Abstract
A radiosynovectomy (RS) should be indicated when recurrent articular bleeds related to chronic hemophilia synovitis (CHS) exist, established by clinical examination, and confirmed by imaging techniques that cannot be constrained with hematological prophylaxis. RS can be performed at any point in life, mainly in adolescents (>13–14 years) and adults. Intraarticular injection (IAI) of a radioactive material in children might be arduous since we need child collaboration which might include general anesthesia. RS is our initial option for management of CHS. For the knee joint we prescribe Yttrium-90, while for the elbow and ankle we prescribe Rhenium-186 (1 to 3 IAIs every 6 months). The procedure is greatly cost efficient when compared to surgical synovectomy. Chemical synovectomy with rifampicin has been reported to be efficacious, inexpensive, simple, and especially practical in developing countries where radioactive materials are not easily available. Rifampicin seems to be more efficacious when it is utilized in small joints (elbows and ankles), than when utilized in bigger ones (knees). When RS and/or chemical synovectomy fail, arthroscopic synovectomy (or open synovectomy in some cases) should be indicated. For us, surgery must be performed after the failure of 3 RSs with 6-month interims. RS is an effective and minimally invasive intervention for treatment of repeated articular bleeds due to CHS. Although it has been published that the risk of cancer does not increase, and that the amount of radioactive material used in RS is insignificant, the issue of chromosomal and/or deoxyribonucleic acid (DNA) changes remains a concern and continued surveillance is critical. As child and adulthood prophylaxis becomes more global, RS might become obsolete in the long-term.
1. Introduction
Hemophilic arthropathy happens because of repeated bleeding into articulations resulting in swelling and degeneration of cartilaginous and osseous tissues in the affected joint. Even though hematological prophylaxis averts arthropathy, it is not always appropriate or accessible [,,,]. The only approach for averting arthropathy in people with hemophilia (PWH) without inhibitors is early primary prophylaxis, although it is not always entirely successful in avoiding articular problems [,,,]. In infants with inhibitors, prophylaxis with bypassing agents (aPCCs and/or RFVIIa) is also recommended to prevent articular complications [,,,]. To prevent joint degeneration in the hemophilic joints due to the impact of blood on the synovial membrane and the cartilage cells, early primary prophylaxis (intravenous infusion of the deficient factor) is the gold standard of treatment of hemophilia. In patients with hemophilia A (deficit of factor VIII), emicizumab prophylaxis has led to greater treatment satisfaction compared with FVIII prophylaxis, reflecting in part the low treatment burden of emicizumab associated with its infrequent, subcutaneous administration. Emicizumab can also be used in patients with inhibitors [,,,].
Articular bleeds cause chondrocyte death and chronic hemophilia synovitis (CHS) resulting in a malicious circle of synovitis-hemartrosis-synovitis. This circle has to be broken quickly to halt or slow the appearance of hemophilic arthropathy. The hypertrophied synovium can be detected through palpation as a hard mass. Removal of the hypertrophied synovial membrane may be performed by using radioisotopes [,,,,,,,,,,,,]. Figure 1 summarizes the mechanism of action of radioactive materials injected intra-articularly (radiosynovectomy-RS) [,,,].

Figure 1.
Mechanism of action of radiosynovectomy (RS).
The objective of RS is to lower the danger of CHS (Figure 2) and recurrent hemarthroses that eventually degenerate the joint (hemophilic arthropathy). Hemophilia is a polyarticular disease, impacting mainly elbows, knees and ankles.
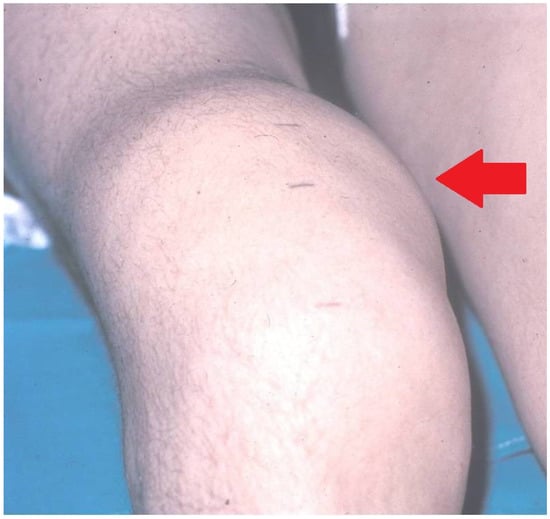
Figure 2.
Serious chronic hemophilia synovitis (CHS) in a hemophilic patient (arrow).
Therefore, it is important to remember that we are facing a multiarticular condition. Confirmation of the problem has to be done with magnetic resonance imaging (MRI) (Figure 3) and/or ultrasonography (US) (Figure 4).
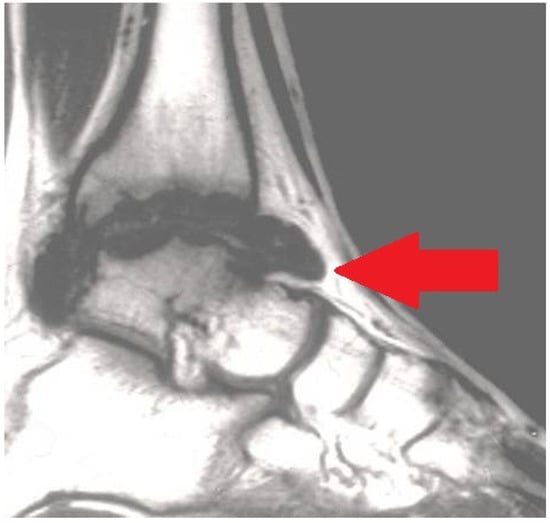
Figure 3.
Magnetic resonance imaging (MRI) exhibiting serious chronic hemophilic synovitis (CHS) of the ankle (arrow).
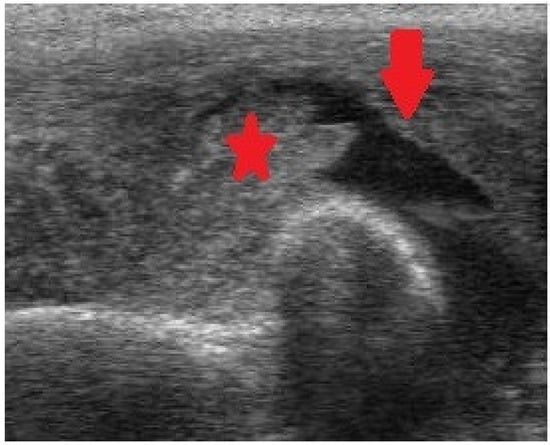
Figure 4.
Ultrasonography (US) of the elbow exhibiting intraarticular fluid (arrow) and intense synovial enlargement (asterisk).
The purpose of this article is to update the function of radiosynovectomy (RS) in the treatment of CHS in PWH.
2. When Should a Radiosynovectomy (RS) Be Indicated?
RS is the elimination of the hypertrophied synovium using an intraarticular injection of a radioisotope. We indicate a RS in the following circumstances [,,,,,]: (1) Two or more events of hemarthrosis in the preceding 6 months; hypertrophied synovium must be confirmed by MRI and/or US. (2) An additional RS must be performed in PWH with two or more episodes of articular bleed in the following 6 months. RS must only be done in specialized hemophilia centers.
MRI and/or US may enhance our prompt detection of CHS, and they can be performed at any stage in life. According to Doria et al. [], even though MRI is the gold standard, US is highly helpful for assessing CHS. MRI can be performed once or twice a year, while US can be carried out as many times as needed. Sierra-Aisa et al. compared US and MRI in PWH []. It was found that US was valuable in uncovering joint bleeds, CHS and articular erosions, with results comparable to those of MRI.
When RS has to be repeated, the procedure is identical to that performed for the first procedure. The result measures have to be obtained 6 months after each RS and then every 6 months until the last follow-up evaluation. The most important result measures are the amount of hemarthroses per month (reduction in hemarthroses), factor use, and the clinical outcome [range of motion (ROM) of the involved joint].
Chemical synovectomy with rifampicin has been reported to be efficacious, inexpensive, simple, and especially practical in developing countries where radioactive materials are not easily available [,,,]. Rifampicin seems to be more efficacious when it is utilized in small joints (elbows and ankles), than when utilized in bigger ones (knees) [,]. When RS and/or chemical synovectomy fail, arthroscopic synovectomy (or open synovectomy in some cases) should be indicated. In a study, 6.3% of articulations required arthroscopic synovectomy or total knee arthroplasty (TKA) [].
3. RS in Individuals with Inhibitors
Patients with inhibitors experience more bleeding episodes. As a consequence, they suffer greater ROM (range of movement) limitation, more movement impairments, more serious orthopedic complications, and poorer quality of life (QoL) [,].
Prophylaxis with bypassing drugs has proved its effectiveness in numerous reports. Up to now, aPCCs (activated prothrombin complex concentrates) and rFVIIa (recombinant factor VII activated) have been utilized in many patients. Both bypassing drugs have shown a decrease in the frequency of bleeding and amelioration of QoL [,,,,,,,,,,,,,,,,,,,,]. In patients with hemophilia A, prophylaxis with subcutaneous emicizumab has also proved its efficacy in many publications [,,,].
When in patients with inhibitors, it is impossible to control recurrent bleeding using bypassing drugs or emicizumab as prophylaxis, a RS must be indicated. A study analyzed four individuals (6 RSs) aged 13 to 17 years who had 7 to 14 bleeding episodes per patient in the previous 12 months) []. No intraarticular bleeding or local inflammatory reaction was noticed during or after treatment, and no radioactivity was detected in the urine. All patients improved both subjectively and objectively. At a 2 year follow-up, the amount of bleeding events per year ranged from 1 to 5, a striking reduction.
In five PWH with inhibitors younger than 15 years, 13 articulations were treated with intraarticular injections of radioactive gold by Lofqvist and Petersson []. Of the 13 articulations injected, a bleeding-free interim of more than 6 months was obtained in 9 patients, of which 6 were free from bleeding for more than a year.
In a study, nine PWH with factor inhibitor aged from 3 to 4 years, 19 joints were treated with RS using radioactive gold []. RS was performed when the antibody titer was low (<10 Bethesda units). At long-term follow-up (range, 18–182 months), results were good in five joints, fair in one joint, and poor in eleven joints. The results were inferior to those for PWH without inhibitor.
We have previously stated that prophylaxis is essential in attempting to avoid the appearance of CHS, and that preeminent treatment for CHS in PWH with inhibitors is RS. With both strategies (prophylaxis and RS), the appearance of serious joint degeneration can be delayed [].
According to Pasta et al., in PWH with inhibitors, a more serious degree of CHS is often observed because treatment is more difficult in this context []. For them, the best management option for recurrent hemarthroses and/or CHS is both chemical synovectomy and RS, with a success percentage of about 80% for both. Nevertheless, RS should be chosen in PWH with inhibitors because it makes it feasible to attain excellent fibrosis of the synovium commonly with one injection; without the necessity of more injections, the risk of recurrent hemarthroses and concentrate use diminishes.
Table 1 summarizes the most important articles on the subject [,,,,,,,,,,,,,,,,].

Table 1.
Reports on radiosynovectomy (RS) in people with hemophilia (PWH) from 2014 to 2022.
4. Technique of Radiosynovectomy
RS must be performed under factor coverage to avoid the risk of hemarthrosis during the procedure. The main radioisotope used in the literature are 90Y (Yttrium-90), 186Rh (Rhenium-186) and 32P (Phosporus-32). All of them give off beta radiation and their therapeutic penetration powers (TPP) in millimeters are 2.8 mm, 1 mm, and 2.2 mm, respectively. In the knee we use Yttrium-90 [185 Megabecquerels (MBq)]. Rhenium-186 is used for elbows (56–74 MBq) and ankles (74 MBq). A small quantity of 99Tc (technetium) is introduced to perform articular scintigraphy after the procedure (to confirm the right dispersion of the radioisotope into the joint) [,,,,,].
We do not use local anesthetic. An ordinary needle is sufficient. When the joint has been accessed, all the fluid (blood or synovial fluid) is evacuated, and only then the radioisotope is introduced. The needle must be removed gradually whilst simultaneously introducing an anti-inflammatory agent (e.g., betamethasone), so as to not cause skin burn. Figure 5 shows the technique and all the elements required for ankle RS.
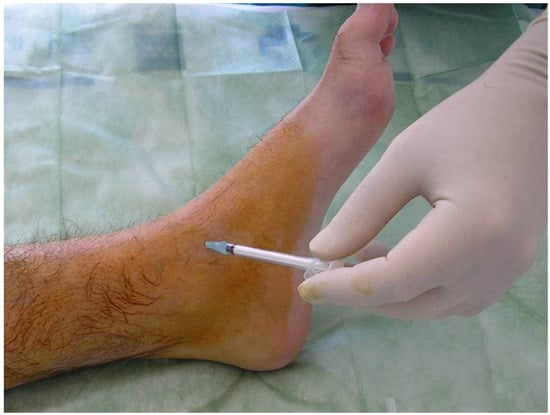
Figure 5.
Radiosynovectomy (RS) of the ankle joint with Rhenium-186 in a haemophilic patients. The needle has to be removed gradually while at the same time introducing an anti-inflammatory agent.
5. Effectiveness of Radiosynovectomy
In relevant articles, 40% to 85% of articulations achieve good clinical outcomes; between 30% and 80% of joints exhibit a reduction in hemarthroses; and 35% to 85% of individuals exhibit a reduction in factor use; in other words, the amount of joint bleeds per month decrease from 3 to 6 on average before the procedure to 1 after the procedure [,,,,,,,,,,,,].
Between 1976 and 2013, we performed 500 RSs (with Yttrium-90 or Rhenium-186) in 443 joints of 345 individuals suffering from CHS []. Their average age was 24 years (range, 6–53) and the follow-up was 18.5 years on average (range: 6 months-38 years). We performed 1 to 3 procedures with a 6-month interim between them. Articular bleeding frequency declined by 64% on average.
In another report, we encountered that decrease of the number of hemarthoses after RS was 68% on average when RS-1 was used, 62% with RS-2, and 61% with RS-3 []. The volume of the synovium declined 31%. World Federation of Hemophilia (WFH) clinical score improved 19%. WFH radiological score did not improve [,]. In one of our reports, we found that knees required more RSs than elbows or ankles, and that the more serious hypertrophied synovial membranes required a greater number of RSs [].
In another report, we found that RS was effective in all cohorts of patients, separately from the existence of inhibitor, the kind of articulation affected, the grade of CHS, and the existence of articular destruction (arthropathy) in the radiological examination []. In our center, we have also demonstrated that each RS performs separately in CHS []. In another report, we found that the variables analyzed improved to an equal grade in articulations with joint destruction in simple radiography (AJDSR) and without AJDSR. No joint without AJDSR required RS-3; this was the only dissimilarity our investigation found between joints without AJDSR and those with AJDSR when RS was performed [].
In 2001, Silva et al. reported 130 RSs utilizing Phosporus-32 with a mean follow-up of 36 months []. Excellent and good outcomes (hemarthrosis decrease from 75% to 100%) were obtained in 79.2% of patients at 6 months to 8 years. No correlation between results and age or degree of arthropathy was found. No complications were observed.
In another report of 125 RSs, 54% got complete arrest of hemarthroses. 73% of patients reported improved mobility of the injected articulation. 79% of patients had a substantial amelioration in QoL attributable to the treated joint. No complications were observed [].
The results of RS with 90Y in 163 joints of PWH were published by Heim et al. []. The median age at the time of the injection was between 11 and 15 years and the median follow-up period was 11 years. Over 80% of PWH reported a decrease in the amount of articular bleeds and 15% experienced full cessation of hemarthroses.
Mortazavi et al. analyzed 66 Phosporus-32 RS in 53 patients []. The mean follow-up was 31 months. The mean age of patients at the time of RS was 16 years. It was found that 77% of individuals experienced a 50% decrease in bleeding incidence after RS.
In 2009, Calegaro et al. evaluated the effectiveness of RS with 153-Sm-HA (185 Mbq) in 31 patients (30 males). Their mean age was 20 years (8 to 34 years). The use of 153 Sm-HA in the treatment of CHS was effective for elbows and ankles, but less effective for knees [].
In 2010, Cho et al. analyzed clinical outcomes and radiologic evaluation of 58 RS (53 patients) utilizing Holmium-166-chitosan complex in PWH []. The mean age of patients was 14 years. The mean follow-up was 33 months. After the injection, the mean frequency of bleeding of the elbow diminished from 3.76 to 0.47 times a month, the knee from 5.87 to 1.12 times a month, and the ankle from 3.62 to 0.73 times per month, respectively.
Turkmen et al. performed 67 Yttrium-90 RS in 67 patients []. Their mean age was 17 years. The mean follow-up was 40 months. It was concluded that Yttrium-90 RS in the knee joint is an important resource for the treatment of CHS, markedly reducing joint bleeding.
6. Complications of Radiosynovectomy
Our reported percentage of adverse events is 1%. The adverse events that we have encountered are the following [,,,,,]: (1) Little skin burns repaired in 1–2 weeks just by cleaning them. They occur when the radioactive material is unintentionally introduced out of the joint (Figure 6); (2) infection (septic arthritis) which requires surgical management (arthrotomy and joint debridement) plus intravenous antibiotics; (3) swelling following injections solved with rest and NSAIDS (Nonsteroidal Anti-inflammatory Drugs). We specifically recommend cyclooxigenase-2 (COX-2) inhibitor inhibitors [,].
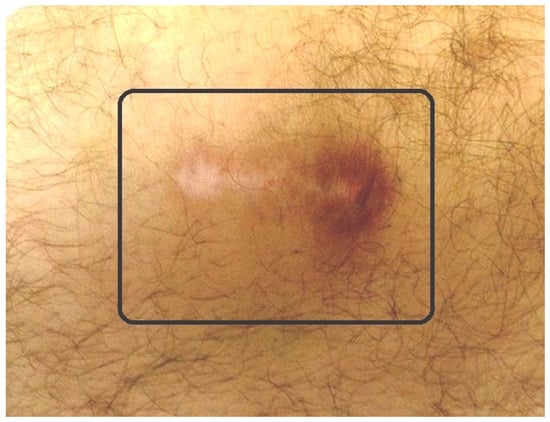
Figure 6.
Small radioactive burn of the adjacent skin (square) after knee radiosynovectomy (RS).
7. Is Radiosynovectomy Safe?
A great number of hemophilic children who may profit from RS for the constraint of CHS do not experience the procedure because there is debate in the literature concerning the safety of radioactive materials after two cases of acute lymphocytic leukemia (ALL) in infants with hemophilia managed with Phosphorus-32 RS were published [].
In 2007, Turkmen et al. studied the genotoxic impact on the peripheral blood lymphocytes possibly caused by Yttrium-90 in children who were experiencing RS for CHS, using chromosomal aberration analysis (CA) and the micronuclei (MN) assay for detecting chromosomal aberrations, as well as the sister chromatid exchanges (SCE) technique for assessed DNA damage []. The outcomes of this investigation indicated that high radiation doses were not attained by peripheral lymphocytes of children who experience Yttrium-90 RS.
No augmentation in the risk of cancer has been reported by Infante-Rivard et al. []. Moreover, they found no dose-response relationship with the amount of radioisotope administered or number of RSs. Table 2 shows that the amount of radiation used is insignificant.

Table 2.
Estimation of the dose of radiation of RS in various situations. Notice that the radiation dose of RS is insignificant.
Infants experiencing knee RS get a radiation dose of approximately 0.74 mSv (90 megabecquerels-MBq) and for elbow and ankle RSs a dose of approximately 0.32 mSv (30–40 MBq). The radiation dose from natural sources is approximately 2 mSv and the recommended limit for patients (apart from natural sources) is 1 mSv/year. The lifetime cancer risk increases about 0.5% per 100 mSv/year.
8. Conclusions
The recommendation for a RS is the existence of recurrent hemarthroses due to CHS (verified clinically and by imaging techniques) that cannot be constrained with hematological prophylaxis. RS can be performed at any age, ideally in teenagers (>13–14 years). We advise Yttrium-90 for the knees and Rhenium-186 for elbows and ankles (1 to 3 RSs with 6-month interim). Chemical synovectomy with rifampicin has been reported to be efficacious, inexpensive, simple, and especially practical in developing countries where radioactive materials are not easily available. Rifampicin seems to be more efficacious when it is utilized in small joints (elbows and ankles), than when utilized in bigger ones (knees). When RS and/or chemical synovectomy fail, arthroscopic synovectomy (or open synovectomy in some cases) should be indicated. For us, surgery must be advised when three RSs with 6-month intervals fail to control CHS. RS is an effective and minimally invasive intervention for treatment of repeated articular bleeds due to CHS.
Author Contributions
All authors participated equally in all tasks: Conceptualization; methodology; writing—original draft preparation; writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Astermark, J. When to start and when to stop primary prophylaxis in patients with severe haemophilia. Haemophilia 2003, 9 (Suppl. S1), 32–36. [Google Scholar] [CrossRef] [PubMed]
- Manco-Johnson, M.J.; Abshire, T.C.; Shapiro, A.D.; Riske, B.; Hacker, M.R.; Kilcoyne, R.; Ingram, J.D.; Manco-Johnson, M.L.; Funk, S.; Jacobson, L.; et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N. Engl. J. Med. 2007, 357, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Berntorp, E.; Fischer, K.; Miners, A. Models of prophylaxis. Haemophilia 2012, 18 (Suppl. S4), 136–140. [Google Scholar] [CrossRef] [PubMed]
- Ljung, R. Hemophilia and prophylaxis. Pediatr. Blood Cancer 2013, 60 (Suppl. S1), S23–S26. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Merchan, E.C.; Valentino, L.A. Emicizumab: Review of the literatura and critical appraisal. Haemophilia 2019, 25, 11–20. [Google Scholar] [CrossRef]
- Kempton, C.; Trask, P.; Parnes, A.; Niggli, M.; Campinha-Bacote, A.; Ucallaghan, M.; O’Connell, N.; Paz-Priel, I.; Mahlangu, J.N. Development and testing of the Satisfaction Questionnaire with Intravenous or Subcutaneous Hemophilia Injection and results from the Phase 3 HAVEN 3 study of emicizumab prophylaxis in persons with haemophilia A without FVIII inhibitors. Haemophilia 2021, 27, 221–228. [Google Scholar] [CrossRef]
- Barg, A.A.; Budnik, I.; Avishai, E.; Brutman-Barazani, T.; Bashari, D.; Misgav, M.; Lubetsky, A.; Kuperman, A.A.; Livnat, T.; Kenet, G. Emicizumab prophylaxis: Prospective longitudinal real-world follow-up and monitoring. Haemophilia 2021, 27, 383–391. [Google Scholar] [CrossRef]
- Mazurkiewicz, Ł.; Czernikiewicz, K.; Rupa-Matysek, J.; Gil, L. Emicizumab: A novel drug in hemophilia A prophylaxis—A narrative review. Expert Rev. Hematol. 2022, 15, 933–942. [Google Scholar] [CrossRef]
- Silva, M.; Luck, J.V.; Siegel, M.E., Jr. 32P chromic phosphate radiosynovectomy for chronic haemophilic synovitis. Haemophilia 2001, 7 (Suppl. S2), 40–49. [Google Scholar] [CrossRef]
- Siegel, H.J.; Luck, J.V.; Siegel, M.E., Jr.; Quinones, C. Phosphate-32 colloid radiosynovectomy in hemophilia: Outcome of 125 procedures. Clin. Orthop. Relat. Res. 2001, 392, 409–417. [Google Scholar] [CrossRef]
- Heim, M.; Goshen, E.; Amit, Y.; Martinowitz, U. Synoviorthesis with radioactive Yttrium in haemophilia: Israel experience. Haemophilia 2001, 7 (Suppl. S2), 36–39. [Google Scholar] [CrossRef]
- Mortazavi, S.M.; Asadollahi, S.; Farzan, M.; Shahriaran, S.; Aghili, M.; Izadyar, S.; Lak, M. (32)P colloid radiosynovectomy in treatment of chronic haemophilic synovitis: Iran experience. Haemophilia 2007, 13, 182–188. [Google Scholar] [CrossRef]
- Calegaro, J.U.; Machado, J.; De Paula, J.C.; De Almeida, J.S.; Casulari, L.A. Clinical evaluation after 1 year of 153-samarium hydroxyapatite synovectomy in patients with haemophilic arthropathy. Haemophilia 2009, 15, 240–246. [Google Scholar] [CrossRef]
- Cho, Y.J.; Kim, K.I.; Chun, Y.S.; Rhyu, K.H.; Kwon, B.K.; Kim, D.Y. Radioisotope synoviorthesis with Holmium-166-chitosan complex in haemophilic arthropath. Radioisotope synoviorthesis with Holmium-166-chitosan complex in haemophilic arthropathy. Haemophilia 2010, 16, 640–646. [Google Scholar]
- De la Corte-Rodriguez, H.; Rodriguez-Merchan, E.C.; Jimenez-Yuste, V. Radiosynovectomy in patients with chronic haemophilic synovitis: When is more than one injection necessary? Eur. J. Haematol. 2011, 86, 430–435. [Google Scholar] [CrossRef]
- De la Corte-Rodriguez, H.; Rodriguez-Merchan, E.C.; Jimenez-Yuste, V. Radiosynovectomy in hemophilia: Quantification of its effectiveness through the assessment of 10 articular parameters. J. Thromb. Haemost. 2011, 9, 928–935. [Google Scholar] [CrossRef]
- De la Corte-Rodriguez, H.; Rodriguez-Merchan, E.C.; Jimenez-Yuste, V. What patient, joint and isotope characteristics influence the response to radiosynovectomy in patients with haemophilia? Haemophilia 2011, 17, e990–e998. [Google Scholar] [CrossRef]
- De la Corte-Rodriguez, H.; Rodriguez-Merchan, E.C.; Jimenez-Yuste, V. Consecutive radiosynovectomy procedures at 6-monthly intervals behave independently in haemophilic synovitis. Blood Transfus. 2013, 11, 254–259. [Google Scholar]
- Rodriguez-Merchan, E.C.; De la Corte-Rodriguez, H.; Jimenez-Yuste, V. Is radiosynovectomy (RS) effective for joints damaged by haemophilia with articular degeneration in simple radiography (ADSR)? Thromb. Res. 2014, 133, 875–879. [Google Scholar] [CrossRef]
- Rodriguez-Merchan, E.C.; De la Corte-Rodriguez, H.; Jimenez-Yuste, V. Radiosynovectomy in haemophilia: Long-term results of 500 procedures performed in a 38-year period. Thromb. Res. 2014, 134, 985–990. [Google Scholar] [CrossRef]
- Knut, L. Radiosynovectomy in the therapeutic management of arthritis. World J. Nucl. Med. 2015, 14, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Savio, E.; Ures, M.C.; Zeledón, P.; Trindade, V.; Paolino, A.; Mockford, V.; Malanga, A.; Fernández, M.; Gaudiano, J. 188Re radiopharmaceuticals for radiosynovectomy: Evaluation and comparison of tin colloid, hydroxyapatite and tin-ferric hydroxide macroaggregates. BMC Nucl. Med. 2004, 4, 1. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Uğur, O.; Gedik, G.K.; Atilla, B.; Rubello, D. Radiosynovectomy: Current status in the management of arthritic conditions. Nucl. Med. Commun. 2008, 29, 755–758. [Google Scholar] [CrossRef]
- Chojnowski, M.M.; Felis-Giemza, A.; Kobylecka, M. Radionuclide synovectomy—Essentials for rheumatologists. Reumatologia 2016, 54, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Doria, A.S.; Keshava, S.N.; Mohanta, A.; Jarrin, J.; Blanchette, V.; Srivastava, A.; Moineddin, R.; Kavitha, M.L.; Hilliard, P.; Poonnoose, P.; et al. Diagnostic accuracy of ultrasound for assessment of hemophilic arthropathy: MRI correlation. AJR Am. J. Roentgenol. 2015, 204, W336–W347. [Google Scholar] [CrossRef]
- Sierra Aisa, C.; Lucía Cuesta, J.F.; Rubio Martínez, A.; Fernández Mosteirín, N.; Iborra Muñoz, A.; Abío Calvete, M.; Guillén Gómez, M.; Moretó Quintana, A.; Rubio Félix, D. Comparison of ultrasound and magnetic resonance imaging for diagnosis and follow-up of joint lesions in patients with haemophilia. Haemophilia 2014, 20, e51–e57. [Google Scholar] [CrossRef]
- Rodriguez-Merchan, E.C.; Caviglia, H.A.; Magallon, M.; Perez-Bianco, R. Chemical synovectomy vs. Radioactive synovectomy for the treatment of chronic haemophilic synovitis: A prospective short-term study. Haemophilia 1997, 3, 118–122. [Google Scholar] [CrossRef]
- Caviglia, H.A.; Fernandez-Palazzi, F.; Galatro, G.; Perez-Bianco, R. Chemical synoviorthesis with rifampicin in haemophilia. Haemophilia 2001, 7 (Suppl. S2), 26–30. [Google Scholar] [CrossRef]
- Rezazadeh, S.; Haghighat, A.; Mahmoodi, M.; Babanezhad, Z.; Karimi, M. Synoviorthesis induced by rifampicin in hemophilic arthropathy: A report of 24 treated joints. Ann. Hematol. 2011, 90, 963–969. [Google Scholar] [CrossRef]
- Suh, H.C.; Kim, D.K.; Kang, S.H.; Seo, K.M.; Kim, H.S.; Lee, J.Y.; Lee, S.Y.; Yoo, K.Y. Clinical and radiological evaluation after chemical synovectomy with rifampicin in hemophilic arthropathy: Korean experience with a 2-week Interval protocol. Ann. Rehabil. Med. 2018, 42, 449–456. [Google Scholar] [CrossRef]
- Soucie, J.M.; Cianfrini, C.; Janco, R.L.; Kulkarni, R.; Hambleton, J.; Evatt, B.; Forsyth, A.; Geraghty, S.; Hoots, K.; Abshire, T.; et al. Joint range-of-motion limitations among young males with hemophilia: Prevalence and risk factors. Blood 2004, 103, 2467–2473. [Google Scholar] [CrossRef]
- Leissinger, C.A. Prophylaxis in haemophilia patients with inhibitors. Haemophilia 2006, 12 (Suppl. S6), 67–72. [Google Scholar] [CrossRef]
- Morfini, M. Articular status of haemophilia patients with inhibitors. Haemophilia 2008, 14 (Suppl. S6), 20–22. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.M.; Lee, W.C.; Joshi, A.V.; Pashos, C.L. Health-related quality of life and productivity impact in haemophilia patients with inhibitors. Haemophilia 2009, 15, 911–917. [Google Scholar] [CrossRef]
- Hilgartner, M.W.; Makipernaa, A.; Dimichele, D.M. Long-term FEIBA prophylaxis does not prevent progression of existing joint disease. Haemophilia 2003, 9, 261–268. [Google Scholar] [CrossRef]
- Young, G.; McDaniel, M.; Nugent, D.J. Prophylactic recombinant factor VIIa in haemophilia patients with inhibitors. Haemophilia 2005, 11, 203–207. [Google Scholar] [CrossRef]
- Leissinger, C.A.; Becton, D.L.; Ewing, N.P.; Valentino, L.A. Prophylactic treatment with activated prothrombin complex concentrate (FEIBA) reduces the frequency of bleeding episodes in paediatric patients with haemophilia A and inhibitors. Haemophilia 2007, 13, 249–255. [Google Scholar] [CrossRef]
- Rodriguez-Merchan, E.C. Some recent developments regarding arthropathy and inhibitors in haemophilia. Haemophilia 2008, 14, 242–247. [Google Scholar] [CrossRef]
- Fischer, K.; Valentino, L.; Ljung, R.; Blanchette, V. Prophylaxis for severe haemophilia: Clinical challenges in the absence as well as in the presence of inhibitors. Haemophilia 2008, 14 (Suppl. S3), 196–201. [Google Scholar] [CrossRef]
- Jimenez-Yuste, V.; Quintana, M.; Alvarez, M.T.; Martin-Salces, M.; Hernandez-Navarro, F. “Primary prophylaxis” with rFVIIa in a patient with severe haemophilia A and inhibitor. Blood Coagul. Fibrinolysis 2008, 19, 719–720. [Google Scholar] [CrossRef]
- Rodriguez-Merchan, E.C. Prevention of haemophilic arthropathy in haemophilic children with inhibitors. Haemophilia 2008, 14 (Suppl. S6), 1–3. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Yuste, V.; Rodriguez-Merchan, E.C.; Alvarez, M.T.; Quintana, M.; Martin-Salces, M.; Hernandez-Navarro, F. Experiences in the prevention of arthropathy in haemophila patients with inhibitors. Haemophilia 2008, 14 (Suppl. S6), 28–35. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Yuste, V.; Alvarez, M.T.; Martín-Salces, M.; Quintana, M.; Rodriguez-Merchan, C.; Lopez-Cabarcos, C.; Velasco, F.; Hernández-Navarro, F. Prophylaxis in 10 patients with severe haemophilia A and inhibitor: Different approaches for different clinical situations. Haemophilia 2009, 15, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Valentino, L.A. The benefits of prophylactic treatment with APCC in patients with haemophilia and high-titre inhibitors: A retrospective case series. Haemophilia 2009, 15, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Manzato, F.; Salvagno, G.L.; Montagnana, M.; Zaffanello, M.; Lippi, G. Prophylaxis in congenital hemophilia with inhibitors: The role of recombinant activated factor VII. Semin. Thromb. Hemost. 2009, 35, 814–819. [Google Scholar] [CrossRef]
- Perry, D.; Berntorp, E.; Tait, C.; Dolan, G.; Holme, P.A.; Laffan, M.; Lassila, R.; Mumford, A.; Pasi, J.; Wilde, J.; et al. FEIBA prophylaxis in haemophilia patients: A clinical update and treatment recommendations. Haemophilia 2010, 16, 80–89. [Google Scholar] [CrossRef]
- Ettingshausen, C.E.; Kreuz, W. Early long-term FEIBA prophylaxis in haemophilia A patients with inhibitor after failing immune tolerance induction: A prospective clinical case series. Haemophilia 2010, 16, 90–100. [Google Scholar] [CrossRef]
- Valentino, L.A. Assessing the benefits of FEIBA prophylaxis in haemophilia patients with inhibitors. Haemophilia 2010, 16, 263–271. [Google Scholar] [CrossRef]
- Young, G.; Auerswald, G.; Jimenez-Yuste, V.; Konkle, B.A.; Lambert, T.; Morfini, M.; Santagostino, E.; Blanchette, V. When should prophylaxis therapy in inhibitor patients be considered? Haemophilia 2011, 17, e849–e857. [Google Scholar] [CrossRef]
- Rodriguez-Merchan, E.C.; Jimenez-Yuste, V.; Aznar, J.A.; Hedner, U.; Knobe, K.; Lee, C.A.; Ljung, R.; Querol, F.; Santagostino, E.; Valentino, L.A.; et al. Joint protection in haemophilia. Haemophilia 2011, 17 (Suppl. S2), 1–23. [Google Scholar] [CrossRef]
- Teitel, J.M.; Sholzberg, M. Current status and future prospects for the prophylactic management of hemophilia patients with inhibitor antibodies. Blood Rev. 2013, 27, 103–109. [Google Scholar] [CrossRef]
- Gringeri, A.; Leissinger, C.; Cortesi, P.A.; Jo, H.; Fusco, F.; Riva, S.; Antmen, B.; Berntorp, E.; Biasoli, C.; Carpenter, S.; et al. Health-related quality of life in patients with haemophilia and inhibitors on prophylaxis with anti-inhibitor complex concentrate: Results from the Pro-FEIBA study. Haemophilia 2013, 19, 736–743. [Google Scholar] [CrossRef]
- Stasyshyn, O.; Antunes, S.; Mamonov, V.; Ye, X.; Epstein, J.; Xiong, Y.; Tangada, S. Prophylaxis with anti-inhibitor coagulant complex improves health-related quality of life in haemophilia patients with inhibitors: Results from FEIBA NF Prophylaxis Study. Haemophilia 2014, 20, 644–650. [Google Scholar] [CrossRef]
- Rivard, G.E.; Girard, M.; Cliche, C.L.; Guay, J.P.; Bélanger, R.; Besner, R. Synoviorthesis in patients with hemophilia and inhibitors. Can. Med. Assoc. J. 1982, 127, 41–42. [Google Scholar]
- Löfqvist, T.; Petersson, C. Synoviorthesis in young patients with hemophilia and inhibitory antibodies. Pediatr. Hematol. Oncol. 1992, 9, 167–170. [Google Scholar] [CrossRef]
- Löfqvist, T.; Petersson, C.; Nilsson, I.M. Radioactive synoviorthesis in patients with hemophilia with factor inhibitor. Clin. Orthop. Relat. Res. 1997, 343, 37–41. [Google Scholar]
- Rodriguez-Merchan, E.C.; Valentino, L.; Quintana, M. Prophylaxis and treatment of chronic synovitis in haemophilia patients with inhibitors. Haemophilia 2007, 13 (Suppl. S3), 45–48. [Google Scholar] [CrossRef]
- Pasta, G.; Mancuso, M.E.; Perfetto, O.S.; Solimeno, L.P. Synoviorthesis in haemophilia patients with inhibitors. Haemophilia 2008, 14 (Suppl. S6), 52–55. [Google Scholar] [CrossRef]
- Ozcan, Z. Radiosynovectomy in hemophilic synovitis. Mol. Imaging Radionucl. Ther. 2014, 23, 1–4. [Google Scholar] [CrossRef]
- Rodriguez-Merchan, E.C. Hemophilic synovitis of the knee: Radiosynovectomy or arthroscopic synovectomy? Expert Rev. Hematol. 2014, 7, 507–511. [Google Scholar] [CrossRef]
- Turkmen, C.; Kilicoglu, O.; Dikici, F.; Bezgal, F.; Kuyumcu, S.; Gorgun, O.; Taser, O.; Zulfikar, B. Survival analysis of Y-90 radiosynovectomy in the treatment of haemophilic synovitis of the knee: A 10-year retrospective review. Haemophilia 2014, 20, e45–e50. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Merchan, E.C.; Valentino, L.A. Safety of radiation exposure after radiosynovectomy in paediatric patients with haemophilia. Haemophilia 2015, 21, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Merchan, E.C.; De la Corte-Rodriguez, H. Radiosynovectomy in haemophilic synovitis of elbows and ankles: Is the effectiveness of yttrium-90 and rhenium-186 different? Thromb. Res. 2016, 140, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Q.; Han, S.Q.; Yuan, Z.; He, Y.T.; Zhang, H.; Zhang, M. Effects of intraarticular (32)P colloid in the treatment of hemophilic synovitis of the knee: A short term clinical study. Indian J. Orthop. 2016, 50, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Y.; Ge, Y.H.; Liu, H.J.; Liu, Y.H.; Zhao, J.J.; Dou, Y.C.; Lei, P.C. Therapeutic response of radiosynovectomy with p-32 colloid in 326 patients with hemophilic arthropathy. Zhonghua Xue Ye Xue Za Zhi 2017, 38, 39–43. [Google Scholar]
- McGuinn, C.; Cheng, D.; Aschman, D.; Carpenter, S.L.; Sidonio, R.; Soni, A.; Tarantino, M.D.; Wheeler, A.P.; Dunn, A.L.; ATHN3 Working Group. Radionuclide synovectomy/synoviorthesis (RS) in patients with bleeding disorders: A review of patient and procedure demographics and functional outcomes in the ATHNdataset. Haemophilia 2017, 23, 926–933. [Google Scholar] [CrossRef]
- Sabet, A.; Strauss, A.C.; Schmolders, J.; Bornemann, R.; Sabet, A.; Oldenburg, J.; Pennekamp, P.H.; Biersack, H.J.; Ezziddin, S. Radiosynoviorthesis in hemophilic arthropathy: Pathologic blood pool imaging on pre-therapeutic bone scintigraphy is not a predictor of treatment success. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 461–467. [Google Scholar] [CrossRef]
- Gallant, R.; McNall-Knapp, R.Y.; Khan, O. Remote arterial vasculitis as a possible complication of Phosphorus-32 Radiosynovectomy. Radiol. Case Rep. 2018, 14, 137–140. [Google Scholar] [CrossRef]
- Rodriguez-Merchan, E.C. Radiosynovectomy in haemophilia. Blood Rev. 2019, 35, 1–6. [Google Scholar] [CrossRef]
- Oliveira, S.; Thomas, S.; Dos Santos, C.L.G.; Berdeguez, M.B.T.; de Sa, L.V.; de Souza, S.A.L. Outpatient treatment for haemophilic arthropathy with radiosynovectomy: Radiation dose to family members. Haemophilia 2019, 25, 509–513. [Google Scholar] [CrossRef]
- Kachooei, A.R.; Heidari, A.; Divband, G.; Zandinezhad, M.E.; Mousavian, A.; Farhangi, H.; Aminzadeh, B.; Zarifian, A.; Bagheri, F.; Badiei, Z. Rhenium-188 radiosynovectomy for chronic haemophilic synovitis: Evaluation of its safety and efficacy in haemophilic patients. Haemophilia 2020, 26, 142–150. [Google Scholar] [CrossRef]
- Koc, B.; Kılıcoglu, O.; Turkmen, C.; Zulfikar, B. Prognostic factors of radiosynovectomy in haemophilia patients with inhibitors: Survival analysis in a 19-year period. Haemophilia 2020, 26, 855–860. [Google Scholar] [CrossRef]
- Ebrahimpour, A.; Ebrahiminasab, M.; Kaseb, M.; Asadollahi, S.; Mortazavi, S.J. Chromic phosphate-32 colloid radiosynovectomy for the treatment of haemophilic synovitis: A long-term follow-up study. Haemophilia 2020, 26, 136–141. [Google Scholar] [CrossRef]
- Magalhães, A.F.; de Oliveira, L.C.O.; Pitella, F.A.; Wichert-Ana, L.; Engel, E.E.; Barbieri, C.H. Yttrium-90 radiosynovectomy in knees and ankles (25 joints in 22 hemophilic patients). Short-term results. Hematol. Transfus. Cell Ther. 2021, 43, 15–20. [Google Scholar] [CrossRef]
- Szerb, I.; Gál, T.; Mikó, I.; Hangody, L. Radiosynoviorthesis in the treatment of posttraumatic joint bleedings of hemophilic patients (concerning hip, knee and ankle joints)-Hungarian experience. Injury 2021, 52 (Suppl. S1), S53–S56. [Google Scholar] [CrossRef]
- Rodriguez-Merchan, E.C.; de la Corte-Rodriguez, H.; Jimenez-Yuste, V. Efficacy of celecoxib in the treatment of joint pain caused by advanced haemophilic arthropathy in adult patients with haemophilia A. Haemophilia 2014, 20, e225–e227. [Google Scholar] [CrossRef]
- Rodriguez-Merchan, E.C. Treatment of musculo-skeletal pain in haemophilia. Blood Rev. 2018, 32, 116–121. [Google Scholar] [CrossRef]
- Turkmen, C.; Ozturk, S.; Unal, S.N.; Zulfikar, B.; Taser, O.; Sanli, Y.; Cefle, K.; Kilicoglu, O.; Palanduz, S. The genotoxic effects in lymphocyte cultures of children treated with radiosynovectomy by using yttrium-90 citrate colloid. Cancer Biother. Radiopharm. 2007, 22, 393–399. [Google Scholar] [CrossRef]
- Infante-Rivard, C.; Rivard, G.E.; Derome, F.; Cusson, A.; Winikoff, R.; Chartrand, R.; Guay, J.P. A retrospective cohort study of cancer incidence among patients treated with radiosynoviorthesis. Haemophilia 2012, 18, 805–809. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).