Ventilation-Induced Lung Injury (VILI) in Neonates: Evidence-Based Concepts and Lung-Protective Strategies
Abstract
1. Introduction
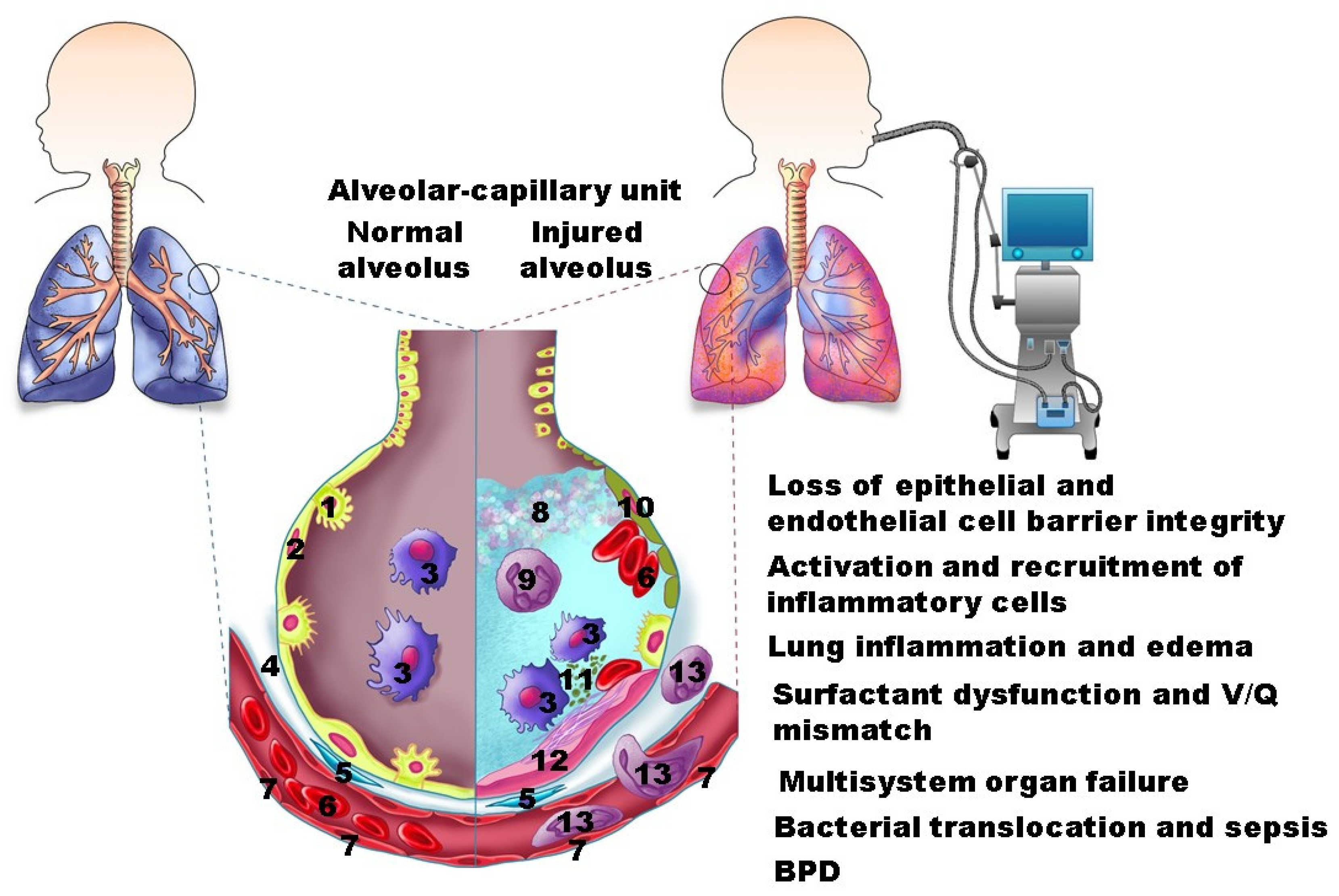
2. Pathology
3. Risk Factors and Pathogenesis
3.1. Ventilator-Related Risk Factors
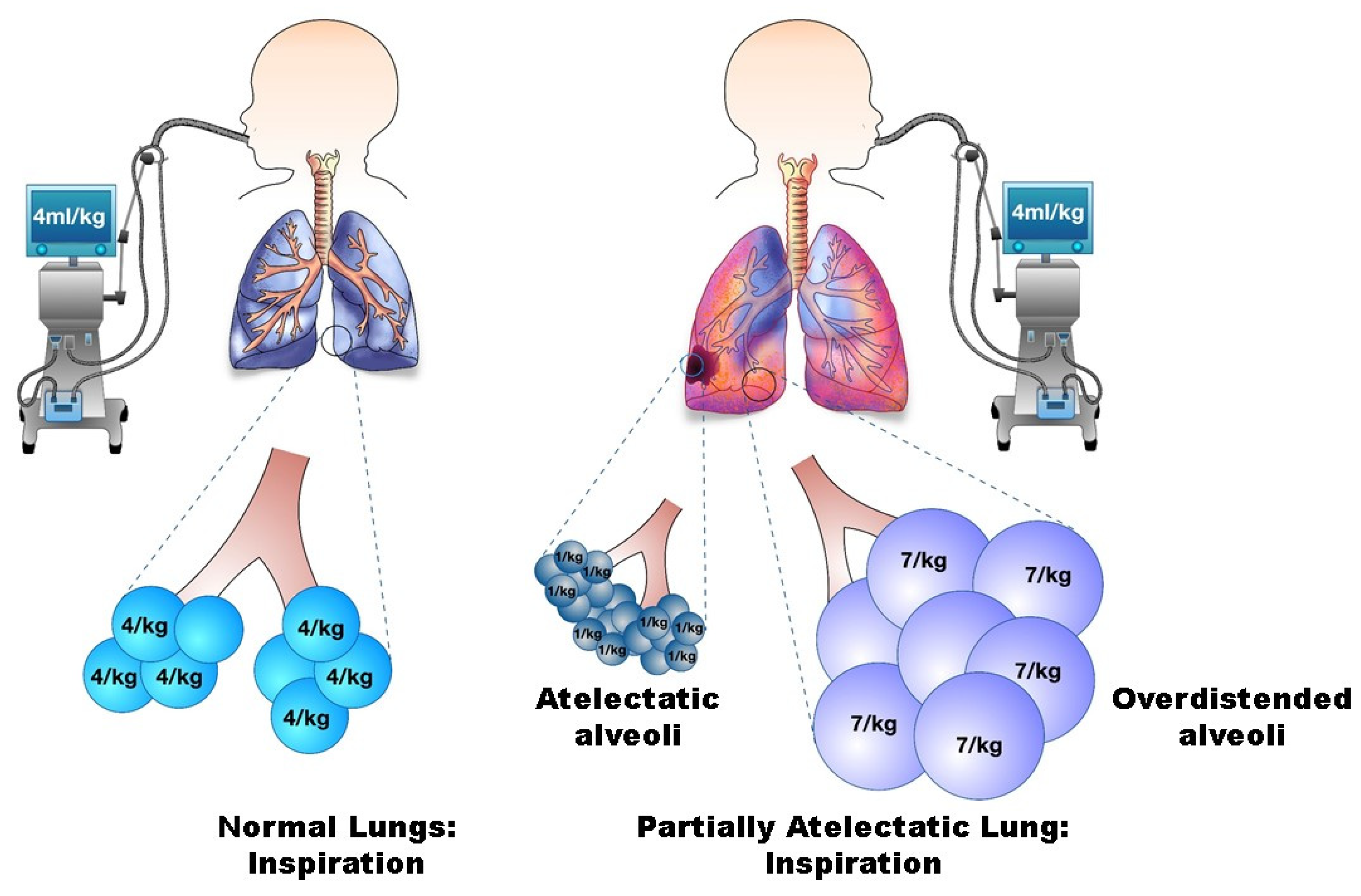
3.1.1. Volutrauma
3.1.2. Barotrauma
What Is More Injurious: Pressure or Volume?
3.1.3. Atelectrauma
3.1.4. Oxidative Stress
3.1.5. Biotrauma
3.1.6. Mechanical Power, Stress and Strain
3.2. Patient-Related Risk Factors
3.2.1. Lung Immaturity
3.2.2. Preexisting Lung Disease
3.2.3. Nutrition
4. Consequences of VILI
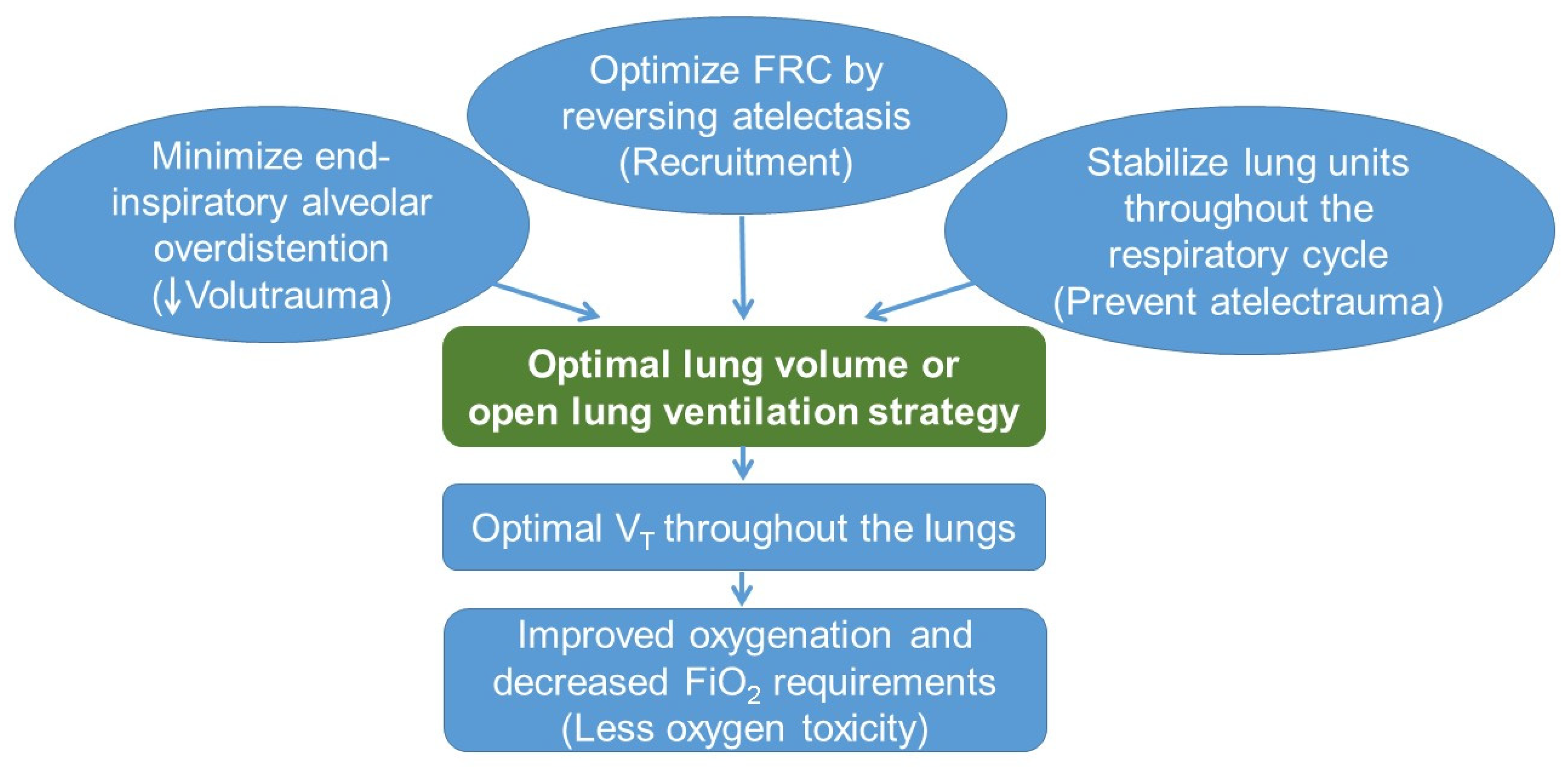
5. Lung-Protective Strategies
5.1. Open Lung Ventilation Strategy
5.2. Preventing Volutrauma
5.3. High-Frequency Ventilation
5.4. Preventing and Reversing Atelectrauma
5.5. Noninvasive Respiratory Support
6. Conclusions
7. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chami, M.; Geoffray, A. Pulmonary sequelae of prematurity: Radiological manifestations. Pediatr. Pulmonol. Suppl. 1997, 16, 51. [Google Scholar] [CrossRef] [PubMed]
- Flor-de-Lima, F.; Rocha, G.; Guimarães, H. Impact of changes in perinatal care on neonatal respiratory outcome and survival of preterm newborns: An overview of 15 years. Crit. Care Res. Pract. 2012, 2012, 643246. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sandri, F.; Plavka, R.; Ancora, G.; Simeoni, U.; Stranak, Z.; Martinelli, S.; Mosca, F.; Nona, J.; Thomson, M.; Verder, H.; et al. Prophylactic or early selective surfactant combined with nCPAP in very preterm infants. Pediatrics 2010, 125, e1402–e1409. [Google Scholar] [CrossRef] [PubMed]
- Artigas, A.; Bernard, G.R.; Carlet, J.; Dreyfuss, D.; Gattinoni, L.; Hudson, L.; Lamy, M.; Marini, J.J.; Matthay, M.A.; Pinsky, M.R.; et al. The American-European Consensus Conference on ARDS, part 2. Ventilatory, pharmacologic, supportive therapy, study design strategies and issues related to recovery and remodeling. Intensive Care Med. 1998, 24, 378–398. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.H.; Gerstmann, D.R.; Jobe, A.H.; Moffitt, S.T.; Slutsky, A.S.; Yoder, B.A. Lung injury in neonates: Causes, strategies for prevention, and long-term consequences. J. Pediatr. 2001, 139, 478–486. [Google Scholar] [CrossRef]
- Slutsky, A.S.; Ranieri, V.M. Ventilator-induced lung injury. N. Engl. J. Med. 2013, 369, 2126–2136. [Google Scholar] [CrossRef]
- Kalikkot Thekkeveedu, R.; Guaman, M.C.; Shivanna, B. Bronchopulmonary dysplasia: A review of pathogenesis and pathophysiology. Respir. Med. 2017, 132, 170–177. [Google Scholar] [CrossRef]
- Jobe, A.H.; Ikegami, M. Mechanisms initiating lung injury in the preterm. Early Hum. Dev. 1998, 53, 81–94. [Google Scholar] [CrossRef]
- Hatch, L.D., 3rd; Clark, R.H.; Carlo, W.A.; Stark, A.R.; Ely, E.W.; Patrick, S.W. Changes in Use of Respiratory Support for Preterm Infants in the US, 2008–2018. JAMA Pediatr. 2021, 175, 1017–1024. [Google Scholar] [CrossRef]
- Keszler, M.; Sant’Anna, G. Mechanical Ventilation and Bronchopulmonary Dysplasia. Clin. Perinatol. 2015, 42, 781–796. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Walsh, M.C.; Carlo, W.A.; Shankaran, S.; Laptook, A.R.; Sánchez, P.J.; Van Meurs, K.P.; Wyckoff, M.; et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA 2015, 314, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Muniraman, H.; Biniwale, M.; Ramanathan, R. A Review on Non-invasive Respiratory Support for Management of Respiratory Distress in Extremely Preterm Infants. Front. Pediatr. 2020, 8, 270. [Google Scholar] [CrossRef] [PubMed]
- Joelsson, J.P.; Ingthorsson, S.; Kricker, J.; Gudjonsson, T.; Karason, S. Ventilator-induced lung-injury in mouse models: Is there a trap? Lab. Anim. Res. 2021, 37, 30. [Google Scholar] [CrossRef] [PubMed]
- Matute-Bello, G.; Downey, G.; Moore, B.B.; Groshong, S.D.; Matthay, M.A.; Slutsky, A.S.; Kuebler, W.M. An official American Thoracic Society workshop report: Features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 2011, 44, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Thille, A.W.; Esteban, A.; Fernández-Segoviano, P.; Rodriguez, J.M.; Aramburu, J.A.; Peñuelas, O.; Cortés-Puch, I.; Cardinal-Fernández, P.; Lorente, J.A.; Frutos-Vivar, F. Comparison of the Berlin definition for acute respiratory distress syndrome with autopsy. Am. J. Respir. Crit. Care Med. 2013, 187, 761–767. [Google Scholar] [CrossRef]
- Jin, S.; Ding, X.; Yang, C.; Li, W.; Deng, M.; Liao, H.; Lv, X.; Pitt, B.R.; Billiar, T.R.; Zhang, L.M.; et al. Mechanical Ventilation Exacerbates Poly (I:C) Induced Acute Lung Injury: Central Role for Caspase-11 and Gut-Lung Axis. Front. Immunol. 2021, 12, 693874. [Google Scholar] [CrossRef] [PubMed]
- Chun, C.D.; Liles, W.C.; Frevert, C.W.; Glenny, R.W.; Altemeier, W.A. Mechanical ventilation modulates Toll-like receptor-3-induced lung inflammation via a MyD88-dependent, TLR4-independent pathway: A controlled animal study. BMC Pulm. Med. 2010, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Tomashefski, J.F., Jr. Pulmonary pathology of acute respiratory distress syndrome. Clin. Chest Med. 2000, 21, 435–466. [Google Scholar] [CrossRef]
- Hughes, K.T.; Beasley, M.B. Pulmonary Manifestations of Acute Lung Injury: More Than Just Diffuse Alveolar Damage. Arch. Pathol. Lab. Med. 2017, 141, 916–922. [Google Scholar] [CrossRef]
- Bachofen, M.; Weibel, E.R. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin. Chest Med. 1982, 3, 35–56. [Google Scholar] [CrossRef]
- Deptula, N.; Royse, E.; Kemp, M.W.; Miura, Y.; Kallapur, S.G.; Jobe, A.H.; Hillman, N.H. Brief mechanical ventilation causes differential epithelial repair along the airways of fetal, preterm lambs. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L412–L420. [Google Scholar] [CrossRef]
- Northway, W.H., Jr.; Rosan, R.C.; Porter, D.Y. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N. Engl. J. Med. 1967, 276, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Husain, A.N.; Siddiqui, N.H.; Stocker, J.T. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum. Pathol. 1998, 29, 710–717. [Google Scholar] [CrossRef]
- Coker, P.J.; Hernandez, L.A.; Peevy, K.J.; Adkins, K.; Parker, J.C. Increased sensitivity to mechanical ventilation after surfactant inactivation in young rabbit lungs. Crit. Care Med. 1992, 20, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, M.; Kallapur, S.G.; Jobe, A.H. Initial responses to ventilation of premature lambs exposed to intra-amniotic endotoxin 4 days before delivery. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 286, L573–L579. [Google Scholar] [CrossRef] [PubMed]
- Altemeier, W.A.; Matute-Bello, G.; Frevert, C.W.; Kawata, Y.; Kajikawa, O.; Martin, T.R.; Glenny, R.W. Mechanical ventilation with moderate tidal volumes synergistically increases lung cytokine response to systemic endotoxin. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 287, L533–L542. [Google Scholar] [CrossRef] [PubMed]
- Carlton, D.P.; Cummings, J.J.; Scheerer, R.G.; Poulain, F.R.; Bland, R.D. Lung overexpansion increases pulmonary microvascular protein permeability in young lambs. J. Appl. Physiol. 1985 1990, 69, 577–583. [Google Scholar] [CrossRef]
- Mokres, L.M.; Parai, K.; Hilgendorff, A.; Ertsey, R.; Alvira, C.M.; Rabinovitch, M.; Bland, R.D. Prolonged mechanical ventilation with air induces apoptosis and causes failure of alveolar septation and angiogenesis in lungs of newborn mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 298, L23–L35. [Google Scholar] [CrossRef]
- Wada, K.; Jobe, A.H.; Ikegami, M. Tidal volume effects on surfactant treatment responses with the initiation of ventilation in preterm lambs. J. Appl. Physiol. 1985 1997, 83, 1054–1061. [Google Scholar] [CrossRef]
- Bjorklund, L.J.; Ingimarsson, J.; Curstedt, T.; John, J.; Robertson, B.; Werner, O.; Vilstrup, C.T. Manual ventilation with a few large breaths at birth compromises the therapeutic effect of subsequent surfactant replacement in immature lambs. Pediatr. Res. 1997, 42, 348–355. [Google Scholar] [CrossRef]
- Dreyfuss, D.; Saumon, G. Ventilator-induced lung injury: Lessons from experimental studies. Am. J. Respir. Crit. Care Med. 1998, 157, 294–323. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Kagalwalla, S.; Cockar, I.; Williams, E.E.; Tamura, K.; Dassios, T.; Greenough, A. Prediction of prolonged ventilator dependence in preterm infants. Eur. J. Pediatr. 2019, 178, 1063–1068. [Google Scholar] [CrossRef]
- Keszler, M.; Abubakar, K. Volume guarantee: Stability of tidal volume and incidence of hypocarbia. Pediatr. Pulmonol. 2004, 38, 240–245. [Google Scholar] [CrossRef]
- Lista, G.; Colnaghi, M.; Castoldi, F.; Condo, V.; Reali, R.; Compagnoni, G.; Mosca, F. Impact of targeted-volume ventilation on lung inflammatory response in preterm infants with respiratory distress syndrome (RDS). Pediatr. Pulmonol. 2004, 37, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Bancalari, E. New Developments in Respiratory Support for Preterm Infants. Am. J. Perinatol. 2019, 36, S13–S17. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.; Wheeler, K.I.; McCallion, N.; Morley, C.J.; Davis, P.G. Volume-targeted versus pressure-limited ventilation in neonates. Cochrane Database Syst. Rev. 2017, 10, CD003666. [Google Scholar] [CrossRef]
- Cannavò, L.; Rulli, I.; Falsaperla, R.; Corsello, G.; Gitto, E. Ventilation, oxidative stress and risk of brain injury in preterm newborn. Ital. J. Pediatr. 2020, 46, 100. [Google Scholar] [CrossRef] [PubMed]
- Webb, H.H.; Tierney, D.F. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am. Rev. Respir. Dis. 1974, 110, 556–565. [Google Scholar]
- Bouhuys, A. Physiology and musical instruments. Nature 1969, 221, 1199–1204. [Google Scholar] [CrossRef]
- Vanhaverbeke, K.; Slaats, M.; Al-Nejar, M.; Everaars, N.; Snoeckx, A.; Spinhoven, M.; El Addouli, H.; Lauwers, E.; Van Eyck, A.; De Winter, B.Y.; et al. Functional respiratory imaging provides novel insights into the long-term respiratory sequelae of bronchopulmonary dysplasia. Eur. Respir. J. 2021, 57. [Google Scholar] [CrossRef]
- Schmidt, A.R.; Ramamoorthy, C. Bronchopulmonary dysplasia. Paediatr. Anaesth. 2021. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, C.T.; Jain, L.; Schmidt, B.; Abman, S.; Bancalari, E.; Aschner, J.L. Bronchopulmonary dysplasia: NHLBI Workshop on the Primary Prevention of Chronic Lung Diseases. Ann. Am. Thorac. Soc. 2014, 11 (Suppl. 3), S146–S153. [Google Scholar] [CrossRef] [PubMed]
- Manti, S.; Galdo, F.; Parisi, G.F.; Napolitano, M.; Decimo, F.; Leonardi, S.; Miraglia Del Giudice, M. Long-term effects of bronchopulmonary dysplasia on lung function: A pilot study in preschool children’s cohort. J. Asthma Off. J. Assoc. Care Asthma 2021, 58, 1186–1193. [Google Scholar] [CrossRef]
- Rahn, H.; Otis, A.B.; Chadwick, L.E.; Fenn, W.O. The pressure-volume diagram of the thorax and lung. Am. J. Physiol. 1946, 146, 161–178. [Google Scholar] [CrossRef]
- Dreyfuss, D.; Soler, P.; Basset, G.; Saumon, G. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am. Rev. Respir. Dis. 1988, 137, 1159–1164. [Google Scholar] [CrossRef]
- Muscedere, J.G.; Mullen, J.B.; Gan, K.; Slutsky, A.S. Tidal ventilation at low airway pressures can augment lung injury. Am. J. Respir. Crit. Care Med. 1994, 149, 1327–1334. [Google Scholar] [CrossRef]
- Taskar, V.; John, J.; Evander, E.; Robertson, B.; Jonson, B. Surfactant dysfunction makes lungs vulnerable to repetitive collapse and reexpansion. Am. J. Respir. Crit. Care Med. 1997, 155, 313–320. [Google Scholar] [CrossRef]
- Williams, E.E.; Greenough, A. Lung Protection During Mechanical Ventilation in the Premature Infant. Clin. Perinatol. 2021, 48, 869–880. [Google Scholar] [CrossRef]
- Morley, C. Continuous distending pressure. Arch. Dis. Child. Fetal Neonatal Ed. 1999, 81, F152–F156. [Google Scholar] [CrossRef]
- Sandhar, B.K.; Niblett, D.J.; Argiras, E.P.; Dunnill, M.S.; Sykes, M.K. Effects of positive end-expiratory pressure on hyaline membrane formation in a rabbit model of the neonatal respiratory distress syndrome. Intensive Care Med. 1988, 14, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Froese, A.B.; McCulloch, P.R.; Sugiura, M.; Vaclavik, S.; Possmayer, F.; Moller, F. Optimizing alveolar expansion prolongs the effectiveness of exogenous surfactant therapy in the adult rabbit. Am. Rev. Respir. Dis. 1993, 148, 569–577. [Google Scholar] [CrossRef]
- McCulloch, P.R.; Forkert, P.G.; Froese, A.B. Lung volume maintenance prevents lung injury during high frequency oscillatory ventilation in surfactant-deficient rabbits. Am. Rev. Respir. Dis. 1988, 137, 1185–1192. [Google Scholar] [CrossRef]
- Bond, D.M.; Froese, A.B. Volume recruitment maneuvers are less deleterious than persistent low lung volumes in the atelectasis-prone rabbit lung during high-frequency oscillation. Crit. Care Med. 1993, 21, 402–412. [Google Scholar] [CrossRef]
- Mead, J.; Takishima, T.; Leith, D. Stress distribution in lungs: A model of pulmonary elasticity. J. Appl. Physiol. 1970, 28, 596–608. [Google Scholar] [CrossRef]
- Berger, T.M.; Fontana, M.; Stocker, M. The journey towards lung protective respiratory support in preterm neonates. Neonatology 2013, 104, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Mathias, M.; Chang, J.; Perez, M.; Saugstad, O. Supplemental Oxygen in the Newborn: Historical Perspective and Current Trends. Antioxidants 2021, 10, 1879. [Google Scholar] [CrossRef] [PubMed]
- Northway, W.H., Jr.; Rosan, R.C.; Shahinian, L., Jr.; Castellino, R.A.; Gyepes, M.T.; Durbridge, T. Radiologic and histologic investigation of pulmonary oxygen toxicity in newborn guinea pigs. Invest. Radiol. 1969, 4, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Frank, L. Antioxidants, nutrition, and bronchopulmonary dysplasia. Clin. Perinatol. 1992, 19, 541–562. [Google Scholar] [CrossRef]
- Berkelhamer, S.K.; Kim, G.A.; Radder, J.E.; Wedgwood, S.; Czech, L.; Steinhorn, R.H.; Schumacker, P.T. Developmental differences in hyperoxia-induced oxidative stress and cellular responses in the murine lung. Free Radic. Biol. Med. 2013, 61, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Cannavò, L.; Perrone, S.; Viola, V.; Marseglia, L.; Di Rosa, G.; Gitto, E. Oxidative Stress and Respiratory Diseases in Preterm Newborns. Int. J. Mol. Sci. 2021, 22, 12504. [Google Scholar] [CrossRef]
- Vento, M.; Moro, M.; Escrig, R.; Arruza, L.; Villar, G.; Izquierdo, I.; Roberts, L.J., 2nd; Arduini, A.; Escobar, J.J.; Sastre, J.; et al. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics 2009, 124, e439–e449. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, V.S.; Chalak, L.F.; Sparks, J.E.; Allen, J.R.; Savani, R.C.; Wyckoff, M.H. Resuscitation of preterm neonates with limited versus high oxygen strategy. Pediatrics 2013, 132, e1488–e1496. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.G.; Tan, A.; O’Donnell, C.P.; Schulze, A. Resuscitation of newborn infants with 100% oxygen or air: A systematic review and meta-analysis. Lancet 2004, 364, 1329–1333. [Google Scholar] [CrossRef]
- Welsford, M.; Nishiyama, C.; Shortt, C.; Isayama, T.; Dawson, J.A.; Weiner, G.; Roehr, C.C.; Wyckoff, M.H.; Rabi, Y. Room Air for Initiating Term Newborn Resuscitation: A Systematic Review With Meta-analysis. Pediatrics 2019, 143, e20181825. [Google Scholar] [CrossRef]
- Escobedo, M.B.; Aziz, K.; Kapadia, V.S.; Lee, H.C.; Niermeyer, S.; Schmölzer, G.M.; Szyld, E.; Weiner, G.M.; Wyckoff, M.H.; Yamada, N.K.; et al. 2019 American Heart Association Focused Update on Neonatal Resuscitation: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Pediatrics 2020, 145, e20191362. [Google Scholar] [CrossRef]
- Wyckoff, M.H.; Wyllie, J.; Aziz, K.; de Almeida, M.F.; Fabres, J.; Fawke, J.; Guinsburg, R.; Hosono, S.; Isayama, T.; Kapadia, V.S.; et al. Neonatal Life Support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 2020, 142, S185–S221. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.V.; Moe-Byrne, T.; Harden, M.; McGuire, W. Lower versus higher oxygen concentration for delivery room stabilisation of preterm neonates: Systematic review. PLoS ONE 2012, 7, e52033. [Google Scholar] [CrossRef] [PubMed]
- Saugstad, O.D.; Aune, D. Optimal oxygenation of extremely low birth weight infants: A meta-analysis and systematic review of the oxygen saturation target studies. Neonatology 2014, 105, 55–63. [Google Scholar] [CrossRef]
- Thamrin, V.; Saugstad, O.D.; Tarnow-Mordi, W.; Wang, Y.A.; Lui, K.; Wright, I.M.; De Waal, K.; Travadi, J.; Smyth, J.P.; Craven, P.; et al. Preterm Infant Outcomes after Randomization to Initial Resuscitation with FiO2 0.21 or 1.0. J. Pediatr. 2018, 201, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, V.; Oei, J.L.; Finer, N.; Rich, W.; Rabi, Y.; Wright, I.M.; Rook, D.; Vermeulen, M.J.; Tarnow-Mordi, W.O.; Smyth, J.P.; et al. Outcomes of delivery room resuscitation of bradycardic preterm infants: A retrospective cohort study of randomised trials of high vs low initial oxygen concentration and an individual patient data analysis. Resuscitation 2021, 167, 209–217. [Google Scholar] [CrossRef]
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Te Pas, A.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; Simeoni, U.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2019 Update. Neonatology 2019, 115, 432–450. [Google Scholar] [CrossRef]
- Vogel, E.R.; Britt, R.D., Jr.; Trinidad, M.C.; Faksh, A.; Martin, R.J.; MacFarlane, P.M.; Pabelick, C.M.; Prakash, Y.S. Perinatal oxygen in the developing lung. Can. J. Physiol. Pharmacol. 2015, 93, 119–127. [Google Scholar] [CrossRef]
- Naik, A.S.; Kallapur, S.G.; Bachurski, C.J.; Jobe, A.H.; Michna, J.; Kramer, B.W.; Ikegami, M. Effects of ventilation with different positive end-expiratory pressures on cytokine expression in the preterm lamb lung. Am. J. Respir. Crit. Care Med. 2001, 164, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, L.N.; Miatto, D.; Hamid, Q.; Govindarajan, A.; Slutsky, A.S. Injurious ventilation induces widespread pulmonary epithelial expression of tumor necrosis factor-alpha and interleukin-6 messenger RNA. Crit. Care Med. 2002, 30, 1693–1700. [Google Scholar] [CrossRef]
- Bohrer, B.; Silveira, R.C.; Neto, E.C.; Procianoy, R.S. Mechanical ventilation of newborns infant changes in plasma pro- and anti-inflammatory cytokines. J. Pediatr. 2010, 156, 16–19. [Google Scholar] [CrossRef]
- Chiumello, D.; Pristine, G.; Slutsky, A.S. Mechanical ventilation affects local and systemic cytokines in an animal model of acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1999, 160, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Haitsma, J.J.; Uhlig, S.; Goggel, R.; Verbrugge, S.J.; Lachmann, U.; Lachmann, B. Ventilator-induced lung injury leads to loss of alveolar and systemic compartmentalization of tumor necrosis factor-alpha. Intensive Care Med. 2000, 26, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Curley, G.F.; Laffey, J.G.; Zhang, H.; Slutsky, A.S. Biotrauma and Ventilator-Induced Lung Injury: Clinical Implications. Chest 2016, 150, 1109–1117. [Google Scholar] [CrossRef]
- Nahum, A.; Hoyt, J.; Schmitz, L.; Moody, J.; Shapiro, R.; Marini, J.J. Effect of mechanical ventilation strategy on dissemination of intratracheally instilled Escherichia coli in dogs. Crit. Care Med. 1997, 25, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, S.J.; Sorm, V.; van ’t Veen, A.; Mouton, J.W.; Gommers, D.; Lachmann, B. Lung overinflation without positive end-expiratory pressure promotes bacteremia after experimental Klebsiella pneumoniae inoculation. Intensive Care Med. 1998, 24, 172–177. [Google Scholar] [CrossRef]
- Cakar, N.; Akinci, O.; Tugrul, S.; Ozcan, P.E.; Esen, F.; Eraksoy, H.; Cagatay, A.; Telci, L.; Nahum, A. Recruitment maneuver: Does it promote bacterial translocation? Crit. Care Med. 2002, 30, 2103–2106. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, P.E.; Cakar, N.; Tugrul, S.; Akinci, O.; Cagatay, A.; Yilmazbayhan, D.; Esen, F.; Telci, L.; Akpir, K. The effects of airway pressure and inspiratory time on bacterial translocation. Anesth. Analg. 2007, 104, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Vasques, F.; Duscio, E.; Cipulli, F.; Romitti, F.; Quintel, M.; Gattinoni, L. Determinants and Prevention of Ventilator-Induced Lung Injury. Crit. Care Clin. 2018, 34, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.F.; Ball, L.; Rocco, P.R.M.; Pelosi, P. Ventilator-induced lung injury during controlled ventilation in patients with acute respiratory distress syndrome: Less is probably better. Expert Rev. Respir. Med. 2018, 12, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Cressoni, M.; Chiumello, D.; Chiurazzi, C.; Brioni, M.; Algieri, I.; Gotti, M.; Nikolla, K.; Massari, D.; Cammaroto, A.; Colombo, A.; et al. Lung inhomogeneities, inflation and [18F]2-fluoro-2-deoxy-D-glucose uptake rate in acute respiratory distress syndrome. Eur. Respir. J. 2016, 47, 233–242. [Google Scholar] [CrossRef]
- Cressoni, M.; Gotti, M.; Chiurazzi, C.; Massari, D.; Algieri, I.; Amini, M.; Cammaroto, A.; Brioni, M.; Montaruli, C.; Nikolla, K.; et al. Mechanical Power and Development of Ventilator-induced Lung Injury. Anesthesiology 2016, 124, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.L.; Ball, L.; Rocco, P.R.M.; Pelosi, P. Power to mechanical power to minimize ventilator-induced lung injury? Intensive Care Med. Exp. 2019, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, A.K.; Klein, M.J.; Modesto, I.A.V.; Emeriaud, G.; Kneyber, M.C.J.; Medina, A.; Cruces, P.; Diaz, F.; Takeuchi, M.; Maddux, A.B.; et al. Mechanical power in pediatric acute respiratory distress syndrome: A PARDIE study. Crit. Care 2022, 26, 2. [Google Scholar] [CrossRef]
- Hubmayr, R.D.; Kallet, R.H. Understanding Pulmonary Stress-Strain Relationships in Severe ARDS and Its Implications for Designing a Safer Approach to Setting the Ventilator. Respir. Care 2018, 63, 219–226. [Google Scholar] [CrossRef]
- Nieman, G.F.; Satalin, J.; Andrews, P.; Habashi, N.M.; Gatto, L.A. Lung stress, strain, and energy load: Engineering concepts to understand the mechanism of ventilator-induced lung injury (VILI). Intensive Care Med. Exp. 2016, 4, 16. [Google Scholar] [CrossRef]
- Makiyama, A.M.; Gibson, L.J.; Harris, R.S.; Venegas, J.G. Stress concentration around an atelectatic region: A finite element model. Respir. Physiol. Neurobiol. 2014, 201, 101–110. [Google Scholar] [CrossRef]
- Marini, J.J. Evolving concepts for safer ventilation. Crit. Care 2019, 23, 114. [Google Scholar] [CrossRef] [PubMed]
- Cressoni, M.; Cadringher, P.; Chiurazzi, C.; Amini, M.; Gallazzi, E.; Marino, A.; Brioni, M.; Carlesso, E.; Chiumello, D.; Quintel, M.; et al. Lung inhomogeneity in patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2014, 189, 149–158. [Google Scholar] [CrossRef]
- Protti, A.; Andreis, D.T.; Milesi, M.; Iapichino, G.E.; Monti, M.; Comini, B.; Pugni, P.; Melis, V.; Santini, A.; Dondossola, D.; et al. Lung anatomy, energy load, and ventilator-induced lung injury. Intensive Care Med. Exp. 2015, 3, 34. [Google Scholar] [CrossRef]
- Jobe, A.H.; Ikegami, M. Lung development and function in preterm infants in the surfactant treatment era. Annu. Rev. Physiol. 2000, 62, 825–846. [Google Scholar] [CrossRef]
- Whitehead, T.; Slutsky, A.S. The pulmonary physician in critical care * 7: Ventilator induced lung injury. Thorax 2002, 57, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Rouby, J.J.; Lherm, T.; Martin de Lassale, E.; Poète, P.; Bodin, L.; Finet, J.F.; Callard, P.; Viars, P. Histologic aspects of pulmonary barotrauma in critically ill patients with acute respiratory failure. Intensive Care Med. 1993, 19, 383–389. [Google Scholar] [CrossRef]
- Goldstein, I.; Bughalo, M.T.; Marquette, C.H.; Lenaour, G.; Lu, Q.; Rouby, J.J. Mechanical ventilation-induced air-space enlargement during experimental pneumonia in piglets. Am. J. Respir. Crit. Care Med. 2001, 163, 958–964. [Google Scholar] [CrossRef]
- Haschke, F.; Binder, C.; Huber-Dangl, M.; Haiden, N. Early-Life Nutrition, Growth Trajectories, and Long-Term Outcome. Nestle Nutr. Inst. Workshop Ser. 2019, 90, 107–120. [Google Scholar]
- Uberos, J.; Lardón-Fernández, M.; Machado-Casas, I.; Molina-Oya, M.; Narbona-López, E. Nutrition in extremely low birth weight infants: Impact on bronchopulmonary dysplasia. Minerva Pediatrica 2016, 68, 419–426. [Google Scholar] [PubMed]
- Klevebro, S.; Westin, V.; Stoltz Sjöström, E.; Norman, M.; Domellöf, M.; Edstedt Bonamy, A.K.; Hallberg, B. Early energy and protein intakes and associations with growth, BPD, and ROP in extremely preterm infants. Clin. Nutr. 2019, 38, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Kuiper-Makris, C.; Selle, J.; Nüsken, E.; Dötsch, J.; Alejandre Alcazar, M.A. Perinatal Nutritional and Metabolic Pathways: Early Origins of Chronic Lung Diseases. Front. Med. 2021, 8, 667315. [Google Scholar] [CrossRef]
- Bancalari, E.; Jain, D. Bronchopulmonary Dysplasia: 50 Years after the Original Description. Neonatology 2019, 115, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Nadimpalli, M.L.; Bourke, C.D.; Robertson, R.C.; Delarocque-Astagneau, E.; Manges, A.R.; Pickering, A.J. Can breastfeeding protect against antimicrobial resistance? BMC Med. 2020, 18, 392. [Google Scholar] [CrossRef]
- Bæk, O.; Ren, S.; Brunse, A.; Sangild, P.T.; Nguyen, D.N. Impaired Neonatal Immunity and Infection Resistance Following Fetal Growth Restriction in Preterm Pigs. Front. Immunol. 2020, 11, 1808. [Google Scholar] [CrossRef]
- Chen, J.H.; Cottrell, E.C.; Ozanne, S.E. Early growth and ageing. Nestle Nutr. Workshop Ser. Paediatr. Programme 2010, 65, 41–50. [Google Scholar]
- Chen, Y.; Fantuzzi, G.; Schoeny, M.; Meier, P.; Patel, A.L. High-Dose Human Milk Feedings Decrease Oxidative Stress in Premature Infant. JPEN J. Parenter. Enter. Nutr. 2019, 43, 126–132. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, Z.; Morales, M.; Wang, Y.; Khafipour, E.; Friel, J. Feeding practice influences gut microbiome composition in very low birth weight preterm infants and the association with oxidative stress: A prospective cohort study. Free Radic. Biol. Med. 2019, 142, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Poindexter, B.B.; Martin, C.R. Impact of Nutrition on Bronchopulmonary Dysplasia. Clin. Perinatol. 2015, 42, 797–806. [Google Scholar] [CrossRef]
- Aschner, J.L.; Bancalari, E.H.; McEvoy, C.T. Can We Prevent Bronchopulmonary Dysplasia? J. Pediatr. 2017, 189, 26–30. [Google Scholar] [CrossRef]
- Pierro, M.; Villamor-Martinez, E.; van Westering-Kroon, E.; Alvarez-Fuente, M.; Abman, S.H.; Villamor, E. Association of the dysfunctional placentation endotype of prematurity with bronchopulmonary dysplasia: A systematic review, meta-analysis and meta-regression. Thorax 2021, 1–8. [Google Scholar] [CrossRef]
- Underwood, M.A.; Lakshminrusimha, S.; Steinhorn, R.H.; Wedgwood, S. Malnutrition, poor post-natal growth, intestinal dysbiosis and the developing lung. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2021, 41, 1797–1810. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.; Lewis, P.L.; Garcia-Pons, T. Intrauterine growth-retarded rat pups show increased susceptibility to pulmonary O2 toxicity. Pediatr. Res. 1985, 19, 281–286. [Google Scholar] [CrossRef]
- Rozance, P.J.; Seedorf, G.J.; Brown, A.; Roe, G.; O’Meara, M.C.; Gien, J.; Tang, J.R.; Abman, S.H. Intrauterine growth restriction decreases pulmonary alveolar and vessel growth and causes pulmonary artery endothelial cell dysfunction in vitro in fetal sheep. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 301, L860–L871. [Google Scholar] [CrossRef] [PubMed]
- Wedgwood, S.; Warford, C.; Agvateesiri, S.C.; Thai, P.; Berkelhamer, S.K.; Perez, M.; Underwood, M.A.; Steinhorn, R.H. Postnatal growth restriction augments oxygen-induced pulmonary hypertension in a neonatal rat model of bronchopulmonary dysplasia. Pediatr. Res. 2016, 80, 894–902. [Google Scholar] [CrossRef]
- Wedgwood, S.; Warford, C.; Agvatisiri, S.R.; Thai, P.N.; Chiamvimonvat, N.; Kalanetra, K.M.; Lakshminrusimha, S.; Steinhorn, R.H.; Mills, D.A.; Underwood, M.A. The developing gut-lung axis: Postnatal growth restriction, intestinal dysbiosis, and pulmonary hypertension in a rodent model. Pediatr. Res. 2020, 87, 472–479. [Google Scholar] [CrossRef]
- Thiess, T.; Lauer, T.; Woesler, A.; Neusius, J.; Stehle, S.; Zimmer, K.P.; Eckert, G.P.; Ehrhardt, H. Correlation of Early Nutritional Supply and Development of Bronchopulmonary Dysplasia in Preterm Infants <1000 g. Front. Pediatr. 2021, 9, 741365. [Google Scholar] [CrossRef] [PubMed]
- Rocha, G.; Guimarães, H.; Pereira-da-Silva, L. The Role of Nutrition in the Prevention and Management of Bronchopulmonary Dysplasia: A Literature Review and Clinical Approach. Int. J. Environ. Res. Public Health 2021, 18, 6245. [Google Scholar] [CrossRef] [PubMed]
- Van Kaam, A.H.; Rimensberger, P.C. Lung-protective ventilation strategies in neonatology: What do we know—What do we need to know? Crit. Care Med. 2007, 35, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Dreyfuss, D.; Saumon, G. Role of tidal volume, FRC, and end-inspiratory volume in the development of pulmonary edema following mechanical ventilation. Am. Rev. Respir. Dis. 1993, 148, 1194–1203. [Google Scholar] [CrossRef]
- Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar]
- Bhuta, T.; Henderson-Smart, D.J. Elective high frequency jet ventilation versus conventional ventilation for respiratory distress syndrome in preterm infants. Cochrane Database Syst. Rev. 2000, 1998, CD000328. [Google Scholar] [CrossRef]
- Bollen, C.W.; Uiterwaal, C.S.; van Vught, A.J. Cumulative metaanalysis of high-frequency versus conventional ventilation in premature neonates. Am. J. Respir. Crit. Care Med. 2003, 168, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Henderson-Smart, D.J.; Bhuta, T.; Cools, F.; Offringa, M. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database Syst. Rev. 2003, CD000104. [Google Scholar] [CrossRef]
- Gommers, D.; Hartog, A.; Schnabel, R.; De Jaegere, A.; Lachmann, B. High-frequency oscillatory ventilation is not superior to conventional mechanical ventilation in surfactant-treated rabbits with lung injury. Eur Respir. J. 1999, 14, 738–744. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vazquez de Anda, G.F.; Hartog, A.; Verbrugge, S.J.; Gommers, D.; Lachmann, B. The open lung concept: Pressure-controlled ventilation is as effective as high-frequency oscillatory ventilation in improving gas exchange and lung mechanics in surfactant-deficient animals. Intensive Care Med. 1999, 25, 990–996. [Google Scholar] [CrossRef]
- Vazquez de Anda, G.F.; Gommers, D.; Verbrugge, S.J.; De Jaegere, A.; Lachmann, B. Mechanical ventilation with high positive end-expiratory pressure and small driving pressure amplitude is as effective as high-frequency oscillatory ventilation to preserve the function of exogenous surfactant in lung-lavaged rats. Crit. Care Med. 2000, 28, 2921–2925. [Google Scholar] [CrossRef]
- Alex, C.G.; Aronson, R.M.; Onal, E.; Lopata, M. Effects of continuous positive airway pressure on upper airway and respiratory muscle activity. J. Appl. Physiol. 1985 1987, 62, 2026–2030. [Google Scholar] [CrossRef]
- Cogswell, J.J.; Hatch, D.J.; Kerr, A.A.; Taylor, B. Effects of continuous positive airway pressure on lung mechanics of babies after operation for congenital heart disease. Arch. Dis. Child. 1975, 50, 799–804. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cotton, R.B.; Lindstrom, D.P.; Kanarek, K.S.; Sundell, H.; Stahlman, M.T. Effect of positive-end-expiratory-pressure on right ventricular output in lambs with hyaline membrane disease. Acta Paediatr. Scand. 1980, 69, 603–606. [Google Scholar] [CrossRef]
- Faridy, E.E. Effect of distension on release of surfactant in excised dogs’ lungs. Respir. Physiol. 1976, 27, 99–114. [Google Scholar] [CrossRef]
- Morley, C.J.; Davis, P.G.; Doyle, L.W.; Brion, L.P.; Hascoet, J.M.; Carlin, J.B. Nasal CPAP or intubation at birth for very preterm infants. N. Engl. J. Med. 2008, 358, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Finer, N.N.; Carlo, W.A.; Walsh, M.C.; Rich, W.; Gantz, M.G.; Laptook, A.R.; Yoder, B.A.; Faix, R.G.; Das, A.; Poole, W.K.; et al. Early CPAP versus surfactant in extremely preterm infants. N. Engl. J. Med. 2010, 362, 1970–1979. [Google Scholar] [PubMed]
- Dunn, M.S.; Kaempf, J.; de Klerk, A.; de Klerk, R.; Reilly, M.; Howard, D.; Ferrelli, K.; O’Conor, J.; Soll, R.F. Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics 2011, 128, e1069–e1076. [Google Scholar] [CrossRef]
- Stevens, T.P.; Harrington, E.W.; Blennow, M.; Soll, R.F. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst. Rev. 2007, CD003063. [Google Scholar] [CrossRef]
- Schmolzer, G.M.; Kumar, M.; Pichler, G.; Aziz, K.; O’Reilly, M.; Cheung, P.Y. Non-invasive versus invasive respiratory support in preterm infants at birth: Systematic review and meta-analysis. BMJ 2013, 347, f5980. [Google Scholar] [CrossRef]
- Respiratory support in preterm infants at birth. Pediatrics 2014, 133, 171–174. [CrossRef]
- Kugelman, A.; Feferkorn, I.; Riskin, A.; Chistyakov, I.; Kaufman, B.; Bader, D. Nasal intermittent mandatory ventilation versus nasal continuous positive airway pressure for respiratory distress syndrome: A randomized, controlled, prospective study. J. Pediatr. 2007, 150, 521–526. [Google Scholar] [CrossRef]
- Bhandari, V.; Finer, N.N.; Ehrenkranz, R.A.; Saha, S.; Das, A.; Walsh, M.C.; Engle, W.A.; VanMeurs, K.P. Synchronized nasal intermittent positive-pressure ventilation and neonatal outcomes. Pediatrics 2009, 124, 517–526. [Google Scholar] [CrossRef]
- Bhandari, V.; Gavino, R.G.; Nedrelow, J.H.; Pallela, P.; Salvador, A.; Ehrenkranz, R.A.; Brodsky, N.L. A randomized controlled trial of synchronized nasal intermittent positive pressure ventilation in RDS. J. Perinatol. 2007, 27, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Meneses, J.; Bhandari, V.; Alves, J.G. Nasal intermittent positive-pressure ventilation vs nasal continuous positive airway pressure for preterm infants with respiratory distress syndrome: A systematic review and meta-analysis. Arch. Pediatr. Adolesc. Med. 2012, 166, 372–376. [Google Scholar] [PubMed]
- Kirpalani, H.; Millar, D.; Lemyre, B.; Yoder, B.A.; Chiu, A.; Roberts, R.S. A trial comparing noninvasive ventilation strategies in preterm infants. N. Engl. J. Med. 2013, 369, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Rüegger, C.M.; Owen, L.S.; Davis, P.G. Nasal Intermittent Positive Pressure Ventilation for Neonatal Respiratory Distress Syndrome. Clin. Perinatol. 2021, 48, 725–744. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalikkot Thekkeveedu, R.; El-Saie, A.; Prakash, V.; Katakam, L.; Shivanna, B. Ventilation-Induced Lung Injury (VILI) in Neonates: Evidence-Based Concepts and Lung-Protective Strategies. J. Clin. Med. 2022, 11, 557. https://doi.org/10.3390/jcm11030557
Kalikkot Thekkeveedu R, El-Saie A, Prakash V, Katakam L, Shivanna B. Ventilation-Induced Lung Injury (VILI) in Neonates: Evidence-Based Concepts and Lung-Protective Strategies. Journal of Clinical Medicine. 2022; 11(3):557. https://doi.org/10.3390/jcm11030557
Chicago/Turabian StyleKalikkot Thekkeveedu, Renjithkumar, Ahmed El-Saie, Varsha Prakash, Lakshmi Katakam, and Binoy Shivanna. 2022. "Ventilation-Induced Lung Injury (VILI) in Neonates: Evidence-Based Concepts and Lung-Protective Strategies" Journal of Clinical Medicine 11, no. 3: 557. https://doi.org/10.3390/jcm11030557
APA StyleKalikkot Thekkeveedu, R., El-Saie, A., Prakash, V., Katakam, L., & Shivanna, B. (2022). Ventilation-Induced Lung Injury (VILI) in Neonates: Evidence-Based Concepts and Lung-Protective Strategies. Journal of Clinical Medicine, 11(3), 557. https://doi.org/10.3390/jcm11030557





