Retrospective Analysis of Sterile Corneal Infiltrates in Patients with Keratoconus after Cross-Linking Procedure
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Examinations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rabinowitz, Y.S. Keratoconus. Surv. Ophthalmol. 1998, 42, 297–319. [Google Scholar] [CrossRef]
- Wollensak, G.; Spoerl, E.; Seiler, T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am. J. Ophthalmol. 2003, 135, 620–627. [Google Scholar] [CrossRef]
- Wollensak, G.; Spoerl, E.; Seiler, T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J. Cataract. Refract. Surg. 2003, 29, 1780–1785. [Google Scholar] [CrossRef]
- Gogri, P.; Parkar, M.; Bhalerao, S.A. Visual outcomes of sterile corneal infiltrates after photorefractive keratectomy. Indian J. Ophthalmol. 2020, 68, 2956–2959. [Google Scholar] [CrossRef]
- Al-Amry, M.A. Severe bilateral paralimbal sterile infiltrates after photorefractive keratectomy. Middle East Afr. J. Ophthalmol. 2014, 21, 83–85. [Google Scholar] [CrossRef]
- Moon, S.W.; Kim, Y.H.; Lee, S.C.; Lee, M.A.; Jin, K.H. Bilateral peripheral infiltrative keratitis after LASIK. Korean J. Ophthalmol. 2007, 21, 172–174. [Google Scholar] [CrossRef][Green Version]
- Angunawela, R.I.; Arnalich-Montiel, F.; Allan, B.D. Peripheral sterile corneal infiltrates and melting after collagen crosslinking for keratoconus. J. Cataract. Refract. Surg. 2009, 35, 606–607. [Google Scholar] [CrossRef]
- Çakmak, S.; Sucu, M.E.; Yildirim, Y.; Kepez Yildiz, B.; Kirgiz, A.; Bektaşoğlu, D.L.; Demirok, A. Complications of accelerated corneal collagen cross-linking: Review of 2025 eyes. Int. Ophthalmol. 2020, 40, 3269–3277. [Google Scholar] [CrossRef]
- Ghanem, R.C.; Netto, M.V.; Ghanem, V.C.; Santhiago, M.R.; Wilson, S.E. Peripheral sterile corneal ring infiltrate after riboflavin-UVA collagen cross-linking in keratoconus. Cornea 2012, 31, 702–705. [Google Scholar] [CrossRef]
- Lam, F.C.; Georgoudis, P.; Nanavaty, M.A.; Khan, S.; Lake, D. Sterile keratitis after combined riboflavin-UVA corneal collagen cross-linking for keratoconus. Eye 2014, 28, 1297–1303. [Google Scholar] [CrossRef]
- Çerman, E.; Özcan, D.Ö.; Toker, E. Sterile corneal infiltrates after corneal collagen cross-linking: Evaluation of risk factors. Acta Ophthalmol. 2017, 95, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Teal, P.; Breslin, C.; Arshinoff, S.; Edmison, D. Corneal subepithelial infiltrates following excimer laser photorefractive keratectomy. J. Cataract. Refract. Surg. 1995, 21, 516–518. [Google Scholar] [CrossRef]
- Mazzotta, C.; Hafezi, F.; Kymionis, G.; Caragiuli, S.; Jacob, S.; Traversi, C.; Barabino, S.; Randleman, J.B. In Vivo Confocal Microscopy after Corneal Collagen Crosslinking. Ocul. Surf. 2015, 13, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Kodavoor, S.K.; Tiwari, N.N.; Ramamurthy, D. Profile of infectious and sterile keratitis after accelerated corneal collagen cross-linking for keratoconus. Oman J. Ophthalmol. 2020, 13, 18–23. [Google Scholar] [CrossRef]
- Mereaux, D.; Knoeri, J.; Jouve, L.; Laroche, L.; Borderie, V.; Bouheraoua, N. Sterile keratitis following standard corneal collagen crosslinking: A case series and literature review. J. Fr. Ophtalmol. 2019, 42, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Javadi, M.A.; Feizi, S. Sterile Keratitis following Collagen Crosslinking. J. Ophthalmic Vis. Res. 2014, 9, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Jain, P.; Gupta, D.; Goyal, J.L. Sterile keratitis after corneal collagen crosslinking in a child. Cont. Lens. Anterior Eye 2012, 35, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ausín, P.; Gutiérrez-Ortega, R.; Arance-Gil, A.; Romero-Jimenez, M.; Fuentes-Páez, G. Keratopathy after cross-linking for keratoconus. Cornea 2011, 30, 1051–1053. [Google Scholar] [CrossRef]
- Mangioris, G.F.; Papadopoulou, D.N.; Balidis, M.O.; Poulas, J.L.; Papadopoulos, N.T.; Seiler, T. Corneal infiltrates after corneal collagen cross-linking. J. Refract. Surg. 2010, 26, 609–611. [Google Scholar] [CrossRef]
- Koller, T.; Mrochen, M.; Seiler, T. Complication and failure rates after corneal crosslinking. J. Cataract. Refract. Surg. 2009, 35, 1358–1362. [Google Scholar] [CrossRef]
- Koppen, C.; Vryghem, J.C.; Gobin, L.; Tassignon, M.J. Keratitis and corneal scarring after UVA/riboflavin cross-linking for keratoconus. J. Refract. Surg. 2009, 25, S819–S823. [Google Scholar] [CrossRef] [PubMed]
- Camesasca, F.I.; Vinciguerra, P.; Seiler, T. Bilateral ring-shaped intrastromal opacities after corneal cross-linking for keratoconus. J. Refract. Surg. 2011, 27, 913–915. [Google Scholar] [CrossRef] [PubMed]
- Uysal, B.S.; Yaman, D.; Sarac, O.; Akcay, E.; Cagil, N. Sterile keratitis after uneventful corneal collagen cross-linking in a patient with Axenfeld-Rieger syndrome. Int. Ophthalmol. 2019, 39, 1169–1173. [Google Scholar] [CrossRef] [PubMed]
- Ficker, L.; Seal, D.; Wright, P. Staphylococcal infection and the limbus: Study of the cell-mediated immune response. Eye 1989, 3 Pt 2, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Garg, P.; Mullick, R.; Annavajjhala, S. Keratitis following laser refractive surgery: Clinical spectrum, prevention and management. Indian J. Ophthalmol. 2020, 68, 2813–2818. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef]
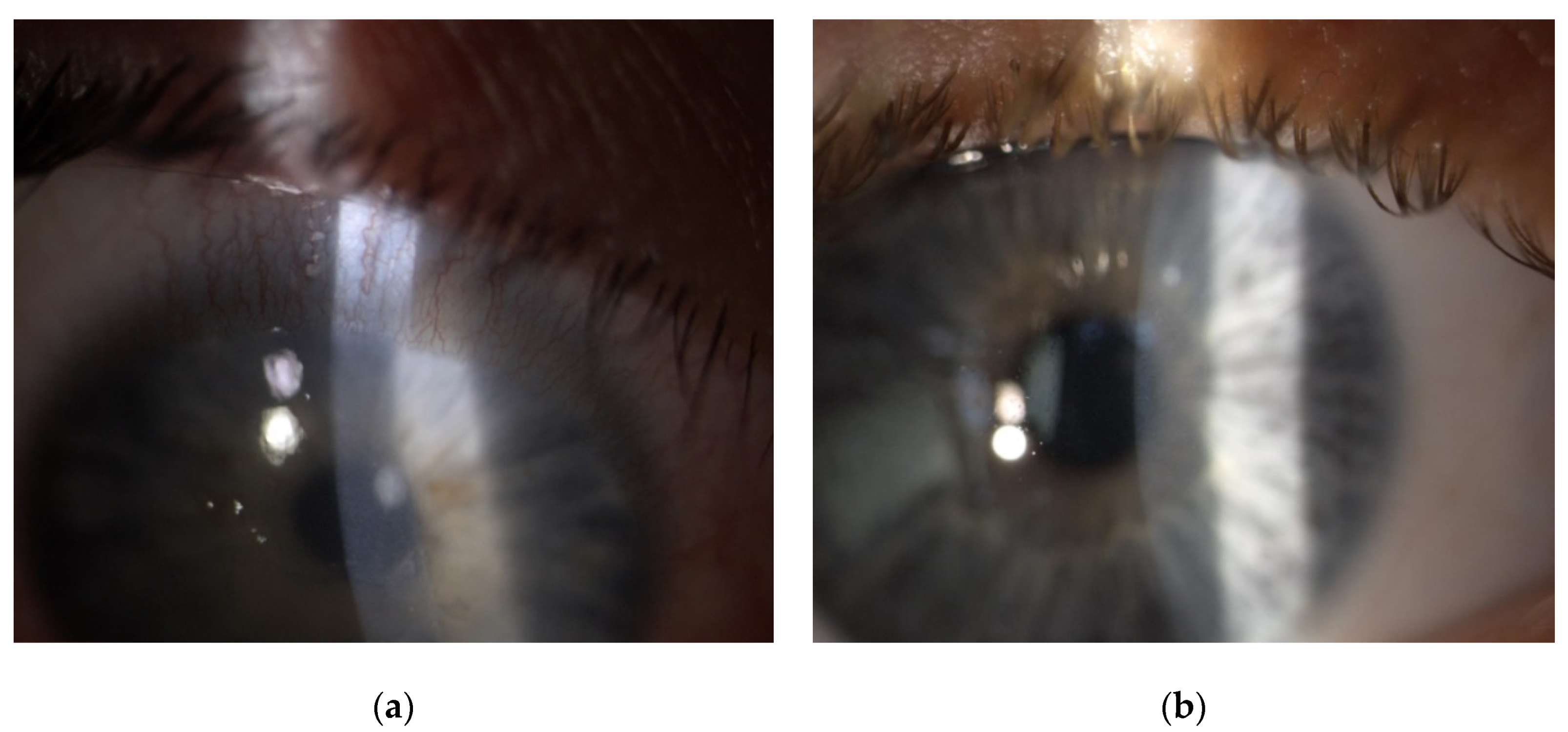
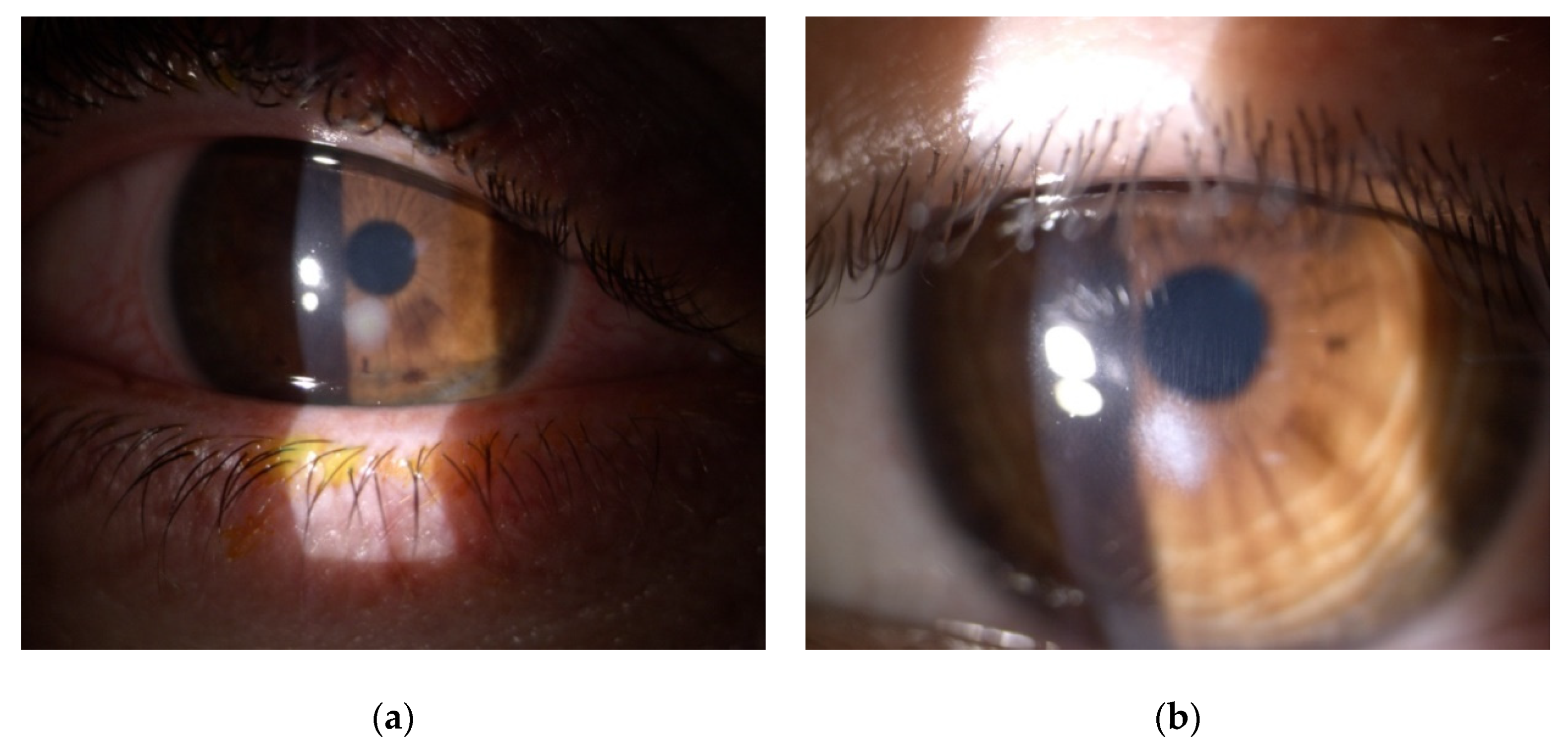
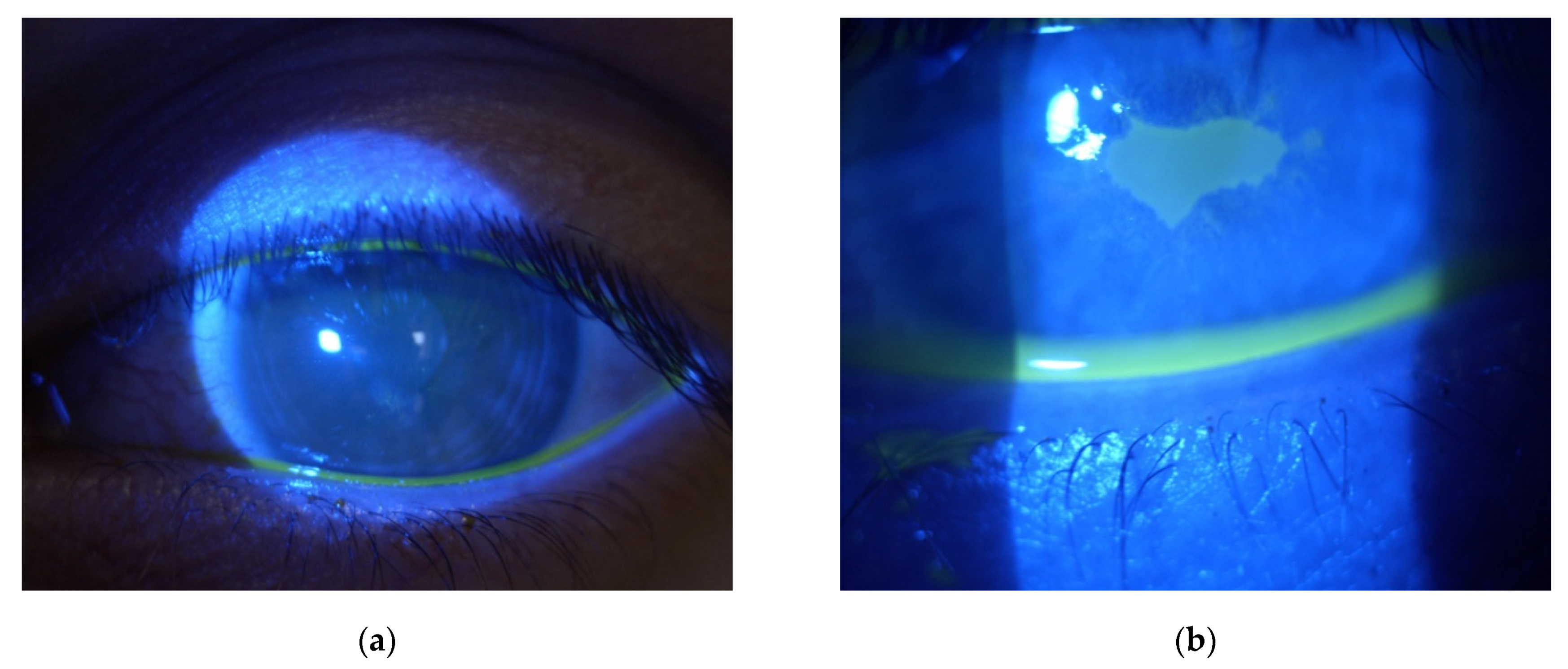
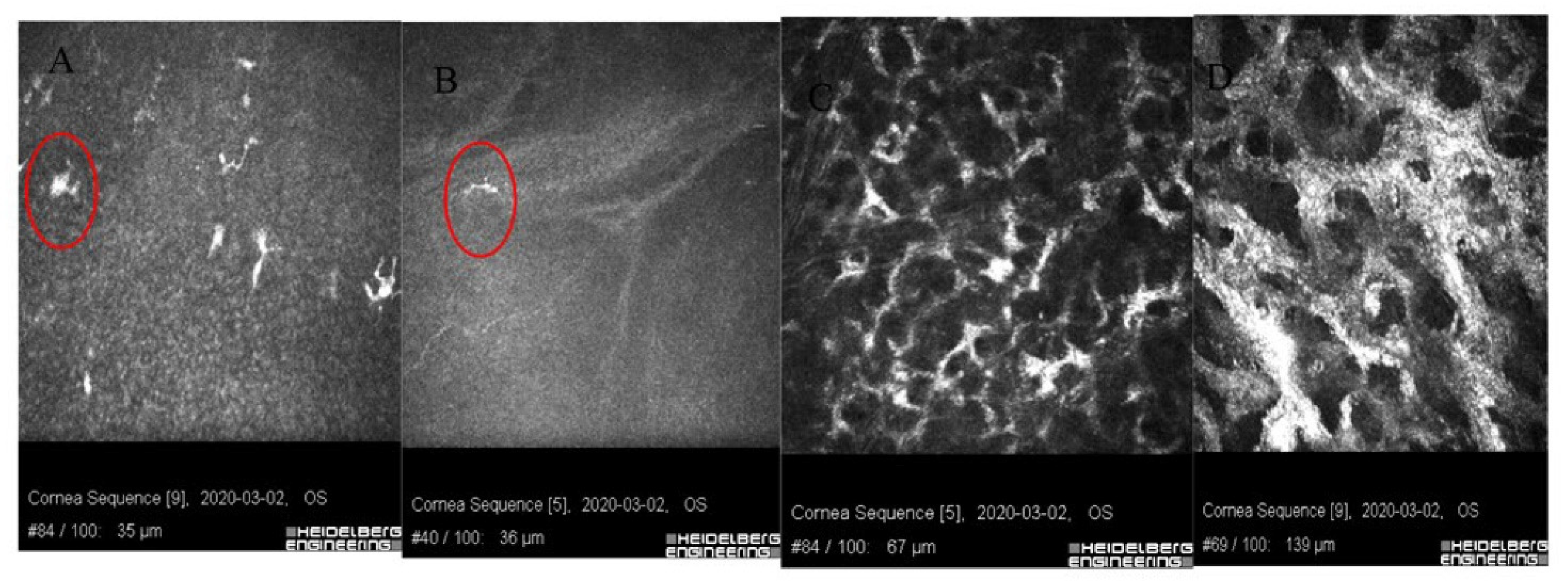


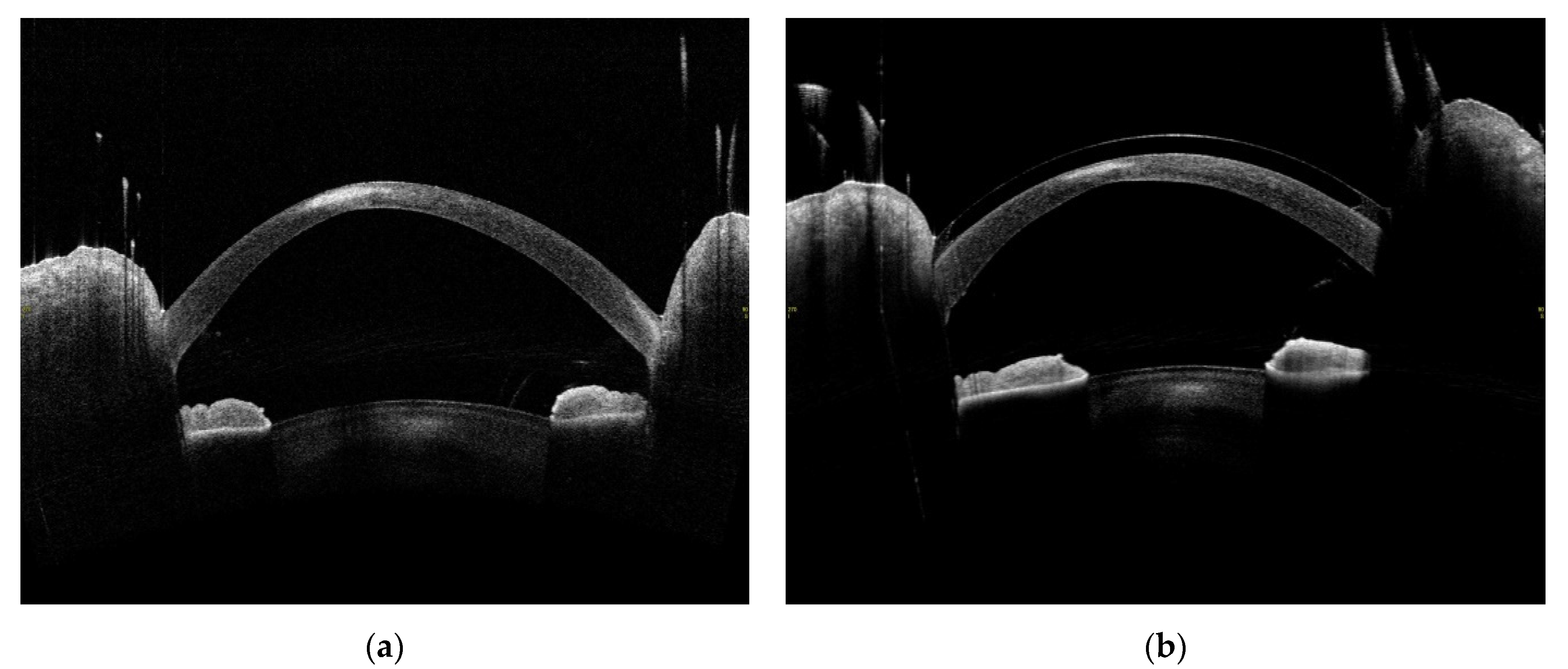
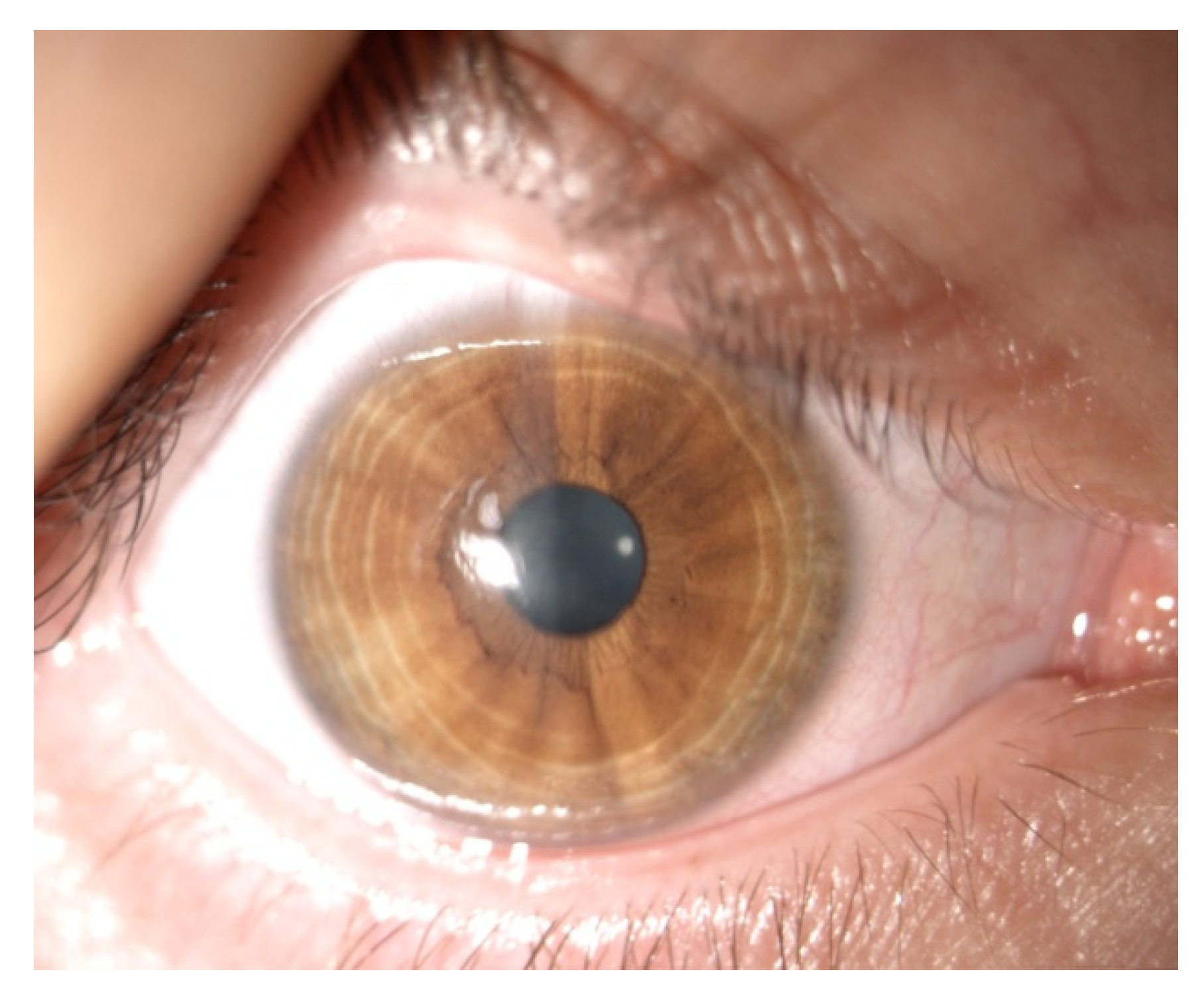
| Patient | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Age | 16 | 17 | 20 | 28 |
| Gender | m | m | m | m |
| Microbiology swab test result | negative | negative | negative | negative |
| Site of infiltrate | paracentral | paracentral | paracentral | paracentral |
| Size of infiltrate (mm) | 1 × 1 | 1 × 1 | 3 × 2.5 | 3 × 2.5 |
| Exacerbation of local symptoms (day) | 3 | 5 | 4 | 5 |
| First check-up(day) | 6 | 7 | 7 | 7 |
| Time of treatment (weeks) | 6 | 4 | 12 | 8 |
| Scar | none | none | present | present |
| Symptoms of allergy, VKC | none | none | none | none |
| Patient | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Degree of keratoconus (Amsler–Krumeich classification) | 3 | 3 | 3–4 | 2–3 |
| Preoperative corneal thickness (µm) max (min) | 475 (449) | 483 (476) | 508 (469) | 561 (518) |
| K1 [D] | 44.1 | 50.1 | 53.2 | 41.3 |
| K2 [D] | 49.0 | 51.8 | 58.6 | 45.5 |
| Kmean [D] | 46.4 | 50.9 | 55.7 | 43.3 |
| Astig [D] | 4.9 | 1.7 | 5.4 | 4.1 |
| BCVA 6 M-postop | 0.3 | 0.3 | 0.6 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krok, M.; Wróblewska-Czajka, E.; Kokot, J.; Micińska, A.; Wylęgała, E.; Dobrowolski, D. Retrospective Analysis of Sterile Corneal Infiltrates in Patients with Keratoconus after Cross-Linking Procedure. J. Clin. Med. 2022, 11, 585. https://doi.org/10.3390/jcm11030585
Krok M, Wróblewska-Czajka E, Kokot J, Micińska A, Wylęgała E, Dobrowolski D. Retrospective Analysis of Sterile Corneal Infiltrates in Patients with Keratoconus after Cross-Linking Procedure. Journal of Clinical Medicine. 2022; 11(3):585. https://doi.org/10.3390/jcm11030585
Chicago/Turabian StyleKrok, Magdalena, Ewa Wróblewska-Czajka, Joanna Kokot, Anna Micińska, Edward Wylęgała, and Dariusz Dobrowolski. 2022. "Retrospective Analysis of Sterile Corneal Infiltrates in Patients with Keratoconus after Cross-Linking Procedure" Journal of Clinical Medicine 11, no. 3: 585. https://doi.org/10.3390/jcm11030585
APA StyleKrok, M., Wróblewska-Czajka, E., Kokot, J., Micińska, A., Wylęgała, E., & Dobrowolski, D. (2022). Retrospective Analysis of Sterile Corneal Infiltrates in Patients with Keratoconus after Cross-Linking Procedure. Journal of Clinical Medicine, 11(3), 585. https://doi.org/10.3390/jcm11030585






