Intensive Postural and Motor Activity Program Reduces Scoliosis Progression in People with Rett Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Outcome Measures
2.3.1. RTT Severity Level
2.3.2. Activity Level
2.3.3. Motor Functioning
2.3.4. Scoliosis Severity
2.4. Procedure
2.5. Statistical Analysis
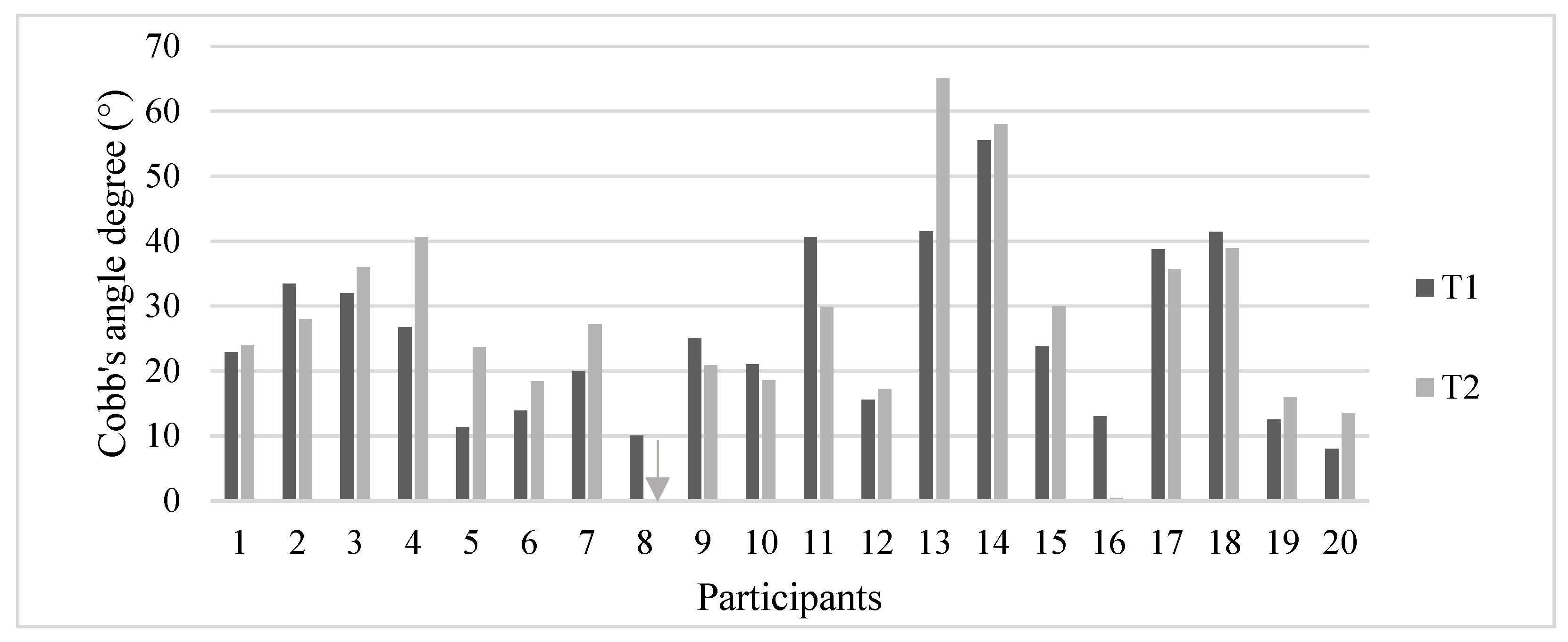
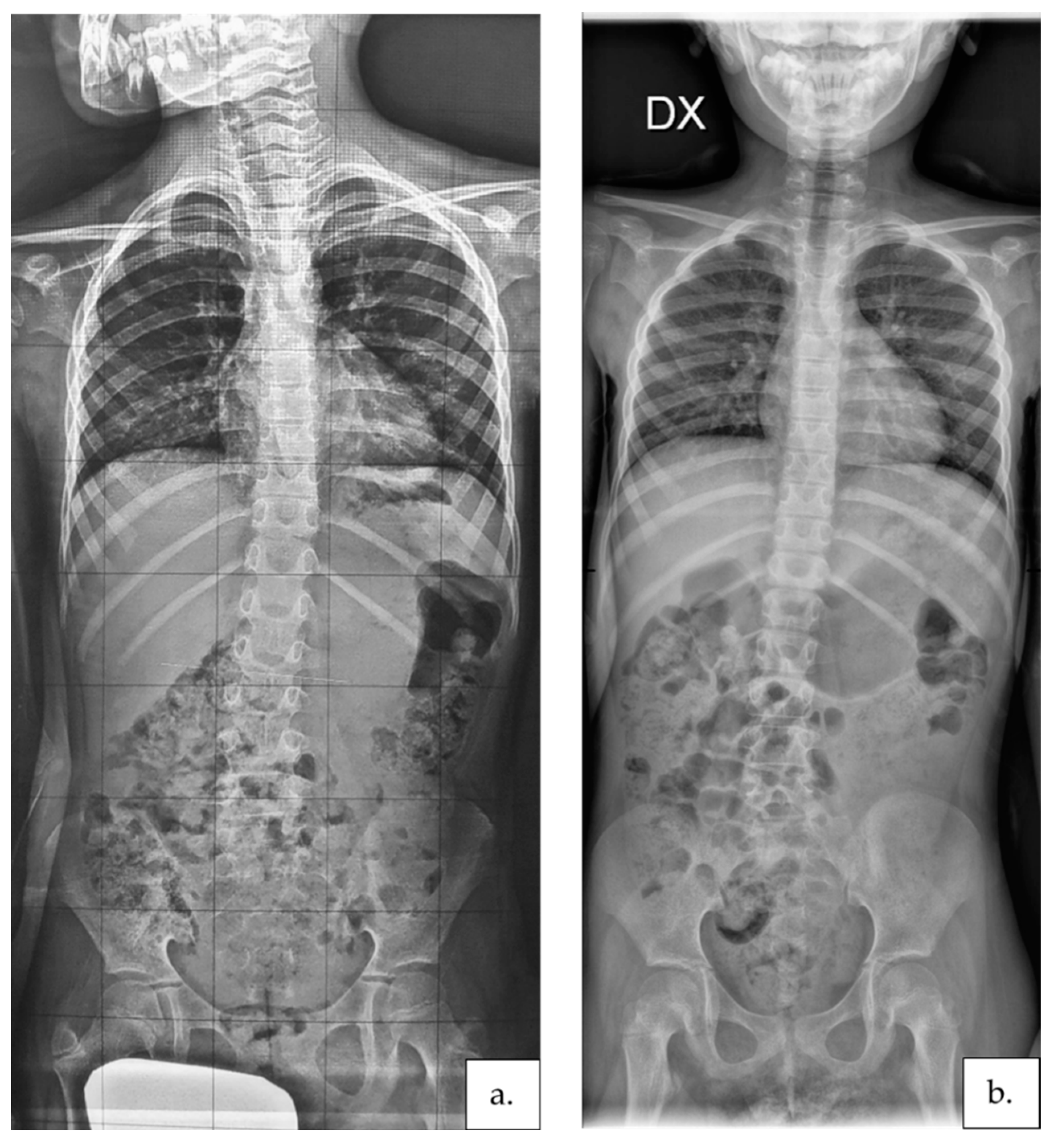
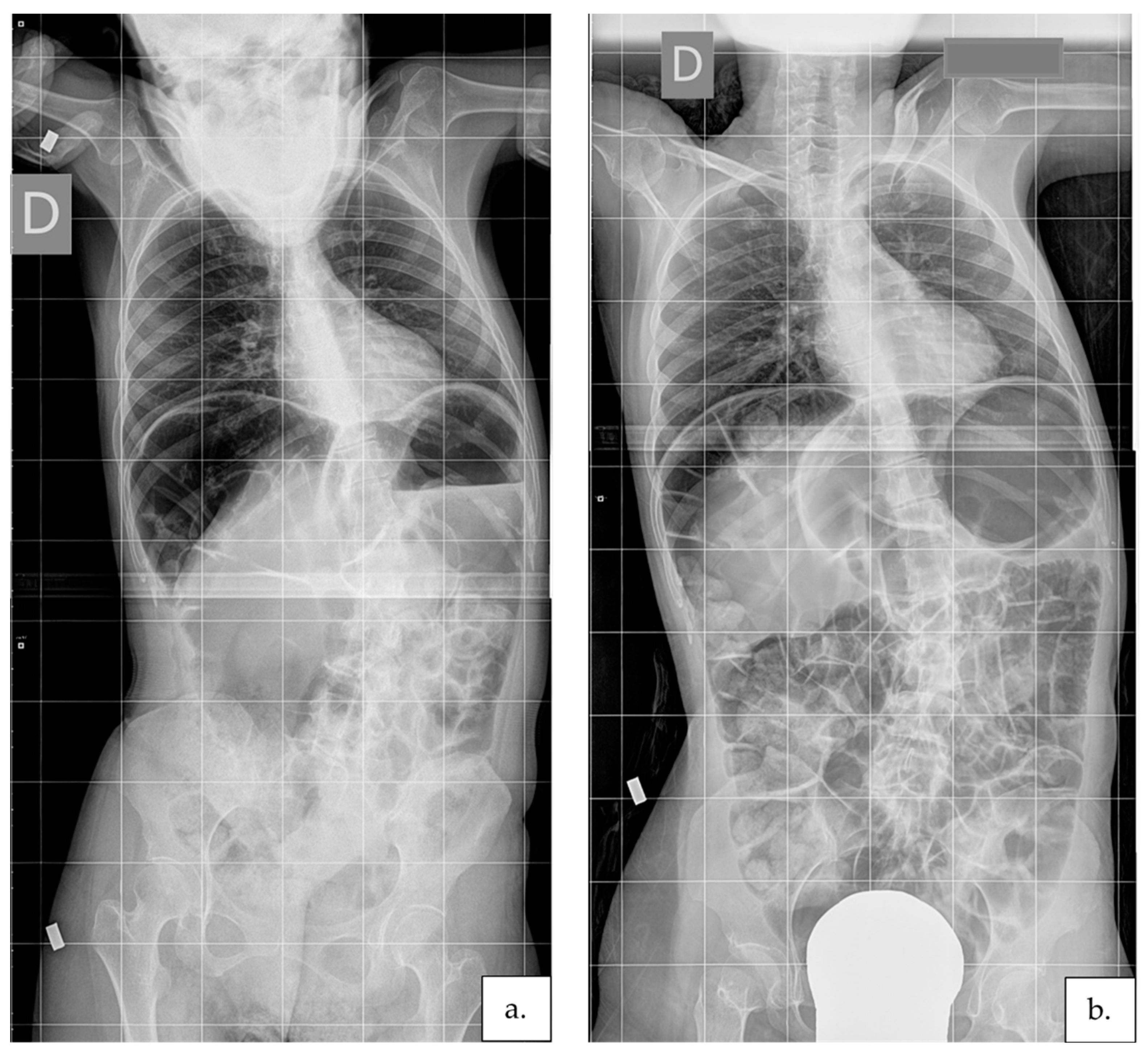
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amir, R.E.; Van den Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [CrossRef]
- Fombonne, E.; Simmons, H.; Ford, T.; Meltzer, H.; Goodman, R. Prevalence of pervasive developmental disorders in the British nationwide survey of child mental health. Int. Rev. Psychiatry 2003, 15, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Skjeldal, O.H.; Von Tetzchner, S.; Aspelund, F.; Herder, G.A.; Lofterød, B. Rett syndrome: Geographic variation in prevalence in Norway. Brain Dev. 1997, 19, 258–261. [Google Scholar] [CrossRef]
- Pini, G.; Milan, M.; Zappella, M. Rett syndrome in Northern Tuscany (Italy): Family tree studies. Clin. Genet. 1996, 50, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Logan, S.W.; Huang, H.H.; Stahlin, K.; Galloway, J.C. Modified ride-on car for mobility and socialization: Single-case study of an infant with down syndrome. Pediatr. Phys. Ther. 2014, 26, 418–4269. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, B.; Witt-Engerström, I.; Opitz, J.M.; Reynolds, J.F. Rett Syndrome: A suggested staging system for describing impairment profile with increasing age towards adolescence. Am. J. Med. Genet. 1986, 25, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Bassett, G.S.; Tolo, V.T. the Incidence and Natural History of Scoliosis in Rett Syndrome. Dev. Med. Child Neurol. 1990, 32, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Ager, S.; Downs, J.; Fyfe, S.; Leonard, H. Parental experiences of scoliosis management in Rett syndrome. Disabil. Rehabil. 2009, 31, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Ager, S.; Fyfe, S.; Christodoulou, J.; Jacoby, P.; Schmitt, L.; Leonard, H. Predictors of scoliosis in Rett syndrome. J. Child Neurol. 2006, 21, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Riise, R.; Brox, J.I.; Sorensen, R.; Skjeldal, O.H. Spinal deformity and disability in patients with Rett syndrome. Dev. Med. Child Neurol. 2011, 53, 653–657. [Google Scholar] [CrossRef]
- Lidström, J.; Stokland, E.; Hagberg, B. Scoliosis in rett syndrome clinical and biological aspects. Spine 1994, 19, 1632–1635. [Google Scholar] [CrossRef] [PubMed]
- Downs, J.; Bergman, A.; Carter, P.; Anderson, A.; Palmer, G.M.; Roye, D.; Van Bosse, H.; Bebbington, A.; Larsson, E.L.; Smith, B.G.; et al. Guidelines for management of scoliosis in rett syndrome patients based on expert consensus and clinical evidence. Spine 2009, 34, E607–E617. [Google Scholar] [CrossRef] [PubMed]
- Percy, A.K.; Lee, H.S.; Neul, J.L.; Lane, J.B.; Skinner, S.A.; Geerts, S.P.; Annese, F.; Graham, J.; McNair, L.; Motil, K.J.; et al. Profiling scoliosis in rett syndrome. Pediatr. Res. 2010, 67, 435–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.J.; Lubicky, J.P.; Hammerberg, K.W. Scoliosis in Rett syndrome. Orthop. Rev. 1994, 23, 931–937. [Google Scholar] [CrossRef]
- Killian, J.T.; Lane, J.B.; Lee, H.S.; Skinner, S.A.; Kaufmann, W.E.; Glaze, D.G.; Neul, J.L.; Percy, A.K. Scoliosis in Rett Syndrome: Progression, Comorbidities, and Predictors. Pediatr. Neurol. 2017, 70, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Harrison, D.J.; Webb, P.J. Scoliosis in the rett syndrome: Natural history and treatment. Brain Dev. 1990, 12, 154–156. [Google Scholar] [CrossRef]
- Downs, J.; Torode, I.; Wong, K.; Ellaway, C.; Elliott, E.J.; Christodoulou, J.; Jacoby, P.; Thomson, M.R.; Izatt, M.T.; Askin, G.N.; et al. The natural history of scoliosis in females with rett syndrome. Spine 2016, 41, 856–863. [Google Scholar] [CrossRef]
- Neul, J.L.; Fang, P.; Barrish, J.; Lane, J.; Caeg, E.B.; Smith, E.O.; Zoghbi, H.; Percy, A.; Glaze, D.G. Specific mutations in methyl-CpG-binding protein 2 confer different severity in Rett syndrome. Neurology 2008, 70, 1313–1321. [Google Scholar] [CrossRef] [Green Version]
- Mehta, J.S.; Gibson, M.J. The Treatment of Neuromuscular Scoliosis. Curr. Orthop. 2003, 17, 313–321. [Google Scholar] [CrossRef]
- Westerlund, L.E.; Gill, S.S.; Jarosz, T.S.; Abel, M.F.; Blanco, J.S. Posterior-only unit rod instrumentation and fusion for neuromuscular scoliosis. Spine 2001, 26, 1984–1989. [Google Scholar] [CrossRef]
- Rocos, B.; Zeller, R. Correcting Scoliosis in Rett Syndrome. Cureus 2021, 13, e15411. [Google Scholar] [CrossRef]
- Barney, C.C.; Merbler, A.M.; Quest, K.; Byiers, B.J.; Wilcox, G.L.; Schwantes, S.; Roiko, S.A.; Feyma, T.; Beisang, A.; Symons, F.J. A case-controlled comparison of postoperative analgesic dosing between girls with Rett syndrome and girls with and without developmental disability undergoing spinal fusion surgery. Pediatr. Anaesth. 2017, 27, 290–299. [Google Scholar] [CrossRef] [Green Version]
- Larsson, E.L.; Aaro, S.; Ahlinder, P.; Normelli, H.; Tropp, H.; Öberg, B. Long-term follow-up of functioning after spinal surgery in patients with Rett syndrome. Eur. Spine J. 2009, 18, 506–511. [Google Scholar] [CrossRef] [Green Version]
- Downs, J.; Young, D.; de Klerk, N.; Bebbington, A.; Baikie, G.; Leonard, H. Impact of scoliosis surgery on activities of daily living in females with Rett syndrome. J. Pediatr. Orthop. 2009, 29, 369–374. [Google Scholar] [CrossRef]
- Downs, J.; Torode, I.; Wong, K.; Ellaway, C.; Elliott, E.J.; Izatt, M.T.; Askin, G.N.; Mcphee, B.I.; Cundy, P.; Leonard, H.; et al. Surgical fusion of early onset severe scoliosis increases survival in Rett syndrome: A cohort study. Dev. Med. Child Neurol. 2016, 58, 632–638. [Google Scholar] [CrossRef] [Green Version]
- Kerr, A.M.; Webb, P.; Prescott, R.J.; Milne, Y. Results of surgery for scoliosis in Rett syndrome. J. Child Neurol. 2003, 18, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.B.; Tsirikos, A.I. Factors influencing the evaluation and management of neuromuscular scoliosis: A review of the literature. J. Back Musculoskelet. Rehabil. 2016, 29, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Weiss, H.R.; Negrini, S.; Rigo, M.; Kotwicki, T.; Hawes, M.C.; Grivas, T.B.; Maruyama, T.; Landauer, F. Indications for conservative management of scoliosis (guidelines). Scoliosis 2006, 1, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotwicki, T.; Jozwiak, M. Conservative management of neuromuscular scoliosis: Personal experience and review of literature. Disabil. Rehabil. 2008, 30, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Rabczuk, T. Scoliosis conservative treatment: A review of literature. J. Craniovertebral Junction Spine 2018, 9, 3. [Google Scholar] [CrossRef]
- Haleem, S.; Nnadi, C. Scoliosis: A review. Pediatr. Child Health 2018, 28, 209–217. [Google Scholar] [CrossRef]
- Ferrari, A.; Ferrara, C.; Balugani, M.; Sassi, S. Severe scoliosis in neurodevelopmental disabilities: Clinical signs and therapeutic proposals. Eur. J. Phys. Rehabil. Med. 2010, 46, 563–579. [Google Scholar]
- Downs, J.; Torode, I.; Ellaway, C.; Jacoby, P.; Bunting, C.; Wong, K.; Christodoulou, J.; Leonard, H. Family satisfaction following spinal fusion in Rett syndrome. Dev. Neurorehabil. 2016, 19, 31–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olafsson, Y.; Saraste, H.; Al-Dabbagh, Z. Brace treatment in neuromuscular spine deformity. Stud. Health Technol. Inform. 1999, 59, 332–335. [Google Scholar] [CrossRef]
- Lotan, M.; Merrick, J.; Carmeli, E. Managing scoliosis in a young child with Rett syndrome: A case study. ScientificWorldJournal. 2005, 5, 264–273. [Google Scholar] [CrossRef]
- Lotan, M.; Downs, J.; Elefant, C. A Pilot Study Delivering Physiotherapy Support for Rett Syndrome Using a Telehealth Framework Suitable for COVID-19 Lockdown. Dev. Neurorehabil. 2021, 429–434. [Google Scholar] [CrossRef]
- Romano, A.; Di Rosa, G.; Tisano, A.; Fabio, R.A.; Lotan, M. Effects of a remotely supervised motor rehabilitation program for individuals with Rett syndrome at home. Disabil. Rehabil. 2021, 1–11. [Google Scholar] [CrossRef]
- Fabio, R.A.; Martinazzoli, C.; Antonietti, A. Development and standardization of the “rars” (Rett assessment rating scale). Life Span Disabil. 2005, 8, 257–281. [Google Scholar]
- Romano, A.; Caprì, T.; Semino, M.; Bizzego, I.; Di Rosa, G.; Fabio, R.A. Gross Motor, Physical Activity and Musculoskeletal Disorder Evaluation Tools for Rett Syndrome: A Systematic Review. Dev. Neurorehabil. 2020, 23, 485–501. [Google Scholar] [CrossRef]
- Stahlhut, M.; Hill, K.; Bisgaard, A.M.; Jensen, A.K.; Andersen, M.; Leonard, H.; Downs, J. Measurement of Sedentary Behaviors or “downtime” in Rett Syndrome. J. Child Neurol. 2017, 32, 1009–1013. [Google Scholar] [CrossRef]
- Lor, L.; Hill, K.; Jacoby, P.; Leonard, H.; Downs, J. A validation study of a modified Bouchard activity record that extends the concept of “uptime” to Rett syndrome. Dev. Med. Child Neurol. 2015, 57, 1137–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodocanachi Roidi, M.L.; Isaias, I.U.; Cozzi, F.; Grange, F.; Scotti, F.M.; Gestra, V.F.; Gandini, A.; Ripamonti, E. A New Scale to Evaluate Motor Function in Rett Syndrome: Validation and Psychometric Properties. Pediatr. Neurol. 2019, 100, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Rodocanachi Roidi, M.L.; Isaias, I.U.; Cozzi, F.; Grange, F.; Scotti, F.M.; Gestra, V.F.; Gandini, A.; Ripamonti, E. Motor function in Rett syndrome: Comparing clinical and parental assessments. Dev. Med. Child Neurol. 2019, 61, 957–963. [Google Scholar] [CrossRef]
- Horng, M.-H.; Kuok, C.-P.; Fu, M.-J.; Lin, C.-J.; Sun, Y.-N. Cobb angle measurement of spine from X-ray images using convolutional neural network. Comput. Math. Methods Med. 2019, 2019, 6357171. [Google Scholar] [CrossRef] [Green Version]
- Hanks, S.B. Motor disabilities in the rett syndrome and physical therapy strategies. Brain Dev. 1990, 12, 157–161. [Google Scholar] [CrossRef]
- Uyanik, M.; Bumin, G.; Kayihan, H. Comparison of different therapy approaches in children with Down syndrome. Pediatr. Int. 2003, 45, 68–73. [Google Scholar] [CrossRef] [Green Version]
- Tomczak, E. The need to report effect size estimates revisited. An overview of some recommended measures of effect size Language and cognition: L2 influence on conceptualization of motion and event construal. View project. Trends Sport Sci. 2014, 21, 19–25. [Google Scholar]
- King, B.M.; Minium, E.W. Statistical Reasoning in the Behavioral Sciences, 7th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; ISBN 9780470134870. [Google Scholar]
- Ottenbacher, K.J. Why Rehabilitation Research Does Not Work (As Well as We Think It Should). Arch. Phys. Med. Rehabil. 1995, 76, 123–129. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: New York, NY, USA, 1988; ISBN 0805802835. [Google Scholar]
- Thompson, B. Effect sizes, confidence intervals, and confidence intervals for effect sizes. Psychol. Sch. 2007, 44, 423–432. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Kinney, A.R.; Eakman, A.M.; Graham, J.E. Novel Effect Size Interpretation Guidelines and an Evaluation of Statistical Power in Rehabilitation Research. Arch. Phys. Med. Rehabil. 2020, 101, 2219–2226. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A. When to use the Bonferroni correction. Ophthalmic Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef]
- Keret, D.; Bassett, G.S.; Bunnell, W.P.; Marks, H.G. Scoliosis in Rett syndrome. J. Pediatr. Orthop. 1988, 8, 138–142. [Google Scholar] [CrossRef]
- Loder, R.T.; Lee, C.L.; Richards, B.S. Orthopedic aspects of Rett syndrome: A multicenter review. J. Pediatr. Orthop. 1989, 9, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Lotan, M.; Isakov, E.; Merrick, J. Improving functional skills and physical fitness in children with Rett syndrome. J. Intellect. Disabil. Res. 2004, 48, 730–735. [Google Scholar] [CrossRef]
- Lotan, M.; Ippolito, E.; Favetta, M.; Romano, A. Skype Supervised, Individualized, Home-Based Rehabilitation Programs for Individuals With Rett Syndrome and Their Families—Parental Satisfaction and Point of View. Front. Psychol. 2021, 12, 3995. [Google Scholar] [CrossRef] [PubMed]
- Lotan, M.; Stahlhut, M.; Romano, A.; Downs, J.; Elefant, C. Family-Centered Telehealth Supporting Motor Skills and Activity in Individuals With Rett Syndrome. In Assistive Technologies for Assessment and Recovery of Neurological Impairments; Stasolla, F., Ed.; IGI Global: Hershey, PA, USA, 2022; pp. 147–171. [Google Scholar]
- Hagberg, B.; Anvret, M.; Wahlstrom, J. Rett Syndrome—Clinical and Biological Aspects: Studies on 130 Swedish Females; MacKeith Press Press: London, UK, 1993; ISBN 0521412838. [Google Scholar]
- Bisgaard, A.M.; Wong, K.; Højfeldt, A.K.; Larsen, J.L.; Schönewolf-Greulich, B.; Rønde, G.; Downs, J.; Stahlhut, M. Decline in gross motor skills in adult Rett syndrome; results from a Danish longitudinal study. Am. J. Med. Genet. Part A 2021, 185, 3683–3693. [Google Scholar] [CrossRef] [PubMed]
- McClure, M.K.; Battaglia, C.; McClure, R.J. The Relationship of Cumulative Motor Asymmetries to Scoliosis in Rett Syndrome. Am. J. Occup. Ther. 1998, 52, 196–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotan, M.; Merrick, J.; Kandel, I.; Morad, M. Aging in persons with Rett syndrome: An updated review. ScientificWorldJournal 2010, 10, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Lotan, M.; Merrick, J. Rett Syndrome: Therapeutic Interventions; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2011; ISBN 978-1-61728-080-1. [Google Scholar]

| Mean (SD) | Median | Range | ||
|---|---|---|---|---|
| Age | 15.6 (8.4) | 13.2 | 3.8–38.3 | |
| RARS | Cognitive | 13.4 (2.8) | 13.5 | 9–18.5 |
| Sensory | 3.2 (1.1) | 3.0 | 2–5.5 | |
| Motor | 10.4 (2.1) | 10.3 | 5.5–13 | |
| Emotional | 3.2 (0.8) | 3.0 | 2–5 | |
| Independence | 10.7 (1.8) | 11.3 | 4.5–12 | |
| Rett characteristics | 23.8 (4.5) | 24.3 | 15–33 | |
| Total | 64.7 (9.5) | 66.0 | 45.5–81.5 | |
| m-BAR | 92.8 (26.1) | 93.5 | 52–145 | |
| Worsening | Improving | All | ||
|---|---|---|---|---|
| No. (%) | 12 (60%) | 8 (40%) | 20 (100%) | |
| Cobb’s angles T1 (°) | Mean (SD) | 23.6 (13.8) | 27.9 (12.5) | 25.3 (13.2) |
| Median | 21.5 | 29.2 | 23.3 | |
| Range | 8–56 | 10–41 | 8–56 | |
| Cobb’s angles T2 (°) | Mean (SD) | 30.8 (16.5) | 21.5 (14.8) | 27.1 (16.1) |
| Median | 25.6 | 24.4 | 25.6 | |
| Range | 14–65 | 0–39 | 0–65 | |
| ∆ Cobb’s angles (°) | Mean (SD) | 7.2 (6.5) | −6.4 (4.1) | 1.7 (8.7) |
| Median | 5.0 | -4.8 | 2.1 | |
| Range | 1–23 | −13–-2.5 | −13–23 | |
| Mean (SD) | Median | Range | p-Value | Effect Size | |||
|---|---|---|---|---|---|---|---|
| RESMES Subscales | Standing | T1 | 3.2 (4) | 1 | 0–11 | 0.098 | \ |
| T2 | 2.6 (3.5) | 0 | 0–10 | ||||
| Sitting | T1 | 1 (2.3) | 0 | 0–9 | 0.066 | \ | |
| T2 | 0.4 (1) | 0 | 0–3 | ||||
| Transition | T1 | 14.6 (6.7) | 16 | 0–24 | 0.034 * | 0.138 | |
| T2 | 14.1 (6.7) | 13.5 | 0–24 | ||||
| Walking | T1 | 7.6 (7) | 5.5 | 0–18 | 0.094 | \ | |
| T2 | 7.1 (6.7) | 4.5 | 0–18 | ||||
| Running | T1 | 4 (0) | 4 | 4–4 | 1.000 | \ | |
| T2 | 4 (0) | 4 | 4–4 | ||||
| Stairs | T1 | 6.5 (1.8) | 6.5 | 2–8 | 0.038 * | 0.071 | |
| T2 | 6.1 (1.9) | 6 | 2–8 | ||||
| RESMES Total | T1 | 36.8 (19.3) | 32.5 | 6–70 | <0.001 * | 0.648 | |
| T2 | 34.3 (18.1) | 28.5 | 6–66 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, A.; Ippolito, E.; Risoli, C.; Malerba, E.; Favetta, M.; Sancesario, A.; Lotan, M.; Moran, D.S. Intensive Postural and Motor Activity Program Reduces Scoliosis Progression in People with Rett Syndrome. J. Clin. Med. 2022, 11, 559. https://doi.org/10.3390/jcm11030559
Romano A, Ippolito E, Risoli C, Malerba E, Favetta M, Sancesario A, Lotan M, Moran DS. Intensive Postural and Motor Activity Program Reduces Scoliosis Progression in People with Rett Syndrome. Journal of Clinical Medicine. 2022; 11(3):559. https://doi.org/10.3390/jcm11030559
Chicago/Turabian StyleRomano, Alberto, Elena Ippolito, Camilla Risoli, Edoardo Malerba, Martina Favetta, Andrea Sancesario, Meir Lotan, and Daniel Sender Moran. 2022. "Intensive Postural and Motor Activity Program Reduces Scoliosis Progression in People with Rett Syndrome" Journal of Clinical Medicine 11, no. 3: 559. https://doi.org/10.3390/jcm11030559
APA StyleRomano, A., Ippolito, E., Risoli, C., Malerba, E., Favetta, M., Sancesario, A., Lotan, M., & Moran, D. S. (2022). Intensive Postural and Motor Activity Program Reduces Scoliosis Progression in People with Rett Syndrome. Journal of Clinical Medicine, 11(3), 559. https://doi.org/10.3390/jcm11030559








