Increased Circulating Levels of Growth Differentiation Factor 15 in Association with Metabolic Disorders in People Living with HIV Receiving Combined Antiretroviral Therapy
Abstract
:1. Introduction
2. Patients and Methods
2.1. Subjects
2.2. Biochemistry Laboratory Measurements
2.3. Body Composition Measurements, the Definition of HALS, Metabolic Syndrome, and Cardiovascular Risk
2.4. GDF15, Cytokine, and Fatty Acid Circulating Levels
2.5. Statistical Analyses
3. Results
3.1. Population Studied
3.2. Antiretroviral Drug Exposure and Immuno-Virological Status
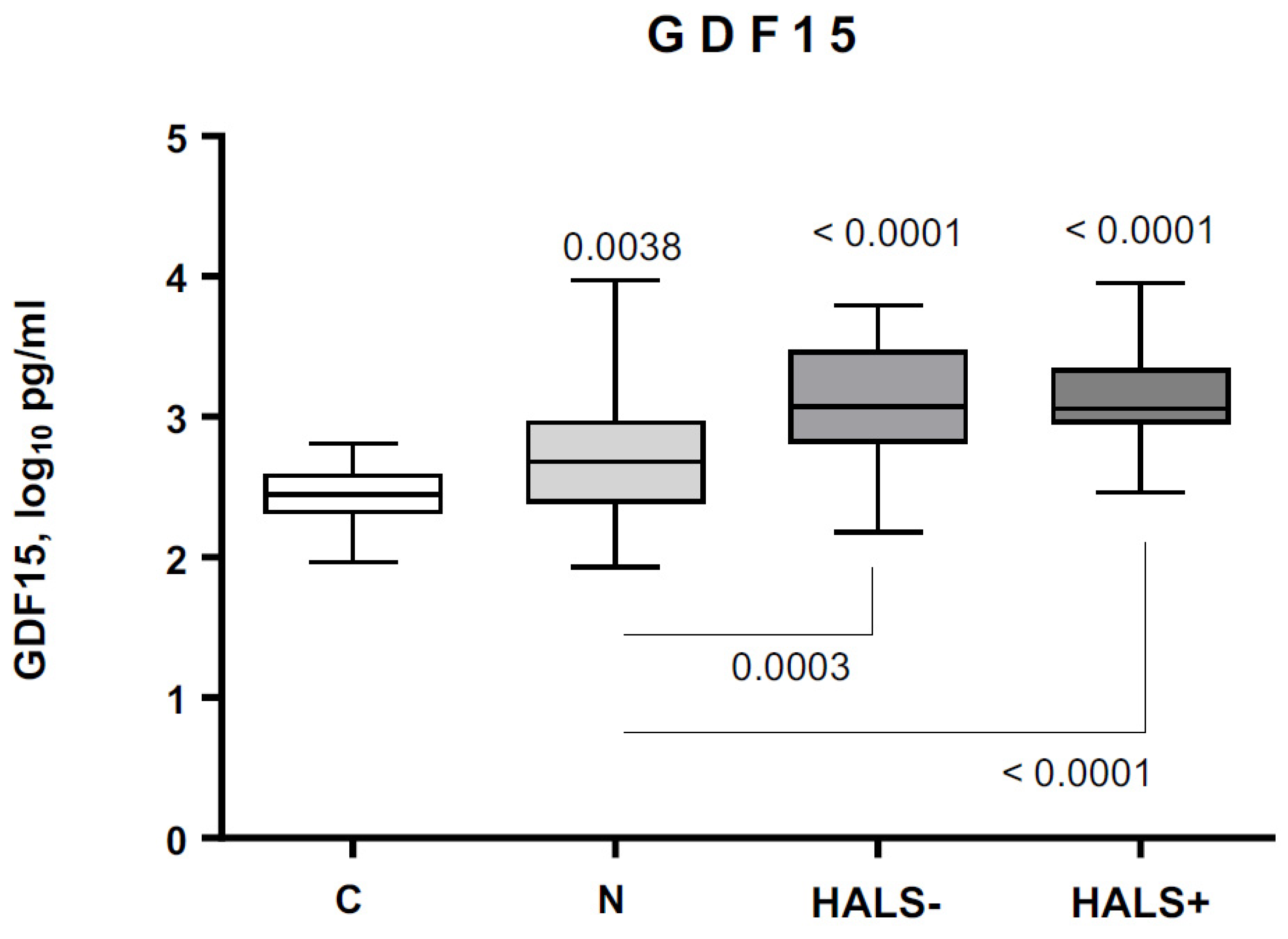
3.3. GDF15 Circulating Levels in Controls and PLWH
3.4. GDF15 Circulating Levels, Fat Mass, HALS, and Metabolic Syndrome
3.5. GDF15 Circulating Levels, Lipids, Glucose Homeostasis, and Inflammation
3.6. GDF15 Circulating Levels and Cardiovascular Risk
3.7. Subgroup Analyses
3.8. Independent Predictors of Cardiovascular Risk
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deeks, S.G.; Lewin, S.R.; Havlir, D.V. The end of AIDS: HIV infection as a chronic disease. Lancet 2013, 382, 1525–1533. [Google Scholar] [CrossRef] [Green Version]
- Brothers, T.D.; Rockwood, K. Biologic aging, frailty, and age-related disease in chronic HIV infection. Curr. Opin. HIV AIDS 2014, 9, 412–418. [Google Scholar] [CrossRef]
- Giannarelli, C.; Klein, R.S.; Badimon, J.J. Cardiovascular implications of HIV-induced dyslipidemia. Atherosclerosis 2011, 219, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Koethe, J.R.; Lagathu, C.; Lake, J.E.; Domingo, P.; Calmy, A.; Falutz, J.; Brown, T.T.; Capeau, J. HIV and antiretroviral therapy-related fat alterations. Nat. Rev. Dis. Primers 2020, 6, 48. [Google Scholar] [CrossRef]
- Cubero, J.M.; Domingo, P.; Sambeat, M.; Ordoñez-Llanos, J.; Rodriguez-Espinosa, J.; Sánchez-Quesada, J.L.; Pérez, A. Prevalence of metabolic syndrome among human immunodeficiency virus-infected subjects is widely influenced by the diagnostic criteria. Metab. Syndr. Relat. Disord. 2011, 9, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Silveira Rossi, J.L.; Barbalho, S.M.; Reverete de Araujo, R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes Metab. Res. Rev. 2021, e3502. [Google Scholar] [CrossRef] [PubMed]
- Triant, V.A.; Lee, H.; Hadigan, C.; Grinspoon, S.K. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J. Clin. Endocrinol. Metab. 2007, 92, 2506–2512. [Google Scholar] [CrossRef] [PubMed]
- Friis-Møller, N.; Sabin, C.A.; Weber, R.; d’Arminio Monforte, A.; El-Sadr, W.M.; Reiss, P.; Thiébaut, R.; Morfeldt, L.; De Wit, S.; Pradier, C.; et al. Combination antiretroviral therapy and the risk of myocardial infarction. N. Engl. J. Med. 2003, 349, 1993–2003. [Google Scholar] [CrossRef] [Green Version]
- Barnes, R.P.; Lacson, J.C.; Bahrami, H. HIV Infection and Risk of Cardiovascular Diseases Beyond Coronary Artery Disease. Curr. Atheroscler. Rep. 2017, 19, 1–9. [Google Scholar] [CrossRef]
- Wang, D.; Day, E.A.; Townsend, L.K.; Djordjevic, D.; Jørgensen, S.B.; Steinberg, G.R. GDF15: Emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat. Rev. Endocrinol. 2021, 17, 592–607. [Google Scholar] [CrossRef]
- Wang, J.; Wei, L.; Yang, X.; Zhong, J. Roles of Growth Differentiation Factor 15 in Atherosclerosis and Coronary Artery Disease. J. Am. Heart Assoc. 2019, 8, e012826. [Google Scholar] [CrossRef]
- Dostálová, I.; Roubícek, T.; Bártlová, M.; Mráz, M.; Lacinová, Z.; Haluzíková, D.; Kaválková, P.; Matoulek, M.; Kasalicky, M.; Haluzík, M. Increased serum concentrations of macrophage inhibitory cytokine-1 in patients with obesity and type 2 diabetes mellitus: The influence of very low calorie diet. Eur. J. Endocrinol. 2009, 161, 397–404. [Google Scholar] [CrossRef] [Green Version]
- Kralisch, S.; Hoffmann, A.; Estrada-Kunz, J.; Stumvoll, M.; Fasshauer, M.; Tönjes, A.; Miehle, K. Increased Growth Differentiation Factor 15 in Patients with Hypoleptinemia-Associated Lipodystrophy. Int. J. Mol. Sci. 2020, 21, 7214. [Google Scholar] [CrossRef] [PubMed]
- Dostálová, I.; Kaválková, P.; Papežová, H.; Domluvilová, D.; Zikán, V.; Haluzík, M. Association of macrophage inhibitory cytokine-1 with nutritional status, body composition and bone mineral density in patients with anorexia nervosa: The influence of partial realimentation. Nutr. Metab. 2010, 7, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.; Alvarez-Guaita, A.; Melvin, A.; Rimmington, D.; Dattilo, A.; Miedzybrodzka, E.L.; Cimino, I.; Maurin, A.C.; Roberts, G.P.; Meek, C.L.; et al. GDF15 Provides an Endocrine Signal of Nutritional Stress in Mice and Humans. Cell Metab. 2019, 29, 707–718.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lockhart, S.M.; Saudek, V.; O’Rahilly, S. GDF15: A Hormone Conveying Somatic Distress to the Brain. Endocr. Rev. 2020, 41, bnaa007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggers, K.M.; Kempf, T.; Venge, P.; Wallentin, L.; Wollert, K.C.; Lindahl, B. Improving long-term risk prediction in patients with acute chest pain: The Global Registry of Acute Coronary Event (GRACE) risk score is enhanced by selected non-necrosis biomarkers. Am. Heart. J. 2010, 160, 88–94. [Google Scholar] [CrossRef]
- Anand, I.S.; Kempf, T.; Rector, T.S.; Tapken, H.; Allhoff, T.; Jantzen, F.; Kuskowski, F.; Cohn, J.N.; Drexler, H.; Wollert, K.C. Serial measurement of growth-differentiation factor-15 in heart failure: Relation to disease severity and prognosis in the Valsartan heart failure trial. Circulation 2010, 122, 1387–1395. [Google Scholar] [CrossRef] [Green Version]
- Domingo, P.; Cabeza, M.C.; Pruvost, A.; Salazar, J.; Gutierrez, M.M.; Mateo, M.G.; Domingo, J.C.; Fernandez, I.; Villarroya, F.; Muñoz, J.; et al. Relationship between HIV/Highly active antiretroviral therapy (HAART)-associated lipodystrophy syndrome and stavudine-triphosphate intracellular levels in patients with stavudine-based antiretroviral regimens. Clin. Infect. Dis. 2010, 50, 1033–1040. [Google Scholar] [CrossRef]
- Lichtenstein, K.A.; Ward, D.J.; Moorman, A.C.; Delaney, K.M.; Young, B.; Palella, F.J.; Rhodes, P.H.; Wood, K.C.; Holmberg, S.D. HIV Outpatient Study Investigators. Clinical assessment of HIV-associated lipodystrophy in an ambulatory population. AIDS 2001, 15, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Young, J.; Xiao, Y.; Moodie, E.E.M.; Abrahamowicz, M.; Klein, M.B.; Bernasconi, E.; Schmid, P.; Calmy, A.; Cavassini, M.; Cusini, A.; et al. Effect of Cumulating Exposure to Abacavir on the Risk of Cardiovascular Disease Events in Patients from the Swiss HIV Cohort Study. J. Acquir. Immune Defic. Syndr. 2015, 69, 413–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anadol, E.; Lust, K.; Boesecke, C.; Schwarze-Zander, C.; Mohr, R.; Wasmuth, J.C.; Rockstroh, J.K.; Trebicka, J. Exposure to previous cART is associated with significant liver fibrosis and cirrhosis in human immunodeficiency virus-infected patients. PLoS ONE 2018, 13, e0191118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control. Revised classification system for HIV infection and expanded surveillance for case definition for AIDS among adolescents and adults. Morb. Mortal. Wkly. Rep. 1993, 41, 1–13. [Google Scholar]
- Domingo, P.; Gutierrez, M.M.; Gallego-Escuredo, J.M.; Torres, F.; Mateo, M.G.; Villarroya, J.; Lamarca, K.; Domingo, J.C.; Vidal, F.; Villarroya, F.; et al. A 48-Week Study of Fat Molecular Alterations in HIV Naive Patients Starting Tenofovir/Emtricitabine with Lopinavir/Ritonavir or Efavirenz. J. Acquir. Immune Defic. Syndr. 2014, 66, 457–465. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, 2182. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting serum glucose and insulin concentrations in man. Diabetologia 1985, 8, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Grundy, S.M.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Agostino, R.B.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008, 1176, 743–753. [Google Scholar] [CrossRef] [Green Version]
- Friis-Møller, N.; Thiébaut, R.; Reiss, P.; Weber, R.; D’Arminio Monforte, A.; De Wit, S.; El-Sadr, W.; Fontas, E.; Worm, S.; Kirk, O.; et al. Predicting the risk of cardiovascular disease in HIV-infected patients: The data collection on adverse effects of anti-HIV drugs study. Eur J. Cardiovasc. Prev. Rehabil. 2010, 17, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Domingo, P.; Fernández, I.; Gallego-Escuredo, J.M.; Torres, F.; Gutiérrez, M.M.; Mateo, M.G.; Villarroya, J.; Giralt, M.; Vidal, F.; Villarroya, F.; et al. Effects of docosahexaenoic acid on metabolic and fat parameters in HIV-infected patients on cART: A randomized, double-blind, placebo-controlled study. Clin. Nutr. 2018, 37, 1340–1347. [Google Scholar] [CrossRef]
- Secemsky, E.A.; Scherzer, R.; Nitta, E.; Wu, A.H.B.; Lange, D.C.; Deeks, S.G.; Martin, J.N.; Snider, J.; Ganz, P.; Hsue, P.Y. Novel biomarkers of cardiac stress, cardiovascular dysfunction, and outcomes in HIV-infected individuals. J. Am. Coll. Cardiol. HF. 2015, 3, 591–599. [Google Scholar] [CrossRef]
- De Filippi, C.; Christenson, R.; Joyce, J.; Park, E.A.; Wu, A.; Fitch, K.V.; Looby, S.E.; Lu, M.T.; Hoffmann, U.; Grinspoon, S.K.; et al. Statin effects on myocardial fibrosis in people with HIV. J. Acquir. Immune Defic. Syndr. 2018, 78, 105–110. [Google Scholar] [CrossRef]
- Scherzer, R.; Shah, S.J.; Secemsky, E.; Butler, J.; Grunfeld, C.; Shlipak, M.G.; Hsue, P.Y. Association of Biomarker Clusters with Cardiac Phenotypes and Mortality in Patients with HIV Infection. Circ. Heart Fail. 2018, 11, e004312. [Google Scholar] [CrossRef]
- Berezin, A.E. Cardiac biomarkers in diabetes mellitus: New dawn for risk stratification? Diabetes Metab. Syndr. 2017, 11 (Suppl. S1), S201–S208. [Google Scholar] [CrossRef] [PubMed]
- Boccara, F.; Lang, S.; Meuleman, C.; Ederhy, S.; Mary-Krause, M.; Costagliola, D.; Capeau, J.; Cohen, A. HIV and coronary heart disease: Time for a better understanding. J. Am. Coll. Cardiol. 2013, 61, 511–523. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.Q.; Ng, K.; Dhillon, O.; Kelly, D.; Quinn, P.; Squire, I.B.; Davies, J.E.; Ng, L.L. Growth differentiation factor-15 as a prognostic marker in patients with acute myocardial infarction. Eur. Heart. J. 2009, 30, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Chambless, L.E.; Cummiskey, C.P.; Cui, G. Several methods to assess improvement in risk prediction models: Extension to survival analysis. Stat. Med. 2011, 30, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Lind, L.; Wallentin, L.; Kempf, T.; Tapken, H.; Quint, A.; Lindahl, B.; Olofsson, S.; Venge, P.; Larsson, A.; Hulthe, J.; et al. Growth-differentiation factor-15 is an independent marker of cardiovascular dysfunction and disease in the elderly: Results from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Eur. Heart J. 2009, 30, 2346–2353. [Google Scholar] [CrossRef] [Green Version]
- Rohatgi, A.; Patel, P.; Das, S.R.; Ayers, C.R.; Khera, A.; Martinez-Rumayor, A.; Berry, J.D.; McGuire, D.K.; de Lemos, J.A. Association of growth differentiation factor-15 with coronary atherosclerosis and mortality in a young, multiethnic population: Observations from the Dallas Heart Study. Clin. Chem. 2012, 58, 172–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schernthaner-Reiter, M.H.; Itariu, B.K.; Krebs, M.; Promintzer-Schifferl, M.; Stulnig, T.M.; Tura, A.; Anderwald, C.H.; Clodi, M.; Ludvik, B.; Pacini, G.; et al. GDF15 reflects beta cell function in obese patients independently of the grade of impairment of glucose metabolism. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 334–342. [Google Scholar] [CrossRef]
- Mullican, S.E.; Rangwala, S.M. Uniting GDF15 and GFRAL: Therapeutic opportunities in obesity and beyond. Trends Endocrinol. Metab. 2018, 29, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Mracek, T.; Gonzalez-Muniesa, P.; Kos, K.; Wilding, J.; Trayhurn, P.; Bing, C. Identification of macrophage inhibitory cytokine-1 in adipose tissue and its secretion as an adipokine by human adipocytes. Endocrinology 2009, 150, 1688–1696. [Google Scholar] [CrossRef]
- Minamino, T.; Orimo, M.; Shimizu, I.; Kunieda, T.; Yokoyama, M.; Ito, T.; Nojima, A.; Nabetani, A.; Oike, Y.; Matsubara, H.; et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat. Med. 2009, 15, 1082–1087. [Google Scholar] [CrossRef]
- Elion, R.A.; Althoff, K.N.; Zhang, J.; Moore, R.D.; Gange, S.J.; Kitahata, M.M.; Crane, H.M.; Drozd, D.R.; Stein, J.H.; Klein, M.B.; et al. Recent abacavir use increases risk of type 1 and type 2 myocardial infarctions among adults with HIV. J. Acquir. Immune Defic. Syndr. 2018, 78, 62–72. [Google Scholar] [CrossRef]
- Savès, M.; Raffi, F.; Capeau, J.; Rozenbaum, W.; Ragnaud, J.M.; Perronne, C.; Basdevant, A.; Leport, C.; Chêne, G.; Antiprotéases Cohorte (APROCO) Study Group. Factors related to lipodystrophy and metabolic alterations in patients with human immunodeficiency virus infection receiving highly active antiretroviral therapy. Clin. Infect. Dis. 2002, 34, 1396–1405. [Google Scholar] [CrossRef] [Green Version]
- Yatsuga, S.; Fujita, Y.; Ishi, A.; Fukumoto, Y.; Arahata, H.; Kakuma, T.; Kojima, T.; Ito, M.; Tanaka, M.; Saiki, R.; et al. Growth differentiation factor 15 as a useful biomarker for mitochondrial disorders. Ann. Neurol. 2015, 78, 814–823. [Google Scholar] [CrossRef] [Green Version]
- Montero, R.; Yubero, D.; Villarroya, J.; Henares, D.; Jou, C.; Rodríguez, M.A.; Ramos, F.; Nascimento, A.; Ortez, C.I.; Campistol, J.; et al. GDF-15 Is Elevated in Children with Mitochondrial Diseases and Is Induced by Mitochondrial Dysfunction. PLoS ONE 2016, 11, e0148709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Sucerquia, L.J.; Blas-Garcia, A.; Marti-Cabrera, M.; Esplugues, J.V.; Apostolova, N. Profile of stress and toxicity gene expression in human hepatic cells treated with Efavirenz. Antiviral Res. 2012, 94, 232–241. [Google Scholar] [CrossRef]
- Monroe, A.K.; Haberlen, S.A.; Post, W.S.; Palella, F.J.; Kinsgley, L.A.; Witt, M.D.; Budoff, M.; Jacobson, L.P.; Brown, T.T. Cardiovascular disease risk scores’ relationship to subclinical cardiovascular disease among HIV-infected and HIV-uninfected men. AIDS 2016, 30, 2075–2084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triant, V.A.; Perez, J.; Regan, S.; Massaro, J.M.; Meigs, J.B.; Grinspoon, S.K.; D’Agostino, R.B. Cardiovascular Risk Prediction Functions Underestimate Risk in HIV Infection. Circulation 2018, 137, 2203–2214. [Google Scholar] [CrossRef] [PubMed]
| Parameter | HIV-1-Infected Patients | p-Value | |||
|---|---|---|---|---|---|
| Controls | Naive | HALS− | HALS+ | ||
| (n = 34) | (n = 49) | (n = 43) | (n = 60) | ||
| Age, years | 40 (39–42) | 37 (31–42) | 43 (39–49) | 45 (41–49) | <0.0001 |
| Sex, male % | 25 (73.5) | 42 (85.7) | 33 (76.7) | 60 (43.6) | 0.4930 |
| Means of transmission, % | <0.0001 | ||||
| HTSX, % | ---- | 15 (30.6) | 15 (34.9) | 23 (38.3) | |
| IDU, % | ---- | 2 (4.1) | 11 (25.6) | 23 (38.3) | |
| MsM, % | ---- | 32 (65.3) | 17 (39.5) | 14 (23.3) | |
| Years of infection | ---- | 2 (1–3) | 12 (7–17.7) | 14 (10–16) | <0.0001 |
| AIDS, % | ---- | 4 (8.1) | 13 (30.2) | 29 (48.3) | <0.0001 |
| HCV infection, % | 0 (0) | 6 (12.2) | 14 (32.5) | 27 (45.0) | 0.0034 |
| HBV infection, % | 0 (0) | 3 (6.1) | 2 (4.6) | 5 (8.3) | 0.3823 |
| CD4 count /mm3 | ---- | 414 (329–606) | 684 (409–908) | 609 (381–839) | 0.0104 |
| CD4 nadir/mm3 | ---- | 364 (256–490) | 161 (82–409) | 119 (22–361) | <0.0001 |
| CD8 count/mm3 | ---- | 826 (540–1298) | 936 (763–1088) | 790 (599–1169) | 0.7098 |
| Serum viral load, log10 | ---- | 4.4 (3.6–5.4) | 2.0 (1.3–2.5) | 2.1 (1.3–2.9) | <0.0001 |
| Undetectable viral load, % | ---- | 0 (0) | 35 (81.4) | 48 (80.0) | <0.0001 |
| Baseline viral load, log10 | ---- | 5.1 (4.2–5.5) | 5.1 (4.4–5.6) | 5.2 (4.0–5.6) | 0.9045 |
| Antiretroviral drug exposure | |||||
| NRTI, m | ---- | ---- | 197 (149–236) | 226 (187–265) | <0.0001 |
| TDF in current regimen, % | ---- | ---- | 25 (58.1) | 29 (48.3) | 0.3251 |
| NNRTI, m | ---- | ---- | 35 (2–52) | 51 (26–69) | 0.0019 |
| PI, m | ---- | ---- | 47 (22–69) | 48 (26–75) | 0.5208 |
| PI in current regimen | ---- | ---- | 25 (59.6) | 15 (30.6) | 0.0400 |
| NNRTI in current regimen | ---- | ---- | 23 (40.3) | 34 (69.3) | |
| Parameter | Controls (n = 34) | HIV-1-Infected Patients | p-Value | ||
|---|---|---|---|---|---|
| Naïve (n = 49) | HALS− (n = 43) | HALS+ (n = 60) | |||
| Smokers, % | 2 (5.9) | 26 (54.1) | 28 (65.1) | 26 (48.3) | <0.0001 |
| Diabetes mellitus, % | 0 (0) | 2 (4.1) | 0 (0) | 10 (16.7) | 0.0011 |
| Metabolic syndrome, % | 0 (0) | 5 (10.2) | 3 (6.9) | 20 (33.3) | <0.0001 |
| Systolic BP, mm Hg | 118 (111–128) | 120 (110–122) | 120 (110–130) | 127 (111–140) | 0.0232 |
| Diastolic BP, mm Hg | 69 (63–77) | 70 (70–80) | 75 (70–80) | 80 (70–81) | 0.0061 |
| Creatinine, mg/dL | 0.84 (0.74–1.02) | 0.95 (0.83–1.09) | 0.86 (0.77–1.02) | 0.89 (0.76–1.07) | 0.5181 |
| eGFR, mL/min/1.73 m2 | 105.2 (89.9–112) | 100 (81.5–114) | 100 (84.4–109.7) | 98 (81.7–110.4) | 0.4745 |
| BMI, kg/m2 | 24.3 (23.5–26.0) | 22.7 (20.8–25.5) | 23.4 (21.2–26.7) | 23.5 (21.7–25.8) | 0.1697 |
| Waist circumference, cm | 89 (81–92) | 83 (77–91) | 88 (83–97) | 86 (80–95) | 0.1217 |
| Waist-to-hip ratio | 0.88 (0.84–0.91) | 0.87 (0.82–0.94) | 0.94 (0.89–0.99) | 0.93 (0.91–1.01) | <0.0001 |
| Total body fat, % | 24.8 (22.2–28.1) | 16.9 (14.7–24.2) | 22.7 (17.5–27.0) | 18.6 (14.5–23.7) | <0.0001 |
| Trunk fat, kg | 9.1 (7.8–10.8) | 5.7 (3.9–9.4) | 7.6 (6.3–10.5) | 8.0 (5.1–12.2) | 0.0064 |
| Appendicular fat, kg | 8.5 (6.5–10.3) | 6.0 (3.4–7.6) | 5.2 (3.3–7.9) | 3.2 (2.2–5.3) | <0.0001 |
| TAFR | 1.1 (0.9–1.3) | 1.1 (0.9–1.3) | 1.4 (1.1–2.2) | 2.3 (1.7–3.0) | <0.0001 |
| Total cholesterol, mmol/L | 5.4 (4.3–5.7) | 4.0 (3.8–5.0) | 4.9 (4.2–5.2) | 5.1 (4.1–6.1) | 0.0015 |
| Triglycerides, mmol/L | 0.83 (0.65–1.09) | 1.0 (0.8–1.3) | 2.0 (1.3–2.5) | 2.0 (1.2–3.1) | <0.0001 |
| HDL-c, mmol/L | 1.5 (1.2–1.6) | 1.0 (0.8–1.3) | 1.2 (1.0–1.5) | 1.2 (0.9–1.4) | <0.0001 |
| LDL-c, mmol/L | 3.3 (2.5–3.9) | 2.4 (2.0–3.4) | 2.7 (1.9–2.9) | 2.9 (2.2–3.6) | 0.0047 |
| Apolipoprotein B, mmol/L | 0.85 (0.77–1.0) | 0.96 (0.87–1.19) | 1.0 (0.79–1.19) | 0.93 (0.82–1.25) | 0.0553 |
| Non-HDL-c, mmol/L | 3.9 (2.8–4.5) | 3.1 (2.4–3.9) | 3.5 (3.0–4.0) | 3.8 (2.9–4.9) | 0.0097 |
| Total cholesterol/HDL-c | 3.4 (2.9–4.5) | 4.1 (3.3–5.0) | 3.7 (3.1–4.9) | 4.2 (3.4–5.2) | 0.0362 |
| MUFAs, % total FA | 23.9 (21.9–26.1) | 26.1 (22.9–27.9) | 28.1 (24.7–31.2) | 28.1 (26.1–32.0) | <0.0001 |
| PUFAs, % total FA | 44.7 (42.3–46.2) | 40.4 (37.7–43.1) | 38.5 (33.1–40.9) | 37.7 (33.9–41.3) | <0.0001 |
| Glucose, mmol/L | 4.8 (4.6–5.2) | 5.0 (4.5–5.5) | 5.2 (4.8–5.5) | 5.5 (5.1–6.1) | <0.0001 |
| Insulin, pmol/L | 27.0 (20.0–52.5) | 29.0 (20.0–53.0) | 48.0 (30.0–83.7) | 85.0 (56.0–135.5) | <0.0001 |
| HOMA1-IR | 0.5 (0.4–1.0) | 0.5 (0.4–0.9) | 0.8 (0.6–1.6) | 1.6 (1.0–2.5) | <0.0001 |
| Adiponectin, µg/mL | 2.9 (1.5–12.5) | 3.8 (1.9–21.6) | 1.9 (1.2–3.7) | 1.3 (0.8–2.8) | <0.0001 |
| Leptin, log10 | 3.7 (3.4–3.9) | 3.1 (2.8–3.4) | 3.6 (3.0–3.9) | 3.4 (2.9–3.8) | 0.0018 |
| C-reactive protein, mmol/L | 0.9 (0.9–1.4) | 2.0 (0.9–5.0) | 1.5 (0.9–5.8) | 2.1 (0.9–3.6) | 0.0008 |
| FGF21, µmol/L | 35.4 (24.9–61.9) | 57.3 (25.6–228.5) | 64.4 (33.7–123.6) | 93 (49.03–125) | <0.0001 |
| Interleukin 6, pg/mL | 107 (65–165) | 156 (97–644) | 126 (70–241) | 128 (65–264) | 0.0427 |
| Interleukin 8, pg/mL | 405.5 (280–550) | 494 (285–719.5) | 429 (295.5–658.5) | 491 (339–898) | 0.0636 |
| TNF-α pg/mL | 345 (257–465.8) | 615 (288.3–949.5) | 399 (307–527.5) | 386 (252–554) | 0.0010 |
| MCP-1, pg/mL | 187 (157.3–240) | 220 (168.5–270.5) | 196 (167–218.5) | 196 (162–230) | 0.1266 |
| GDF15, ng/mL | 0.31 (0.15–0.4) | 0.55 (0.33–0.94) | 1.03 (0.64–2.55) | 1.19 (0.8–1.73) | 0.0039 |
| Serum GDF15 (pg/L) (Crude Analysis) | Age-Adjusted Serum GDF15 | |||
|---|---|---|---|---|
| r | p | r | p | |
| Age | 0.453 | <0.001 | ||
| Fasting insulin | 0.327 | <0.001 | 0.280 | 0.002 |
| HOMA1-IR | 0.326 | <0.001 | 0.272 | 0.003 |
| FGF21 | 0.422 | <0.001 | 0.425 | <0.001 |
| MUFAs | 0.479 | <0.001 | 0.417 | <0.001 |
| PUFAs | −0.492 | <0.001 | −0.468 | <0.001 |
| LDL cholesterol | −0.212 | 0.047 | −0.278 | 0.003 |
| Diastolic BP | 0.241 | 0.006 | 0.243 | 0.009 |
| Years of infection | 0.513 | <0.001 | 0.459 | <0.001 |
| NRTI exposure | 0.575 | <0.001 | 0.434 | <0.001 |
| NNRTI exposure | 0.151 | 0.002 | 0.141 | 0.372 |
| PI exposure | 0.417 | <0.001 | 0.392 | <0.001 |
| Interleukin-6 | 0.1882 | 0.031 | 0.285 | 0.013 |
| Interleukin-8 | 0.424 | <0.001 | 0.371 | <0.001 |
| TNF-α | 0.329 | 0.004 | 0.215 | 0.003 |
| Leptin | −0.165 | 0.063 | −0.203 | 0.029 |
| C reactive protein | 0.289 | 0.001 | 0.318 | 0.001 |
| Framingham CVR | 0.377 | <0.001 | 0.152 | 0.105 |
| 10-yr. Framingham CVR | 5-yr. D:A:D CVR | |||||||
|---|---|---|---|---|---|---|---|---|
| Crude Analysis | Age-Adjusted | Crude Analysis | Age-Adjusted | |||||
| r | p | r | p | r | p | r | p | |
| Age | 0.719 | <0.001 | 0.671 | <0.001 | ||||
| Systolic BP | 0.427 | <0.001 | 0.362 | <0.001 | 0.233 | 0.081 | 0.120 | 0.285 |
| Diastolic BP | 0.259 | <0.001 | 0.234 | 0.005 | 0.015 | 0.139 | 0.045 | 0.689 |
| WHR | 0.139 | 0.058 | −0.005 | 0.949 | 0.213 | 0.015 | 0.296 | 0.007 |
| Trunk fat | 0.190 | 0.010 | 0.096 | 0.249 | 0.230 | 0.005 | 0.226 | 0.043 |
| TAFR | 0.522 | <0.001 | 0.268 | 0.001 | 0.496 | <0.001 | 0.331 | 0.003 |
| HOMA1-IR | 0.296 | <0.001 | 0.148 | 0.076 | 0.292 | <0.001 | 0.323 | 0.003 |
| Triglycerides | 0.388 | <0.001 | 0.358 | <0.001 | 0.306 | <0.001 | 0.419 | <0.001 |
| Total cholesterol/HDL-c ratio | 0.288 | <0.001 | 0.405 | <0.001 | 0.114 | 0.161 | 0.289 | 0.009 |
| NRTI exposure | 0.346 | <0.001 | 0.024 | 0.779 | 0.348 | <0.001 | 0.115 | 0.308 |
| NNRTI exposure | 0.307 | <0.001 | 0.061 | 0.463 | 0.252 | 0.002 | 0.104 | 0.354 |
| PI exposure | 0.376 | <0.001 | 0.171 | 0.039 | 0.358 | <0.001 | 0.293 | 0.008 |
| C reactive protein | 0.285 | <0.001 | 0.160 | 0.048 | 0.207 | 0.012 | 0.065 | 0.567 |
| FGF21 | 0.096 | 0.252 | 0.014 | 0.854 | 0.188 | 0.025 | 0.262 | 0.018 |
| GDF15 | 0.412 | <0.001 | 0.016 | 0.853 | 0.050 | 0.598 | 0.061 | 0.429 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domingo, P.; Mateo, M.G.; Villarroya, J.; Cereijo, R.; Torres, F.; Domingo, J.C.; Campderrós, L.; Gallego-Escuredo, J.M.; Gutierrez, M.d.M.; Mur, I.; et al. Increased Circulating Levels of Growth Differentiation Factor 15 in Association with Metabolic Disorders in People Living with HIV Receiving Combined Antiretroviral Therapy. J. Clin. Med. 2022, 11, 549. https://doi.org/10.3390/jcm11030549
Domingo P, Mateo MG, Villarroya J, Cereijo R, Torres F, Domingo JC, Campderrós L, Gallego-Escuredo JM, Gutierrez MdM, Mur I, et al. Increased Circulating Levels of Growth Differentiation Factor 15 in Association with Metabolic Disorders in People Living with HIV Receiving Combined Antiretroviral Therapy. Journal of Clinical Medicine. 2022; 11(3):549. https://doi.org/10.3390/jcm11030549
Chicago/Turabian StyleDomingo, Pere, María Gracia Mateo, Joan Villarroya, Rubén Cereijo, Ferran Torres, Joan C. Domingo, Laura Campderrós, José M. Gallego-Escuredo, María del Mar Gutierrez, Isabel Mur, and et al. 2022. "Increased Circulating Levels of Growth Differentiation Factor 15 in Association with Metabolic Disorders in People Living with HIV Receiving Combined Antiretroviral Therapy" Journal of Clinical Medicine 11, no. 3: 549. https://doi.org/10.3390/jcm11030549
APA StyleDomingo, P., Mateo, M. G., Villarroya, J., Cereijo, R., Torres, F., Domingo, J. C., Campderrós, L., Gallego-Escuredo, J. M., Gutierrez, M. d. M., Mur, I., Corbacho, N., Vidal, F., Villarroya, F., & Giralt, M. (2022). Increased Circulating Levels of Growth Differentiation Factor 15 in Association with Metabolic Disorders in People Living with HIV Receiving Combined Antiretroviral Therapy. Journal of Clinical Medicine, 11(3), 549. https://doi.org/10.3390/jcm11030549







