The Rationale and Current Status of Endotoxin Adsorption in the Treatment of Septic Shock
Abstract
:1. Introduction
2. Endotoxin, Lipopolysaccharide
3. Lipopolysaccharide Sensing Pathways
3.1. Toll-like Receptor 4–Myeloid Differentiation Protein 2 (TLR4-MD-2) Pathway
3.2. Transient Receptor Potential (TRP) Ion Channels
3.3. Intracellular LPS Sensing
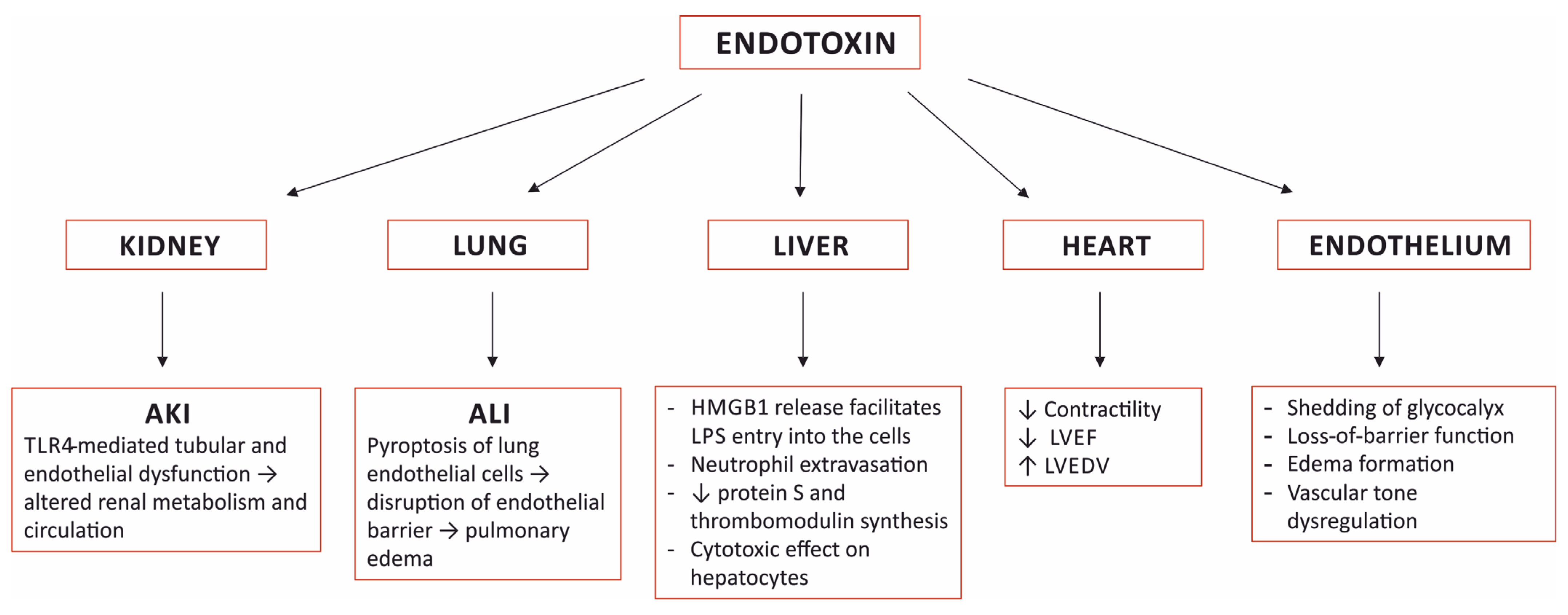
4. Organ Damage Caused by Sensing Endotoxin
4.1. The Kidney
4.2. The Lung
4.3. The Heart
4.4. The Liver
4.5. The Vascular Endothelium
5. Endotoxin Removal
5.1. Randomized Controlled Trials of Endotoxin Adsorption Therapies
5.2. Systematic Reviews and Meta-Analyses
6. COVID-19 and Endotoxemia
7. Investigating Aspects of Endotoxin Removal
7.1. Timing of the Initiation of Endotoxin Adsorption
7.2. Extended Endotoxin Adsorption Treatment
7.3. Endotoxin Removal Treatment Guided by Measuring the Endotoxin Level
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Markwart, R.; Saito, H.; Harder, T.; Tomczyk, S.; Cassini, A.; Fleischmann-Struzek, C.; Reichert, F.; Eckmanns, T.; Allegranzi, B. Epidemiology and Burden of Sepsis Acquired in Hospitals and Intensive Care Units: A Systematic Review and Meta-Analysis. Intensive Care Med. 2020, 46, 1536–1551. [Google Scholar] [CrossRef] [PubMed]
- Putzu, A.; Schorer, R.; Lopez-Delgado, J.C.; Cassina, T.; Landoni, G. Blood Purification and Mortality in Sepsis and Septic Shock. Anesthesiology 2019, 131, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L. Septic Shock. In Textbook of Critical Care; Vincent, J.-L., Abraham, E., Moore, F.A., Kochanek, P.M., Fink, M.P., Eds.; Elsevier: Philadelphia, PA, USA, 2017; pp. 843–848. ISBN 978-0-323-37638-9. [Google Scholar]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Rietschel, E.T.; Cavaillon, J.-M. Endotoxin and Anti-Endotoxin: The Contribution of the Schools of Koch and Pasteur: Life, Milestone-Experiments and Concepts of Richard Pfeiffer (Berlin) and Alexandre Besredka (Paris). J. Endotoxin Res. 2002, 8, 3–16. [Google Scholar] [CrossRef]
- Heine, H.; Rietschel, E.T.; Ulmer, A.J. The Biology of Endotoxin. Mol. Biotechnol. 2001, 19, 279–296. [Google Scholar] [CrossRef]
- Caroff, M.; Novikov, A. Lipopolysaccharides: Structure, Function and Bacterial Identification. OCL 2020, 27, 31. [Google Scholar] [CrossRef]
- Lundstedt, E.; Kahne, D.; Ruiz, N. Assembly and Maintenance of Lipids at the Bacterial Outer Membrane. Chem. Rev. 2021, 121, 5098–5123. [Google Scholar] [CrossRef]
- Raetz, C.R.H.; Whitfield, C. Lipopolysaccharide Endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef] [Green Version]
- Bertani, B.; Ruiz, N. Function and Biogenesis of Lipopolysaccharides. EcoSal Plus 2018, 8. [Google Scholar] [CrossRef]
- Munford, R.S. Sensing Gram-Negative Bacterial Lipopolysaccharides: A Human Disease Determinant? Infect. Immun. 2008, 76, 454–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- d’Hennezel, E.; Abubucker, S.; Murphy, L.O.; Cullen, T.W. Total Lipopolysaccharide from the Human Gut Microbiome Silences Toll-Like Receptor Signaling. MSystems 2017, 2, e00046-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adelman, M.W.; Woodworth, M.H.; Langelier, C.; Busch, L.M.; Kempker, J.A.; Kraft, C.S.; Martin, G.S. The Gut Microbiome’s Role in the Development, Maintenance, and Outcomes of Sepsis. Crit. Care 2020, 24, 278. [Google Scholar] [CrossRef] [PubMed]
- Montminy, S.W.; Khan, N.; McGrath, S.; Walkowicz, M.J.; Sharp, F.; Conlon, J.E.; Fukase, K.; Kusumoto, S.; Sweet, C.; Miyake, K.; et al. Virulence Factors of Yersinia Pestis Are Overcome by a Strong Lipopolysaccharide Response. Nat. Immunol. 2006, 7, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, R.F.; Sá-Correia, I.; Valvano, M.A. Lipopolysaccharide Modification in Gram-Negative Bacteria during Chronic Infection. FEMS Microbiol. Rev. 2016, 40, 480–493. [Google Scholar] [CrossRef]
- Guerville, M.; Boudry, G. Gastrointestinal and Hepatic Mechanisms Limiting Entry and Dissemination of Lipopolysaccharide into the Systemic Circulation. Am. J. Physiol. -Gastrointest. Liver Physiol. 2016, 311, G1–G15. [Google Scholar] [CrossRef] [Green Version]
- Kell, D.B.; Pretorius, E. On the Translocation of Bacteria and Their Lipopolysaccharides between Blood and Peripheral Locations in Chronic, Inflammatory Diseases: The Central Roles of LPS and LPS-Induced Cell Death. Integr. Biol. 2015, 7, 1339–1377. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, M.; Pallot, G.; Jalil, A.; Tavernier, A.; Dusuel, A.; Le Guern, N.; Lagrost, L.; Pais de Barros, J.-P.; Choubley, H.; Bergas, V.; et al. Intra-Abdominal Lipopolysaccharide Clearance and Inactivation in Peritonitis: Key Roles for Lipoproteins and the Phospholipid Transfer Protein. Front. Immunol. 2021, 12, 622935. [Google Scholar] [CrossRef]
- Murch, O.; Collin, M.; Hinds, C.J.; Thiemermann, C. Lipoproteins in Inflammation and Sepsis. I. Basic Science. Intensive Care Med. 2007, 33, 13–24. [Google Scholar] [CrossRef]
- Han, Y.-H.; Onufer, E.J.; Huang, L.-H.; Sprung, R.W.; Davidson, W.S.; Czepielewski, R.S.; Wohltmann, M.; Sorci-Thomas, M.G.; Warner, B.W.; Randolph, G.J. Enterically Derived High-Density Lipoprotein Restrains Liver Injury through the Portal Vein. Science 2021, 373, eabe6729. [Google Scholar] [CrossRef]
- Garcia, M.A.; Nelson, W.J.; Chavez, N. Cell–Cell Junctions Organize Structural and Signaling Networks. Cold Spring Harb. Perspect Biol. 2018, 10, a029181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariès, P.; Huet, O. Ileus in the Critically Ill: Causes, Treatment and Prevention. Minerva. Anestesiol. 2020, 86, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, H.; Ono, S.; Mochizuki, H. Role of Translocation of Pathogen-Associated Molecular Patterns in Sepsis. Dig. Surg. 2009, 26, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Haussner, F.; Chakraborty, S.; Halbgebauer, R.; Huber-Lang, M. Challenge to the Intestinal Mucosa During Sepsis. Front. Immunol. 2019, 10, 891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palsson-McDermott, E.M.; O’Neill, L.A.J. Signal Transduction by the Lipopolysaccharide Receptor, Toll-like Receptor-4. Immunology 2004, 113, 153–162. [Google Scholar] [CrossRef]
- Rosadini, C.V.; Kagan, J.C. Early Innate Immune Responses to Bacterial LPS. Curr. Opin. Immunol. 2017, 44, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Aluri, J.; Cooper, M.A.; Schuettpelz, L.G. Toll-Like Receptor Signaling in the Establishment and Function of the Immune System. Cells 2021, 10, 1374. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Meseguer, V.; Alpizar, Y.A.; Luis, E.; Tajada, S.; Denlinger, B.; Fajardo, O.; Manenschijn, J.-A.; Fernández-Peña, C.; Talavera, A.; Kichko, T.; et al. TRPA1 Channels Mediate Acute Neurogenic Inflammation and Pain Produced by Bacterial Endotoxins. Nat. Commun. 2014, 5, 3125. [Google Scholar] [CrossRef] [Green Version]
- Alpizar, Y.A.; Boonen, B.; Sanchez, A.; Jung, C.; López-Requena, A.; Naert, R.; Steelant, B.; Luyts, K.; Plata, C.; De Vooght, V.; et al. TRPV4 Activation Triggers Protective Responses to Bacterial Lipopolysaccharides in Airway Epithelial Cells. Nat. Commun. 2017, 8, 1059. [Google Scholar] [CrossRef]
- Mazgaeen, L.; Gurung, P. Recent Advances in Lipopolysaccharide Recognition Systems. IJMS 2020, 21, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathinam, V.A.K.; Zhao, Y.; Shao, F. Innate Immunity to Intracellular LPS. Nat. Immunol. 2019, 20, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Zamyatina, A.; Heine, H. Lipopolysaccharide Recognition in the Crossroads of TLR4 and Caspase-4/11 Mediated Inflammatory Pathways. Front. Immunol. 2020, 11, 585146. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.T.; Xiong, S.; Ye, Z.; Hong, Z.; Di, A.; Tsang, K.M.; Gao, X.; An, S.; Mittal, M.; Vogel, S.M.; et al. Caspase-11–Mediated Endothelial Pyroptosis Underlies Endotoxemia-Induced Lung Injury. J. Clin. Investig. 2017, 127, 4124–4135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Coopersmith, C.M. Dying as a Pathway to Death in Sepsis. Anesthesiology 2018, 129, 238–240. [Google Scholar] [CrossRef]
- Hoste, E.A.J.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of Acute Kidney Injury in Critically Ill Patients: The Multinational AKI-EPI Study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Uchino, S. Acute Renal Failure in Critically Ill PatientsA Multinational, Multicenter Study. JAMA 2005, 294, 813. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.-L.; Sakr, Y.; Sprung, C.L.; Ranieri, V.M.; Reinhart, K.; Gerlach, H.; Moreno, R.; Carlet, J.; Le Gall, J.-R.; Payen, D. Sepsis in European Intensive Care Units: Results of the SOAP Study. Crit. Care Med. 2006, 34, 344–353. [Google Scholar] [CrossRef]
- Kellum, J.A.; Chawla, L.S.; Keener, C.; Singbartl, K.; Palevsky, P.M.; Pike, F.L.; Yealy, D.M.; Huang, D.T.; Angus, D.C. The Effects of Alternative Resuscitation Strategies on Acute Kidney Injury in Patients with Septic Shock. Am. J. Respir. Crit. Care Med. 2016, 193, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Fenhammar, J.; Rundgren, M.; Forestier, J.; Kalman, S.; Eriksson, S.; Frithiof, R. Toll-Like Receptor 4 Inhibitor TAK-242 Attenuates Acute Kidney Injury in Endotoxemic Sheep. Anesthesiology 2011, 114, 1130–1137. [Google Scholar] [CrossRef] [Green Version]
- Anderberg, S.B.; Luther, T.; Frithiof, R. Physiological Aspects of Toll-like Receptor 4 Activation in Sepsis-Induced Acute Kidney Injury. Acta Physiol. 2017, 219, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Kalakeche, R.; Hato, T.; Rhodes, G.; Dunn, K.W.; El-Achkar, T.M.; Plotkin, Z.; Sandoval, R.M.; Dagher, P.C. Endotoxin Uptake by S1 Proximal Tubular Segment Causes Oxidative Stress in the Downstream S2 Segment. J. Am. Soc. Nephrol. 2011, 22, 1505–1516. [Google Scholar] [CrossRef] [PubMed]
- Ince, C.; Mayeux, P.R.; Nguyen, T.; Gomez, H.; Kellum, J.A.; Ospina-Tascón, G.A.; Hernandez, G.; Murray, P.; De Backer, D. The Endothelium in Sepsis. Shock 2016, 45, 259–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czaikoski, P.G.; Mota, J.M.S.C.; Nascimento, D.C.; Sônego, F.; Castanheira, F.V.e.S.; Melo, P.H.; Scortegagna, G.T.; Silva, R.L.; Barroso-Sousa, R.; Souto, F.O.; et al. Neutrophil Extracellular Traps Induce Organ Damage during Experimental and Clinical Sepsis. PLoS ONE 2016, 11, e0148142. [Google Scholar] [CrossRef] [Green Version]
- Gomez, H.; Ince, C.; De Backer, D.; Pickkers, P.; Payen, D.; Hotchkiss, J.; Kellum, J.A. A Unified Theory of Sepsis-Induced Acute Kidney Injury: Inflammation, Microcirculatory Dysfunction, Bioenergetics, and the Tubular Cell Adaptation to Injury. Shock 2014, 41, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Boyd, J.; Mathur, S.; Wang, Y.; Bateman, R.; Walley, K. Toll-like Receptor Stimulation in Cardiomyoctes Decreases Contractility and Initiates an NF-ΚB Dependent Inflammatory Response. Cardiovasc. Res. 2006, 72, 384–393. [Google Scholar] [CrossRef]
- Suffredini, A.F.; Fromm, R.E.; Parker, M.M.; Brenner, M.; Kovacs, J.A.; Wesley, R.A.; Parrillo, J.E. The Cardiovascular Response of Normal Humans to the Administration of Endotoxin. N. Engl. J. Med. 1989, 321, 280–287. [Google Scholar] [CrossRef]
- Martin, L.; Derwall, M.; Al Zoubi, S.; Zechendorf, E.; Reuter, D.A.; Thiemermann, C.; Schuerholz, T. The Septic Heart. Chest 2019, 155, 427–437. [Google Scholar] [CrossRef]
- Li, W.; Deng, M.; Loughran, P.A.; Yang, M.; Lin, M.; Yang, C.; Gao, W.; Jin, S.; Li, S.; Cai, J.; et al. LPS Induces Active HMGB1 Release From Hepatocytes Into Exosomes Through the Coordinated Activities of TLR4 and Caspase-11/GSDMD Signaling. Front. Immunol. 2020, 11, 229. [Google Scholar] [CrossRef]
- Deng, M.; Tang, Y.; Li, W.; Wang, X.; Zhang, R.; Zhang, X.; Zhao, X.; Liu, J.; Tang, C.; Liu, Z.; et al. The Endotoxin Delivery Protein HMGB1 Mediates Caspase-11-Dependent Lethality in Sepsis. Immunity 2018, 49, 740–753.e7. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, Y. Neutrophil-Induced Liver Injury and Interactions Between Neutrophils and Liver Sinusoidal Endothelial Cells. Inflammation 2021, 44, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Danner, R.L.; Elin, R.J.; Hosseini, J.M.; Wesley, R.A.; Reilly, J.M.; Parillo, J.E. Endotoxemia in Human Septic Shock. Chest 1991, 99, 169–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, J.C.; Foster, D.; Vincent, J.; Cook, D.J.; Cohen, J.; Dellinger, R.P.; Opal, S.; Abraham, E.; Brett, S.J.; Smith, T.; et al. Diagnostic and Prognostic Implications of Endotoxemia in Critical Illness: Results of the MEDIC Study. J. Infect. Dis. 2004, 190, 527–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, D.J.; Derzko, A.; Foster, D.; Seely, A.J.E.; Brunet, F.; Romaschin, A.D.; Marshall, J.C. Daily Varialtion in Endotoxin Levels Is Associated with Increased Organ Failure in Critically Ill Patients. Shock 2007, 28, 524–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamik, B.; Smiechowicz, J.; Jakubczyk, D.; Kübler, A. Elevated Serum PCT in Septic Shock With Endotoxemia Is Associated With a Higher Mortality Rate. Medicine 2015, 94, e1085. [Google Scholar] [CrossRef]
- Valenza, F.; Fagnani, L.; Coppola, S.; Froio, S.; Sacconi, F.; Tedesco, C.; Maffioletti, M.; Pizzocri, M.; Salice, V.; Ranzi, M.; et al. Prevalence of Endotoxemia after Surgery and Its Association with ICU Length of Stay. Crit. Care 2009, 13, R102. [Google Scholar] [CrossRef] [Green Version]
- Opal, S.M.; Scannon, P.J.; Vincent, J.; White, M.; Carroll, S.F.; Palardy, J.E.; Parejo, N.A.; Pribble, J.P.; Lemke, J.H. Relationship between Plasma Levels of Lipopolysaccharide (LPS) and LPS-Binding Protein in Patients with Severe Sepsis and Septic Shock. J. Infect. Dis. 1999, 180, 1584–1589. [Google Scholar] [CrossRef] [Green Version]
- Klein, D.J.; Foster, D.; Walker, P.M.; Bagshaw, S.M.; Mekonnen, H.; Antonelli, M. Polymyxin B Hemoperfusion in Endotoxemic Septic Shock Patients without Extreme Endotoxemia: A Post Hoc Analysis of the EUPHRATES Trial. Intensive Care Med. 2018, 44, 2205–2212. [Google Scholar] [CrossRef] [Green Version]
- Adamik, B.; Zielinski, S.; Smiechowicz, J.; Kübler, A. Endotoxin Elimination in Patients with Septic Shock: An Observation Study. Arch. Immunol. Ther. Exp. 2015, 63, 475–483. [Google Scholar] [CrossRef] [Green Version]
- Yoseph, B.P.; Klingensmith, N.J.; Liang, Z.; Breed, E.R.; Burd, E.M.; Mittal, R.; Dominguez, J.A.; Petrie, B.; Ford, M.L.; Coopersmith, C.M. Mechanisms of Intestinal Barrier Dysfunction in Sepsis. Shock 2016, 46, 52–59. [Google Scholar] [CrossRef]
- Adamik, B.; Kübler, A.; Gozdzik, A.; Gozdzik, W. Prolonged Cardiopulmonary Bypass Is a Risk Factor for Intestinal Ischaemic Damage and Endotoxaemia. Heart Lung Circ. 2017, 26, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Assimakopoulos, S.F.; Triantos, C.; Thomopoulos, K.; Fligou, F.; Maroulis, I.; Marangos, M.; Gogos, C.A. Gut-Origin Sepsis in the Critically Ill Patient: Pathophysiology and Treatment. Infection 2018, 46, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Fink, M.P.; Delude, R.L. Epithelial Barrier Dysfunction: A Unifying Theme to Explain the Pathogenesis of Multiple Organ Dysfunction at the Cellular Level. Crit. Care Clin. 2005, 21, 177–196. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, R.V.; Straube, R.C.; Sanders, C.; Smith, S.M.; Smith, C.R. Treatment of Septic Shock with Human Monoclonal Antibody HA-1A: A Randomized, Double-Blind, Placebo-Controlled Trial. Ann. Intern. Med. 1994, 121, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C. E5 Murine Monoclonal Antiendotoxin Antibody in Gram-Negative SepsisA Randomized Controlled Trial. JAMA 2000, 283, 1723. [Google Scholar] [CrossRef]
- Phillip Dellinger, R.; Tomayko, J.F.; Angus, D.C.; Opal, S.; Cupo, M.A.; McDermott, S.; Ducher, A.; Calandra, T.; Cohen, J. Efficacy and Safety of a Phospholipid Emulsion (GR270773) in Gram-Negative Severe Sepsis: Results of a Phase II Multicenter, Randomized, Placebo-Controlled, Dose-Finding Clinical Trial. Crit. Care Med. 2009, 37, 2929–2938. [Google Scholar] [CrossRef]
- Levin, M.; Quint, P.A.; Goldstein, B.; Barton, P.; Bradley, J.S.; Shemie, S.; Yeh, T.; Kim, S.S.; Cafaro, D.P.; Scannon, P.J.; et al. Recombinant Bactericidal/Permeability-Increasing Protein (RBPI21) as Adjunctive Treatment for Children with Severe Meningococcal Sepsis: A Randomised Trial. Lancet 2000, 356, 961–967. [Google Scholar] [CrossRef]
- Opal, S.M.; Laterre, P.-F.; Francois, B.; LaRosa, S.P.; Angus, D.C.; Mira, J.-P.; Wittebole, X.; Dugernier, T.; Perrotin, D.; Tidswell, M.; et al. Effect of Eritoran, an Antagonist of MD2-TLR4, on Mortality in Patients With Severe Sepsis: The ACCESS Randomized Trial. JAMA 2013, 309, 1154. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Shoji, H.; Koga, N. Endotoxin Adsorption by Polymyxin B Immobilized Fiber Column in Patients with Systemic Inflammatory Response Syndrome: The Japanese Experience. Ther. Apher. Dial. 2003, 7, 252–258. [Google Scholar] [CrossRef]
- Shimizu, T.; Miyake, T.; Kitamura, N.; Tani, M.; Endo, Y. Endotoxin Adsorption: Direct Hemoperfusion with the Polymyxin B-Immobilized Fiber Column (PMX). Transfus. Apher. Sci. 2017, 56, 682–688. [Google Scholar] [CrossRef]
- Cruz, D.N.; Perazella, M.A.; Bellomo, R.; de Cal, M.; Polanco, N.; Corradi, V.; Lentini, P.; Nalesso, F.; Ueno, T.; Ranieri, V.M.; et al. Effectiveness of Polymyxin B-Immobilized Fiber Column in Sepsis: A Systematic Review. Crit. Care 2007, 11, R47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, J.-L.; Laterre, P.-F.; Cohen, J.; Burchardi, H.; Bruining, H.; Lerma, F.A.; Wittebole, X.; De Backer, D.; Brett, S.; Marzo, D.; et al. A Pilot-Controlled Study of a Polymyxin B-Immobilized Hemoperfusion Cartridge in Patients with Severe Sepsis Secondary to Intra-Abdominal Infection. Shock 2005, 23, 400–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz, D.N.; Antonelli, M.; Fumagalli, R.; Foltran, F.; Brienza, N.; Donati, A.; Malcangi, V.; Petrini, F.; Volta, G.; Bobbio Pallavicini, F.M.; et al. Early Use of Polymyxin B Hemoperfusion in Abdominal Septic Shock: The EUPHAS Randomized Controlled Trial. JAMA 2009, 301, 2445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, J.-L. Polymyxin B Hemoperfusion and Mortality in Abdominal Septic Shock. JAMA 2009, 302, 1968. [Google Scholar] [CrossRef]
- Pickkers, P.; Russell, J.A. Treatment with a Polymyxin B Filter to Capture Endotoxin in Sepsis Patients: Is There a Signal for Therapeutic Efficacy? Intensive Care Med. 2019, 45, 282–283. [Google Scholar] [CrossRef] [Green Version]
- The ABDOMIX Group; Payen, D.M.; Guilhot, J.; Launey, Y.; Lukaszewicz, A.C.; Kaaki, M.; Veber, B.; Pottecher, J.; Joannes-Boyau, O.; Martin-Lefevre, L.; et al. Early Use of Polymyxin B Hemoperfusion in Patients with Septic Shock Due to Peritonitis: A Multicenter Randomized Control Trial. Intensive Care Med. 2015, 41, 975–984. [Google Scholar] [CrossRef] [Green Version]
- Antonelli, M.; Cutuli, S.L.; Ronco, C. Polymyxin B Hemoperfusion in Septic Shock: Just Look at the Evidence! Intensive Care Med. 2015, 41, 1731–1732. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Bagshaw, S.M.; Antonelli, M.; Foster, D.M.; Klein, D.J.; Marshall, J.C.; Palevsky, P.M.; Weisberg, L.S.; Schorr, C.A.; Trzeciak, S.; et al. Effect of Targeted Polymyxin B Hemoperfusion on 28-Day Mortality in Patients With Septic Shock and Elevated Endotoxin Level: The EUPHRATES Randomized Clinical Trial. JAMA 2018, 320, 1455. [Google Scholar] [CrossRef] [Green Version]
- Romaschin, A.D.; Obiezu-Forster, C.V.; Shoji, H.; Klein, D.J. Novel Insights into the Direct Removal of Endotoxin by Polymyxin B Hemoperfusion. Blood Purif. 2017, 44, 193–197. [Google Scholar] [CrossRef]
- Fujii, T.; Ganeko, R.; Kataoka, Y.; Furukawa, T.A.; Featherstone, R.; Doi, K.; Vincent, J.-L.; Pasero, D.; Robert, R.; Ronco, C.; et al. Polymyxin B-Immobilized Hemoperfusion and Mortality in Critically Ill Adult Patients with Sepsis/Septic Shock: A Systematic Review with Meta-Analysis and Trial Sequential Analysis. Intensive Care Med. 2018, 44, 167–178. [Google Scholar] [CrossRef]
- Kuriyama, A.; Katsura, M.; Urushidani, S.; Takada, T. Impact of Polymyxin B Hemoperfusion in the Treatment of Patients with Sepsis and Septic Shock: A Meta-Analysis of Randomized Controlled Trials. Ann. Transl. Med. 2018, 6, 206. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Tu, Y.-K.; Lee, C.-T.; Chao, A.; Huang, C.-H.; Wang, M.-J.; Yeh, Y.-C. Effects of Polymyxin B Hemoperfusion on Mortality in Patients With Severe Sepsis and Septic Shock: A Systemic Review, Meta-Analysis Update, and Disease Severity Subgroup Meta-Analysis. Crit. Care Med. 2017, 45, e858–e864. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, C.; Mao, Z.; Qi, S.; Song, R.; Zhou, F. Effectiveness of Polymyxin B-Immobilized Hemoperfusion against Sepsis and Septic Shock: A Systematic Review and Meta-Analysis. J. Crit. Care 2021, 63, 187–195. [Google Scholar] [CrossRef] [PubMed]
- The EUPHAS 2 Collaborative Group; Cutuli, S.L.; Artigas, A.; Fumagalli, R.; Monti, G.; Ranieri, V.M.; Ronco, C.; Antonelli, M. Polymyxin-B Hemoperfusion in Septic Patients: Analysis of a Multicenter Registry. Ann. Intensive Care 2016, 6, 77. [Google Scholar] [CrossRef] [Green Version]
- Iwagami, M.; Yasunaga, H.; Noiri, E.; Horiguchi, H.; Fushimi, K.; Matsubara, T.; Yahagi, N.; Nangaku, M.; Doi, K. Potential Survival Benefit of Polymyxin B Hemoperfusion in Septic Shock Patients on Continuous Renal Replacement Therapy: A Propensity-Matched Analysis. Blood Purif. 2016, 42, 9–17. [Google Scholar] [CrossRef]
- Iwagami, M.; Yasunaga, H.; Doi, K.; Horiguchi, H.; Fushimi, K.; Matsubara, T.; Yahagi, N.; Noiri, E. Postoperative Polymyxin B Hemoperfusion and Mortality in Patients with Abdominal Septic Shock: A Propensity-Matched Analysis. Crit. Care Med. 2014, 42, 1187–1193. [Google Scholar] [CrossRef]
- Sirivongrangson, P.; Kulvichit, W.; Payungporn, S.; Pisitkun, T.; Chindamporn, A.; Peerapornratana, S.; Pisitkun, P.; Chitcharoen, S.; Sawaswong, V.; Worasilchai, N.; et al. Endotoxemia and Circulating Bacteriome in Severe COVID-19 Patients. Intensive Care Med. Exp. 2020, 8, 72. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Wimmers, F.; Mok, C.K.P.; Perera, R.A.P.M.; Scott, M.; Hagan, T.; Sigal, N.; Feng, Y.; Bristow, L.; Tak-Yin Tsang, O.; et al. Systems Biological Assessment of Immunity to Mild versus Severe COVID-19 Infection in Humans. Science 2020, 369, 1210–1220. [Google Scholar] [CrossRef]
- Khan, S.; Bolotova, O.; Sahib, H.; Foster, D.; Mallipattu, S.K. Endotoxemia in Critically Ill Patients with COVID-19. Blood Purif. 2021, 1–7. [Google Scholar] [CrossRef]
- Assimakopoulos, S.F.; Mastronikolis, S.; De Lastic, A.-L.; Aretha, D.; Papageorgiou, D.; Chalkidi, T.; Oikonomou, I.; Triantos, C.; Mouzaki, A.; Marangos, M. Intestinal Barrier Biomarker ZO1 and Endotoxin Are Increased in Blood of Patients With COVID-19-Associated Pneumonia. In Vivo 2021, 35, 2483–2488. [Google Scholar] [CrossRef]
- Oliva, A.; Cammisotto, V.; Cangemi, R.; Ferro, D.; Miele, M.C.; De Angelis, M.; Cancelli, F.; Pignatelli, P.; Venditti, M.; Pugliese, F.; et al. Low-Grade Endotoxemia and Thrombosis in COVID-19. Clin. Transl. Gastroenterol. 2021, 12, e00348. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Cutuli, S.L.; Ferrer, R.; Antonelli, M.; Ronco, C.; The COVID-19 EUPHAS2 Collaborative Group. Polymyxin B Hemoperfusion in Coronavirus Disease 2019 Patients with Endotoxic Shock: Case Series from EUPHAS2 Registry. Artif. Organs 2021, 45, E187–E194. [Google Scholar] [CrossRef] [PubMed]
- Peerapornratana, S.; Sirivongrangson, P.; Tungsanga, S.; Tiankanon, K.; Kulvichit, W.; Putcharoen, O.; Kellum, J.A.; Srisawat, N. Endotoxin Adsorbent Therapy in Severe COVID-19 Pneumonia. Blood Purif. 2021, 51, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, D.; Ishikane, M.; Asai, Y.; Izumi, S.; Takasaki, J.; Katsuoka, H.; Kondo, I.; Ide, S.; Nakamura, K.; Nakamoto, T.; et al. Direct Hemoperfusion Using a Polymyxin B-immobilized Polystyrene Column for COVID-19. J. Clin. Apher. 2021, 36, 313–321. [Google Scholar] [CrossRef]
- Takeyama, N.; Noguchi, H.; Hirakawa, A.; Kano, H.; Morino, K.; Obata, T.; Sakamoto, T.; Tamai, F.; Ishikura, H.; Kase, Y.; et al. Time to Initiation of Treatment with Polymyxin B Cartridge Hemoperfusion in Septic Shock Patients. Blood Purif. 2012, 33, 252–256. [Google Scholar] [CrossRef]
- Chihara, S.; Masuda, Y.; Tatsumi, H.; Nakano, K.; Shimada, T.; Murohashi, T.; Yamakage, M. Early Induction of Direct Hemoperfusion with a Polymyxin-B Immobilized Column Is Associated with Amelioration of Hemodynamic Derangement and Mortality in Patients with Septic Shock. J. Artif. Organs 2017, 20, 71–75. [Google Scholar] [CrossRef]
- Tanaka, T.; Tabata, T.; Fujino, K.; Tsujita, Y.; Eguchi, Y. Impact of Timing of Polymyxin B-immobilized Fiber Column Direct Hemoperfusion on Outcome in Patients with Septic Shock: A Single-center Observational Study. Acute Med. Surg. 2020, 7, e446. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, C.; Hara, Y.; Kuriyama, N.; Nakamura, T.; Nishida, O. Clinical Effects of a Longer Duration of Polymyxin B-Immobilized Fiber Column Direct Hemoperfusion Therapy for Severe Sepsis and Septic Shock: Longer Duration of PMX-DHP Therapy. Apher. Dial. 2015, 19, 316–323. [Google Scholar] [CrossRef]
- Miyamoto, K.; Kawazoe, Y.; Kato, S. Prolonged Direct Hemoperfusion Using a Polymyxin B Immobilized Fiber Cartridge Provides Sustained Circulatory Stabilization in Patients with Septic Shock: A Retrospective Observational before-after Study. J. Intensive Care 2017, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- Mitaka, C.; Kusao, M.; Kawagoe, I.; Satoh, D.; Iba, T.; Ronco, C. Impact of Extended Duration of Polymyxin B-Immobilized Fiber Column Direct Hemoperfusion on Hemodynamics, Vasoactive Substance Requirement, and Pulmonary Oxygenation in Patients with Sepsis: An Observational Study. Blood Purif. 2021, 51, 62–69. [Google Scholar] [CrossRef]
- Novelli, G.; Ferretti, G.; Ruberto, F.; Morabito, V.; Pugliese, F. Early Management of Endotoxemia Using the Endotoxin Activity Assay and Polymyxin B-Based Hemoperfusion. In Contributions to Nephrology; Ronco, C., Piccinni, P., Rosner, M.H., Eds.; KARGER: Basel, Switzerland, 2010; Volume 167, pp. 91–101. ISBN 978-3-8055-9484-4. [Google Scholar]
- Rachoin, J.-S.; Foster, D.; Giese, R.; Weisberg, L.S.; Klein, D.J. Importance of Endotoxin Clearance in Endotoxemic Septic Shock: An Analysis from the Evaluating Use of PolymyxinB Hemoperfusion in a Randomized Controlled Trial of Adults Treated for Endotoxemic Septic Shock (EUPHRATES) Trial. Crit. Care Explor. 2020, 2, e0083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śmiechowicz, J. The Rationale and Current Status of Endotoxin Adsorption in the Treatment of Septic Shock. J. Clin. Med. 2022, 11, 619. https://doi.org/10.3390/jcm11030619
Śmiechowicz J. The Rationale and Current Status of Endotoxin Adsorption in the Treatment of Septic Shock. Journal of Clinical Medicine. 2022; 11(3):619. https://doi.org/10.3390/jcm11030619
Chicago/Turabian StyleŚmiechowicz, Jakub. 2022. "The Rationale and Current Status of Endotoxin Adsorption in the Treatment of Septic Shock" Journal of Clinical Medicine 11, no. 3: 619. https://doi.org/10.3390/jcm11030619
APA StyleŚmiechowicz, J. (2022). The Rationale and Current Status of Endotoxin Adsorption in the Treatment of Septic Shock. Journal of Clinical Medicine, 11(3), 619. https://doi.org/10.3390/jcm11030619





