina, N.A.; Petrova, A.O.; Sergienko, V.I. Antiaggregant effect of taurine chloramines in the presence of serum albumin. Bull. Exp. Biol. Med. 2009, 147, 704–707. [Google Scholar] [CrossRef]
- Roshchupkin, D.I.; Murina, M.A.; Sergienko, V.I. Covalent chloramine inhibitors of blood platelet functions: Computational indices for their reactivity and antiplatelet activity. Biofizika 2011, 56, 945–954. [Google Scholar] [CrossRef]
- Murina, M.A.; Roshchupkin, D.I.; Kondrashova, K.V.; Sergienko, V.I. Inhibition of Plasma Coagulation and Platelet Aggregation with Structural Analogs of Taurine Chloramine. Bull. Exp. Biol. Med. 2014, 157, 207–210. [Google Scholar] [CrossRef]
- Almazov, V.A.; Gurevich, V.S.; Mikhaĭlova, I.A.; Strel’tsova, E.N. Effect of taurine on Ca, Mg-ATPase activity in human platelets and on their aggregation. Bull. Exp. Biol. Med. 1985, 100, 398–400. (In Russian) [Google Scholar] [CrossRef]
- Raghu, C.N.; Manikeri, S.R.; Sheth, U.K.; Dadkar, V.N. Probable mode of taurine action. Indian J. Exp. Biol. 1982, 20, 481–483. [Google Scholar]
- Thomas, G.; Lucas, F.V.; Schumacher, O.P.; Skrinska, V. Behavior of intracellular glutathione during platelet thromboxane synthesis in diabetes. Prostaglandins. Leukot. Med. 1986, 22, 117–128. [Google Scholar] [CrossRef]
- Franconi, F.; Miceli, M.; Bennardini, F.; Mattana, A.; Covarrubias, J.; Seghieri, G. Taurine potentiates the antiaggregatory action of aspirin and indomethacin. Adv. Exp. Med. Biol. 1992, 315, 181–186. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. Sub-optimal taurine status may promote platelet hyperaggregability in vegetarians. Med. Hypotheses 2004, 63, 426–433. [Google Scholar] [CrossRef]
- Franconi, F.; Bennardini, F.; Mattana, A.; Miceli, M.; Ciuti, M.; Milan, M.; Gironi, A.; Bartomomei, G.; Anichini, R.; Seghieri, G. Taurine levels in plasma and platelets in insulin-dependent and non-insulin-dependent diabetes mellitus: Correlation with platelet aggregation. Adv. Exp. Med. Biol. 1994, 359, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Franconi, F.; Bennardini, F.; Mattana, A.; Miceli, M.; Ciuti, M.; Milan, M.; Gironi, A.; Anichini, R.; Seghieri, G. Plasma and platelet taurine are reduced in subjects with insulin-dependent diabetes mellitus: Effects of taurine supplementation. Am. J. Clin. Nutr. 1995, 61, 1115–1119. [Google Scholar] [CrossRef]
- Geggel, H.S.; Ament, M.E.; Heckenlively, J.R.; Martin, D.A.; Kopple, J.D. Nutritional requirement for taurine in patients receiving long-term parenteral nutrition. N. Engl. J. Med. 1985, 312, 142–146. [Google Scholar] [CrossRef]
- Trachtman, H.; Del Pizzo, R.; Futterweit, S.; Levine, D.; Rao, P.S.; Valderrama, E.; Sturman, J.A. Taurine attenuates renal disease in chronic puromycin aminonucleoside nephropathy. Am. J. Physiol. Physiol. 1992, 262, F117–F123. [Google Scholar] [CrossRef]
- Douglas, J.T.; Shah, M.; Lowe, G.D.; Belch, J.J.; Forbes, C.D.; Prentice, C.R. Plasma fibrinopeptide A and beta-thromboglobulin in pre-eclampsia and pregnancy hypertension. Thromb. Haemost. 1982, 47, 54–55. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, M. The role of coagulation and fibrinolysis system in pathogenesis of toxemia of pregnancy. Nihon Sanka Fujinka Gakkai Zasshi 1988, 40, 1000–1009. [Google Scholar]
- Ornaghi, S.; Mueller, M.; Barnea, E.R.; Paidas, M.J. Thrombosis during pregnancy: Risks, prevention, and treatment for mother and fetus-harvesting the power of omic technology, biomarkers and in vitro or in vivo models to facilitate the treatment of thrombosis. Birth Defects Res. C Embryo. Today 2015, 105, 209–225. [Google Scholar] [CrossRef]
- Valera, M.C.; Parant, O.; Vayssiere, C.; Arnal, J.F.; Payrastre, B. Physiologic and pathologic changes of platelets in pregnancy. Platelets 2010, 21, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, C.; Larsen, J.B.; Fuglsang, J.; Hvas, A.-M. Platelet function in preeclampsia—A systematic review and meta-analysis. Platelets 2019, 30, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Namba, K.; Ejiri, K.; Kanemori, H.; Kudo, T.; Sekiba, K. Effect of taurine concentration on platelet aggregation in gestosis patients with edema, proteinuria and hypertension. Acta. Med. Okayama 1992, 46, 241–247. [Google Scholar] [PubMed]
- White, J.G.; Rao, G.H.; Gerrard, J.M. Effects of the lonophore A23187 on blood platelets I. Influence on aggregation and secretion. Am. J. Pathol. 1974, 77, 135–149. [Google Scholar] [PubMed]
- Atahanov, S.E.; Elizarova, E.P. Modulation of receptor-dependent increase of calcium ions in human platelets by taurine. Arzneimittelforschung 1992, 42, 1311–1313. [Google Scholar]
- Santhakumar, A.; Fozzard, N.; Perkins, A.; Singh, I. The Synergistic Effect of Taurine and Caffeine on Platelet Activity and Hemostatic Function. Food Public Health 2013, 3, 147–153. [Google Scholar] [CrossRef]
- Roshchupkin, D.I.; Murina, M.A.; Adnoral, N.V.; Kravchenko, N.N.; Sergienko, V.I. Inhibition of platelet function by biogenic chloramines. Fiziol. Cheloveka 1998, 24, 113–120. [Google Scholar]
- Roshchupkin, D.I.; Berzhitskaia, V.V.; Sokolov, A.; Murina, M.A. Changes in the initial platelet aggregation during storage of the platelet concentrate and exposure to the myeloperoxidase reaction products. Bull. Eksp. Biol. Med. 1997, 124, 523–526. [Google Scholar]
- Murina, M.A.; Roshchupkin, D.I.; Kravchenko, N.N.; Sadovnikov, V.B.; Sergienko, V.I. The anti-aggregatory effect of chloramine derivatives of amino acids on platelets in the presence of plasma. Biofizika 1997, 42, 1279–1285. [Google Scholar]
- Rpshchupkin, D.I.; Berzhitskaia, V.V.; Murina, M.A. Difference in inhibitory actions of products of the myeloperoxidase- catalyzed reaction on initial aggregation of activated platelets. Biofizika 1998, 43, 323–328. [Google Scholar]
- Elizarova, E.P. Effects of taurine on calcium in platelets and their aggregation. Adv. Exp. Med. Biol. 1996, 403, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, B.; Li, Y.; Sun, F.; Li, P.; Xia, W.; Zhou, X.; Li, Q.; Wang, X.; Chen, J.; et al. Taurine Supplementation Lowers Blood Pressure and Improves Vascular Function in Prehypertension: Randomized, Double-Blind, Placebo-Controlled Study. Hypertension 2016, 67, 541–549. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; Okeefe, J.H.; McCarty, M.F. Boosting endogenous production of vasoprotective hydrogen sulfide via supplementation with taurine and N-acetylcysteine: A novel way to promote cardiovascular health. Open Heart 2017, 4, e000600. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; O’Keefe, J.H.; DiNicolantonio, J.J. A diet rich in taurine, cysteine, folate, B12 and betaine may lessen risk for Alzheimer’s disease by boosting brain synthesis of hydrogen sulfide. Med. Hypotheses 2019, 132, 109356. [Google Scholar] [CrossRef] [PubMed]
- Baseggio Conrado, A.; Capuozzo, E.; Mosca, L.; Francioso, A.; Fontana, M. Thiotaurine: From Chemical and Biological Properties to Role in H2S Signaling. Adv. Exp. Med. Biol. 2019, 1155, 755–771. [Google Scholar] [CrossRef] [PubMed]
- Pircher, J.; Fochler, F.; Czermak, T.; Mannell, H.; Kraemer, B.F.; Wörnle, M.; Sparatore, A.; Del Soldato, P.; Pohl, U.; Krötz, F. Hydrogen Sulfide–Releasing Aspirin Derivative ACS14 Exerts Strong Antithrombotic Effects In Vitro and In Vivo. Arter. Thromb. Vasc. Biol. 2012, 32, 2884–2891. [Google Scholar] [CrossRef]
- Gao, L.; Cheng, C.; Sparatore, A.; Zhang, H.; Wang, C. Hydrogen sulfide inhibits human platelet aggregation in vitro in part by interfering gap junction channels: Effects of ACS14, a hydrogen sulfide-releasing aspirin. Heart Lung Circ. 2015, 24, 77–85. [Google Scholar] [CrossRef][Green Version]
- Grambow, E.; Mueller-Graf, F.; Delyagina, E.; Frank, M.; Kuhla, A.; Vollmar, B. Effect of the hydrogen sulfide donor GYY4137 on platelet activation and microvascular thrombus formation in mice. Platelets 2014, 25, 166–174. [Google Scholar] [CrossRef]
- Zhong, L.; Lv, L.; Yang, J.; Liao, X.; Yu, J.; Wang, R.; Zhou, P. Inhibitory Effect of Hydrogen Sulfide on Platelet Aggregation and the Underlying Mechanisms. J. Cardiovasc. Pharmacol. 2014, 64, 481–487. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, X.; Shi, S.; Li, H.; Gao, F.; Zhong, X.; Wang, Y. Exogenous hydrogen sulfide protects from endothelial cell damage, platelet activation, and neutrophils extracellular traps formation in hyperhomocysteinemia rats. Exp. Cell Res. 2018, 370, 434–443. [Google Scholar] [CrossRef]
- Zhang, H.; Bai, Z.; Zhu, L.; Liang, Y.; Fan, X.; Li, J.; Wen, H.; Shi, T.; Zhao, Q.; Wang, Z. Hydrogen sulfide donors: Therapeutic potential in anti-atherosclerosis. Eur. J. Med. Chem. 2020, 205, 112665. [Google Scholar] [CrossRef] [PubMed]
- Fennessy, F.; Moneley, D.; Wang, J.; Kelly, C.; Bouchier-Hayes, D. Taurine and Vitamin C Modify Monocyte and Endothelial Dysfunction in Young Smokers. Circulation 2003, 107, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Jiang, D.-J.; Huang, H.; Jia, S.-J.; Jiang, J.-L.; Hu, C.-P.; Li, Y.-J. Taurine protects against low-density lipoprotein-induced endothelial dysfunction by the DDAH/ADMA pathway. Vasc. Pharmacol. 2007, 46, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xu, X.; Yang, J.; Wu, G.; Sun, C.; Lv, Q. Antihypertensive Effect of Taurine in Rat. Adv. Exp. Med. Biol. 2009, 643, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Maia, A.R.; Batista, T.M.; Victorio, J.A.; Clerici, S.P.; Delbin, M.A.; Carneiro, E.M.; Davel, A.P. Taurine supplementation reduces blood pressure and prevents endothelial dysfunction and oxidative stress in post-weaning protein-restricted rats. PLoS ONE. 2014, 9, e105851. [Google Scholar] [CrossRef]
- Yamamoto, J.; Akabane, S.; Yoshimi, H.; Nakai, M.; Ikeda, M. Effects of taurine on stress-evoked hemodynamic and plasma catecholamine changes in spontaneously hypertensive rats. Hypertension 1985, 7, 913–922. [Google Scholar] [CrossRef]
- Tanabe, Y.; Urata, H.; Kiyonaga, A.; Ikeda, M.; Tanaka, H.; Shindo, M.; Arakawa, K. Changes in Serum Concentrations of Taurine and Other Amino Acids in Clinical Antihypertensive Exercise Therapy. Clin. Exp. Hypertens. Part A Theory Pract. 1989, 11, 149–165. [Google Scholar] [CrossRef]
- Fujita, T.; Sato, Y.; Ando, K. Changes in cardiac and hypothalamic noradrenergic activity with taurine in DOCA-salt rats. Am. J. Physiol. Circ. Physiol. 1986, 251, H926–H933. [Google Scholar] [CrossRef] [PubMed]
- Chanh, P.H.; Chahine, R.; Dossou-Gbete, V.; Navarro-Delmasure, C. Taurine and icosanoids in the heart. Prostaglandins Leukot. Med. 1987, 28, 243–254. [Google Scholar] [CrossRef]
- Wettstein, M. Cytoprotection by the osmolytes betaine and taurine in ischemia- reoxygenation injury in the perfused rat liver. Hepatology 1997, 26, 1560–1566. [Google Scholar] [CrossRef]
- Liu, Y.; Niu, L.; Zhang, W.; Cui, L.; Zhang, X.; Liang, Y.; Zhang, M. Effects of taurine on contractions of the porcine coronary artery. Pharmacol. Rep. 2009, 61, 681–689. [Google Scholar] [CrossRef]
- Hu, D.H. Effects of taurine enhancement of synthesis of TxA2 and PGI2 in cultured intra-pulmonary arteriolar smooth muscle cells under acute hypoxia. Sheng Li Xue Bao 1998, 50, 465–468. (In Chinese) [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roşca, A.E.; Vlădăreanu, A.-M.; Mirica, R.; Anghel-Timaru, C.-M.; Mititelu, A.; Popescu, B.O.; Căruntu, C.; Voiculescu, S.E.; Gologan, Ş.; Onisâi, M.; et al. Taurine and Its Derivatives: Analysis of the Inhibitory Effect on Platelet Function and Their Antithrombotic Potential. J. Clin. Med. 2022, 11, 666. https://doi.org/10.3390/jcm11030666
Roşca AE, Vlădăreanu A-M, Mirica R, Anghel-Timaru C-M, Mititelu A, Popescu BO, Căruntu C, Voiculescu SE, Gologan Ş, Onisâi M, et al. Taurine and Its Derivatives: Analysis of the Inhibitory Effect on Platelet Function and Their Antithrombotic Potential. Journal of Clinical Medicine. 2022; 11(3):666. https://doi.org/10.3390/jcm11030666
Chicago/Turabian StyleRoşca, Adrian Eugen, Ana-Maria Vlădăreanu, Radu Mirica, Cristina-Mihaela Anghel-Timaru, Alina Mititelu, Bogdan Ovidiu Popescu, Constantin Căruntu, Suzana Elena Voiculescu, Şerban Gologan, Minodora Onisâi, and et al. 2022. "Taurine and Its Derivatives: Analysis of the Inhibitory Effect on Platelet Function and Their Antithrombotic Potential" Journal of Clinical Medicine 11, no. 3: 666. https://doi.org/10.3390/jcm11030666
APA StyleRoşca, A. E., Vlădăreanu, A.-M., Mirica, R., Anghel-Timaru, C.-M., Mititelu, A., Popescu, B. O., Căruntu, C., Voiculescu, S. E., Gologan, Ş., Onisâi, M., Iordan, I., & Zăgrean, L. (2022). Taurine and Its Derivatives: Analysis of the Inhibitory Effect on Platelet Function and Their Antithrombotic Potential. Journal of Clinical Medicine, 11(3), 666. https://doi.org/10.3390/jcm11030666








