The Profile of Markers of Bone Turnover, Inflammation and Extracellular Neutrophil Traps on Bone Mass in Haemophilia and the Development of Haemophilic Arthropathy
Abstract
1. Introduction
2. Materials and Methods
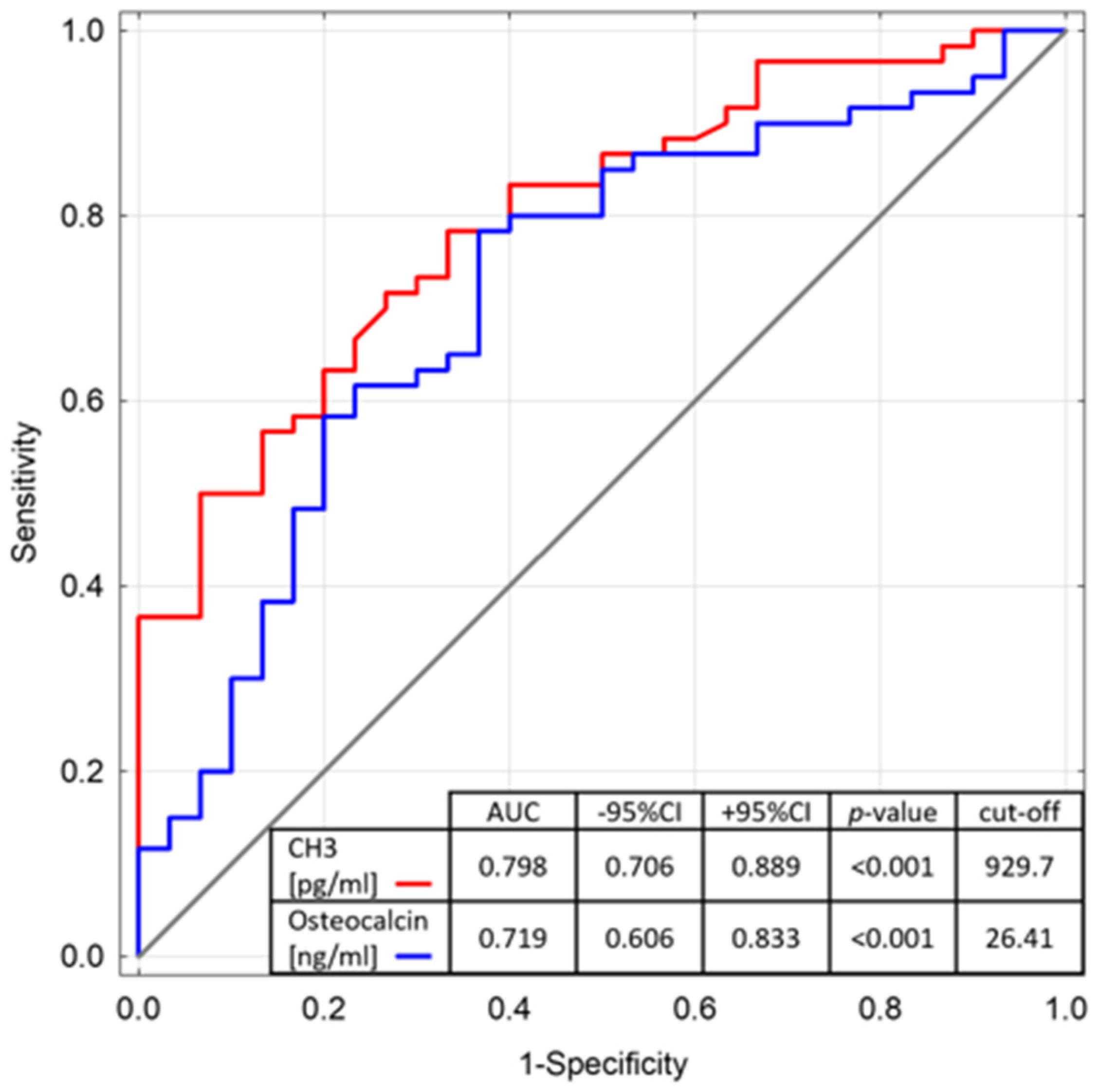
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamal, A.F.; Waryudi, A.; Kurniawan, A.; Lubis, A.M.; Gatot, D. Various Surgical Treatment of Hemophilic Pseudotumor: A Case Series. Arch. Bone Jt. Surg. 2019, 7, 514–522. [Google Scholar]
- Cox, D.P.; Solar, A.; Huang, J.; Chigurupati, R. Pseudotumor of the Mandible as First Presentation of Hemophilia in a 2-Year-Old Male: A Case Report and Review of Jaw Pseudotumors of Hemophilia. Head Neck Pathol. 2011, 5, 226–232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kwon, A.-Y.; Huh, K.-H.; Yi, W.-J.; Symkhampha, K.S.; Heo, M.-S.; Lee, S.-S.; Choi, S.-C. Haemophilic Pseudotumour in Two Parts of the Maxilla: Case Report. Dentomaxillofac. Radiol. 2016, 45, 20150440. [Google Scholar] [CrossRef]
- Rodriguez-Merchan, E.C. Hemophilic Pseudotumors: Diagnosis and Management. Arch. Bone Jt. Surg. 2020, 8, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Martinoli, C.; Della Casa Alberighi, O.; Di Minno, G.; Graziano, E.; Molinari, A.C.; Pasta, G.; Russo, G.; Santagostino, E.; Tagliaferri, A.; Tagliafico, A.; et al. Development and Definition of a Simplified Scanning Procedure and Scoring Method for Haemophilia Early Arthropathy Detection with Ultrasound (HEAD-US). Thromb. Haemost. 2013, 109, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Gualtierotti, R.; Solimeno, L.P.; Peyvandi, F. Hemophilic Arthropathy: Current Knowledge and Future Perspectives. J. Thromb. Haemost. JTH 2021, 19, 2112–2121. [Google Scholar] [CrossRef]
- Sackstein, P.; Cooper, P.; Kessler, C. The Role of Total Ankle Replacement in Patients with Haemophilia and End-Stage Ankle Arthropathy: A Review. Haemoph. Off. J. World Fed. Hemoph. 2021, 27, 184–191. [Google Scholar] [CrossRef]
- van Vulpen, L.F.D.; Holstein, K.; Martinoli, C. Joint Disease in Haemophilia: Pathophysiology, Pain and Imaging. Haemoph. Off. J. World Fed. Hemoph. 2018, 24 (Suppl. S6), 44–49. [Google Scholar] [CrossRef]
- Melchiorre, D.; Manetti, M.; Matucci-Cerinic, M. Pathophysiology of Hemophilic Arthropathy. J. Clin. Med. 2017, 6, 63. [Google Scholar] [CrossRef]
- Hill, K.; Fearn, M.; Williams, S.; Mudge, L.; Walsh, C.; McCarthy, P.; Walsh, M.; Street, A. Effectiveness of a Balance Training Home Exercise Programme for Adults with Haemophilia: A Pilot Study. Haemoph. Off. J. World Fed. Hemoph. 2010, 16, 162–169. [Google Scholar] [CrossRef]
- Carrasco, J.J.; Pérez-Alenda, S.; Casaña, J.; Soria-Olivas, E.; Bonanad, S.; Querol, F. Physical Activity Monitoring and Acceptance of a Commercial Activity Tracker in Adult Patients with Haemophilia. Int. J. Environ. Res. Public Health 2019, 16, 3851. [Google Scholar] [CrossRef]
- Linari, S.; Montorzi, G.; Bartolozzi, D.; Borderi, M.; Melchiorre, D.; Benelli, M.; Morfini, M. Hypovitaminosis D and Osteopenia/Osteoporosis in a Haemophilia Population: A Study in HCV/HIV or HCV Infected Patients. Haemoph. Off. J. World Fed. Hemoph. 2013, 19, 126–133. [Google Scholar] [CrossRef]
- Czajkowska, S.; Rupa-Matysek, J.; Wojtasińska, E.; Nijakowski, K.; Gil, L.; Surdacka, A.; Kulczyk, T. Potential Biochemical Markers and Radiomorphometric Indices as Predictors of Reduced Bone Mass in Patients with Congenital Hemophilia. J. Clin. Med. 2022, 11, 3391. [Google Scholar] [CrossRef] [PubMed]
- Sobh, M.M.; Abdalbary, M.; Elnagar, S.; Nagy, E.; Elshabrawy, N.; Abdelsalam, M.; Asadipooya, K.; El-Husseini, A. Secondary Osteoporosis and Metabolic Bone Diseases. J. Clin. Med. 2022, 11, 2382. [Google Scholar] [CrossRef] [PubMed]
- Linari, S.; Melchiorre, D.; Pieri, L.; Tofani, L.; Fanelli, A.; Brogi, M.; Castaman, G. Low Bone Mass and Hypovitaminosis D in Haemophilia: A Single-Centre Study in Patients with Severe and Moderate Haemophilia A and B. Haemoph. Off. J. World Fed. Hemoph. 2020, 26, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Anagnostis, P.; Karras, S.N.; Vakalopoulou, S.; Terpos, E. Haemophilia and Low Bone Mass. Ok, but What about Fracture Risk? Haemoph. Off. J. World Fed. Hemoph. 2016, 22, 11–14. [Google Scholar] [CrossRef]
- Sossa Melo, C.L.; Wandurraga, E.A.; Peña, A.M.; Jiménez, S.I.; Salazar, L.A.; Ochoa, M.E.; Luna-Gonzalez, M.L.; Ortiz, M.L.; Morales, K.; Ayala-Castillo, M.; et al. Low Bone Mineral Density and Associated Factors in Patients with Haemophilia in Colombia. Haemoph. Off. J. World Fed. Hemoph. 2018, 24, e222–e229. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, C.S. Hemophilia, Low Bone Mass, and Osteopenia/Osteoporosis. Transfus. Apher. Sci. Off. J. World Apher. Assoc. Off. J. Eur. Soc. Haemapheresis 2008, 38, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Gerstner, G.; Damiano, M.L.; Tom, A.; Worman, C.; Schultz, W.; Recht, M.; Stopeck, A.T. Prevalence and Risk Factors Associated with Decreased Bone Mineral Density in Patients with Haemophilia. Haemoph. Off. J. World Fed. Hemoph. 2009, 15, 559–565. [Google Scholar] [CrossRef]
- Flaherty, L.M.; Schoeppe, J.; Kruse-Jarres, R.; Konkle, B.A. Balance, Falls, and Exercise: Beliefs and Experiences in People with Hemophilia: A Qualitative Study. Res. Pract. Thromb. Haemost. 2018, 2, 147–154. [Google Scholar] [CrossRef]
- Recht, M.; Liel, M.S.; Turner, R.T.; Klein, R.F.; Taylor, J.A. The Bone Disease Associated with Factor VIII Deficiency in Mice Is Secondary to Increased Bone Resorption. Haemoph. Off. J. World Fed. Hemoph. 2013, 19, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Baud’huin, M.; Duplomb, L.; Téletchéa, S.; Charrier, C.; Maillasson, M.; Fouassier, M.; Heymann, D. Factor VIII-von Willebrand Factor Complex Inhibits Osteoclastogenesis and Controls Cell Survival. J. Biol. Chem. 2009, 284, 31704–31713. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Merchan, E.C.; Valentino, L.A. Increased Bone Resorption in Hemophilia. Blood Rev. 2019, 33, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Olmos-Martínez, J.M.; Hernández, J.L.; Fábrega, E.; Olmos, J.M.; Crespo, J.; González-Macías, J. Bone Mineral Density and Trabecular Bone Score in Treatment-Naïve Patients with Non-Cirrhotic Hepatitis C Virus Infection. Arch. Osteoporos. 2020, 15, 72. [Google Scholar] [CrossRef]
- Mousavi, S.H.; Khairkhah, N.; Bahri, T.D.; Anvar, A.; Saraji, A.A.; Behnava, B.; Alavian, S.M.; Namvar, A. First Report of Prevalence of Blood-Borne Viruses (HBV, HCV, HIV, HTLV-1 and Parvovirus B19) Among Hemophilia Patients in Afghanistan. Sci. Rep. 2019, 9, 7259. [Google Scholar] [CrossRef]
- Kucharska, M.; Zaleska-Dorobisz, U.; Szymczak, A.; Inglot, M.; Rymer, W.; Zalewska, M.; Małyszczak, K.; Kuliszkiewicz-Janus, M.; Inglot, M. Stage of Liver Fibrosis in Patients with Congenital Bleeding Disorders and Infected with Hepatitis C Virus. Pol. Arch. Intern. Med. 2017, 127, 412–417. [Google Scholar] [CrossRef]
- Carbone, L.D.; Ortiz Kaemena, M.F.; Elam, R.E. Heart Failure and Osteoporosis: An Association That Merits Further Study. Pol. Arch. Intern. Med. 2020, 130, 928–929. [Google Scholar] [CrossRef]
- Nijakowski, K.; Surdacka, A. Salivary Biomarkers for Diagnosis of Inflammatory Bowel Diseases: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 7477. [Google Scholar] [CrossRef] [PubMed]
- Ohnuma, K.; Kasagi, S.; Uto, K.; Noguchi, Y.; Nakamachi, Y.; Saegusa, J.; Kawano, S. MicroRNA-124 Inhibits TNF-α- and IL-6-Induced Osteoclastogenesis. Rheumatol. Int. 2019, 39, 689–695. [Google Scholar] [CrossRef]
- Kaminski, T.W.; Brzoska, T.; Tutuncuoglu, E.; Ragni, M.V.; Sundd, P. Neutrophil Extracellular Traps Promote Joint Injury in Hemophilia. Blood 2020, 136, 43. [Google Scholar] [CrossRef]
- Anagnostis, P.; Vakalopoulou, S.; Slavakis, A.; Charizopoulou, M.; Kazantzidou, E.; Chrysopoulou, T.; Vyzantiadis, T.-A.; Moka, E.; Agapidou, A.; Garipidou, V. Reduced Bone Mineral Density in Patients with Haemophilia A and B in Northern Greece. Thromb. Haemost. 2012, 107, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.; Wong, P.; Egan, B.; Speller, T.; Cameron, F.; Jones, G.; Ekert, H.; Monagle, P. Reduced Bone Density among Children with Severe Hemophilia. Pediatrics 2004, 114, e177–e181. [Google Scholar] [CrossRef] [PubMed]
- Iorio, A.; Fabbriciani, G.; Marcucci, M.; Brozzetti, M.; Filipponi, P. Bone Mineral Density in Haemophilia Patients. A Meta-Analysis. Thromb. Haemost. 2010, 103, 596–603. [Google Scholar] [CrossRef]
- Atalay, S.; Elci, A.; Kayadibi, H.; Onder, C.B.; Aka, N. Diagnostic Utility of Osteocalcin, Undercarboxylated Osteocalcin, and Alkaline Phosphatase for Osteoporosis in Premenopausal and Postmenopausal Women. Ann. Lab. Med. 2012, 32, 23–30. [Google Scholar] [CrossRef]
- Christoforidis, A.; Economou, M.; Papadopoulou, E.; Kazantzidou, E.; Farmaki, E.; Tzimouli, V.; Tsatra, I.; Gompakis, N.; Athanassiou-Metaxa, M. Comparative Study of Dual Energy X-ray Absorptiometry and Quantitative Ultrasonography with the Use of Biochemical Markers of Bone Turnover in Boys with Haemophilia. Haemoph. Off. J. World Fed. Hemoph. 2011, 17, e217–e222. [Google Scholar] [CrossRef] [PubMed]
- Christoforidis, A.; Economou, M.; Farmaki, E.; Tzimouli, V.; Gombakis, N.; Athanassiou-Metaxa, M. Increased Osteoclastic Activity as Shown by Increased SRANK-L/OPG Ratio in Boys with Hemophilia. Ann. Hematol. 2010, 89, 837–838. [Google Scholar] [CrossRef]
- Alioglu, B.; Selver, B.; Ozsoy, H.; Koca, G.; Ozdemir, M.; Dallar, Y. Evaluation of Bone Mineral Density in Turkish Children with Severe Haemophilia A: Ankara Hospital Experience. Haemoph. Off. J. World Fed. Hemoph. 2012, 18, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Pergantou, H.; Papakonstantinou, O.; Xafaki, P.; Athanasopoulou, H.; Balanika, A.; Kattamis, A.; Doulgeraki, A. Uncoupling of Bone Turnover May Compromise Skeletal Health of Young Patients With Haemophilia A. J. Clin. Densitom. Off. J. Int. Soc. Clin. Densitom. 2021, 25, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Katsarou, O.; Terpos, E.; Chatzismalis, P.; Provelengios, S.; Adraktas, T.; Hadjidakis, D.; Kouramba, A.; Karafoulidou, A. Increased Bone Resorption Is Implicated in the Pathogenesis of Bone Loss in Hemophiliacs: Correlations with Hemophilic Arthropathy and HIV Infection. Ann. Hematol. 2010, 89, 67–74. [Google Scholar] [CrossRef]
- Gebetsberger, J.; Schirmer, M.; Wurzer, W.J.; Streif, W. Low Bone Mineral Density in Hemophiliacs. Front. Med. 2022, 9, 794456. [Google Scholar] [CrossRef]
- Serological Biomarkers in Hemophilic Arthropathy: Can They Be Used to Monitor Bleeding and Ongoing Progression of Blood-Induced Joint Disease in Patients with Hemophilia? PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/31796337/ (accessed on 24 May 2022).
- Hoots, W.K. Pathogenesis of Hemophilic Arthropathy. Semin. Hematol. 2006, 43, S18–S22. [Google Scholar] [CrossRef]
- Marcadet, L.; Bouredji, Z.; Argaw, A.; Frenette, J. The Roles of RANK/RANKL/OPG in Cardiac, Skeletal, and Smooth Muscles in Health and Disease. Front. Cell Dev. Biol. 2022, 10, 903657. [Google Scholar] [CrossRef]
- Ziolkowska, M.; Kurowska, M.; Radzikowska, A.; Luszczykiewicz, G.; Wiland, P.; Dziewczopolski, W.; Filipowicz-Sosnowska, A.; Pazdur, J.; Szechinski, J.; Kowalczewski, J.; et al. High Levels of Osteoprotegerin and Soluble Receptor Activator of Nuclear Factor Kappa B Ligand in Serum of Rheumatoid Arthritis Patients and Their Normalization after Anti-Tumor Necrosis Factor Alpha Treatment. Arthritis Rheum. 2002, 46, 1744–1753. [Google Scholar] [CrossRef]
- Castaman, G.; Melchiorre, D.; Linari, S. Clinical, Instrumental, Serological and Histological Findings Suggest That Hemophilia B May Be Less Severe than Hemophilia A. Blood 2015, 126, 4682. [Google Scholar] [CrossRef]
- Srivastava, A.; Brewer, A.K.; Mauser-Bunschoten, E.P.; Key, N.S.; Kitchen, S.; Llinas, A.; Ludlam, C.A.; Mahlangu, J.N.; Mulder, K.; Poon, M.C.; et al. Guidelines for the Management of Hemophilia. Haemoph. Off. J. World Fed. Hemoph. 2013, 19, e1–e47. [Google Scholar] [CrossRef] [PubMed]
- Program Polityki Zdrowotnej. Narodowy Program Leczenia Chorych na Hemofilię i Pokrewne Skazy Krwotoczne. Available online: https://www.gov.pl/web/zdrowie/narodowy-program-leczenia-chorych-na-hemofilie-i-pokrewne-skazy-krwotoczne-na-lata-2019-2023 (accessed on 24 May 2022).
- Manios, Y.; Moschonis, G.; Lambrinou, C.P.; Mavrogianni, C.; Tsirigoti, L.; Hoeller, U.; Roos, F.F.; Bendik, I.; Eggersdorfer, M.; Celis-Morales, C.; et al. Associations of Vitamin D Status with Dietary Intakes and Physical Activity Levels among Adults from Seven European Countries: The Food4Me Study. Eur. J. Nutr. 2018, 57, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Płudowski, P.; Karczmarewicz, E.; Bayer, M.; Carter, G.; Chlebna-Sokół, D.; Czech-Kowalska, J.; Dębski, R.; Decsi, T.; Dobrzańska, A.; Franek, E.; et al. Practical Guidelines for the Supplementation of Vitamin D and the Treatment of Deficits in Central Europe-Recommended Vitamin D Intakes in the General Population and Groups at Risk of Vitamin D Deficiency. Endokrynol. Pol. 2013, 64, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Media, M. Neutrophil Extracellular Traps Promote Joint Injury in Hemophilia. ISTH Congr. Abstr. 2020, 136, 990. [Google Scholar]
- Zawilski, J.; Dudek, A.; Lisiński, P. Wyniki stosowania nowych metod rehabilitacji u pacjentów z artropatią hemofilową. Acta Haematol. Pol. 2017, 48, 28–34. [Google Scholar] [CrossRef]
- Darby, S.C.; Kan, S.W.; Spooner, R.J.; Giangrande, P.L.F.; Hill, F.G.H.; Hay, C.R.M.; Lee, C.A.; Ludlam, C.A.; Williams, M. Mortality Rates, Life Expectancy, and Causes of Death in People with Hemophilia A or B in the United Kingdom Who Were Not Infected with HIV. Blood 2007, 110, 815–825. [Google Scholar] [CrossRef]
- Gomez-Rosas, P. Extensive Characterization of the Hemostatic Derangement Occurring in COVID-19 Patients Admitted to the Bergamo Hospital. Blood 2020, 136, 36. [Google Scholar] [CrossRef]
| n | % | ||
|---|---|---|---|
| Type of Haemophilia | A | 48 | 80.0 |
| B | 12 | 20.0 | |
| Routine Management | On-demand therapy | 27 | 45.0 |
| Secondary prophylactic therapy | 33 | 55.0 | |
| Severity of Haemophilia | Severe | 42 | 70.0 |
| Moderate | 9 | 15.0 | |
| Mild | 9 | 15.0 | |
| Study Group n = 60 | Control Group n = 30 | p-Value | |
|---|---|---|---|
| M [Q1–Q3] | M [Q1–Q3] | ||
| IL-6 [pg/mL] | 214.1 [158.4–322.8] | 216.5 [163.4–267.3] | 0.716 |
| CH3 [pg/mL] | 1019.2 [909.8–1130.1] | 859.3 [798.0–934.3] | <0.001 * |
| CICP [ng/mL] | 173.1 [129.4–210.8] | 165.8 [145.9–176.6] | 0.250 |
| PINP [ng/mL] | 1057.7 [905.9–1198.7] | 1121.4 [992.6–1256.7] | 0.154 |
| BALP [U/L] | 292.8 [261.8–350.9] | 328.1 [263.7–354.0] | 0.138 |
| BGLAP [ng/mL] | 32.4 [26.6–37.6] | 24.3 [21.5–29.6] | <0.001 * |
| On-Demand Therapy n = 27 | Prophylactic Therapy n = 33 | p-Value | |
|---|---|---|---|
| M [Q1–Q3] | M [Q1–Q3] | ||
| IL-6 [pg/mL] | 181.4 [155.0–354.6] | 244.8 [169.3–316.8] | 0.305 |
| CH3 [pg/mL] | 1114.4 [934.3–1149.8] | 952.5 [875.6–1057.4] | 0.008 * |
| CICP [ng/mL] | 191.2 [160.2–224.3] | 161.6 [126.4–189.6] | 0.015 * |
| PINP [ng/mL] | 1117.6 [882.9–1214.6] | 1030.9 [927.5–1178.9] | 0.888 |
| BALP [U/L] | 322.5 [281.3–356.4] | 280.9 [254.2–338.0] | 0.038 * |
| BGLAP [ng/mL] | 28.0 [24.5–32.9] | 35.9 [28.9–39.9] | 0.017 * |
| Vitamin D [ng/mL] | 21.1 [16.3–24.7] | 17.5 [14.1–25.2] | 0.142 |
| Calcium [mmol/L] | 2.5 [2.5–2.6] | 2.5 [2.4–2.6] | 0.175 |
| Ferritin [ng/mL] | 93.4 [42.0–175.7] | 93.0 [65.2–236.9] | 0.357 |
| Mild and Moderate n = 18 | Severe n = 42 | p-Value | |
|---|---|---|---|
| M [Q1–Q3] | M [Q1–Q3] | ||
| IL-6 [pg/mL] | 164.6 [155.0–228.3] | 247.0 [169.3–328.7] | 0.131 |
| CH3 [pg/mL] | 1048.9 [959.4–1145.2] | 971.4 [875.6–1101.3] | 0.118 |
| CICP [ng/mL] | 188.3 [163.1–224.3] | 166.2 [126.4–208.8] | 0.070 |
| PINP [ng/mL] | 1113.8 [889.2–1194.2] | 1015.6 [912.2–1212.0] | 0.955 |
| BALP [U/L] | 311.0 [281.3–348.1] | 288.7 [250.7–352.7] | 0.208 |
| BGLAP [ng/mL] | 27.1 [24.5–36.3] | 32.7 [27.9–38.5] | 0.183 |
| Vitamin D [ng/mL] | 20.7 [17.9–24.7] | 19.0 [14.6–24.6] | 0.348 |
| Calcium [mmol/L] | 2.5 [2.5–2.6] | 2.5 [2.4–2.6] | 0.354 |
| Ferritin [ng/mL] | 65.0 [42.0–115.9] | 98.7 [68.5–228.8] | 0.055 |
| With Arthropathy n = 42 | Without Arthropathy n = 18 | p-Value | |
|---|---|---|---|
| M [Q1–Q3] | M [Q1–Q3] | ||
| IL-6 [pg/mL] | 254.1 [169.3–362.5] | 164.6 [135.8–211.4] | 0.020 * |
| CH3 [pg/mL] | 957.4 [880.7–1101.3] | 1062.6 [987.3–1146.3] | 0.108 |
| CICP [ng/mL] | 167.8 [127.1–212.1] | 182.6 [163.1–207.3] | 0.397 |
| PINP [ng/mL] | 1015.6 [908.4–1178.9] | 1157.2 [882.9–1214.6] | 0.429 |
| BALP [U/L] | 284.0 [254.2–338.0] | 332.3 [291.0–353.9] | 0.028 * |
| Osteocalcin [ng/mL] | 34.9 [28.0–39.9] | 26.7 [23.3–30.3] | 0.002 * |
| Vitamin D [ng/mL] | 19.1 [15.0–25.8] | 21.1 [17.0–23.5] | 0.525 |
| Calcium [mmol/L] | 2.5 [2.4–2.6] | 2.5 [2.5–2.6] | 0.388 |
| Ferritin [ng/mL] | 99.6 [0.23–0.63] | 66.4 [44.9–112.4] | 0.148 |
| antyHBs Positive n = 48 | antyHBs Negative n = 6 | p-Value | |
|---|---|---|---|
| M [Q1–Q3] | M [Q1–Q3] | ||
| IL-6 [pg/mL] | 247.0 [168.4–358.1] | 149.4 [132.0–211.1] | 0.107 |
| CH3 [pg/mL] | 997.0 [908.7–1131.5] | 1013.0 [910.3–1114.4] | 0.869 |
| CICP [ng/mL] | 169.5 [129.4–210.5] | 169.3 [122.0–189.9] | 0.670 |
| PINP [ng/mL] | 1083.2 [913.5–1213.3] | 916.1 [882.9–1057.7] | 0.137 |
| BALP [U/L] | 298.0 [258.5–351.8] | 290.2 [289.1–303.4] | 0.527 |
| BGLAP [ng/mL] | 32.7 [27.9–38.7] | 24.3 [23.3–27.3] | 0.006 * |
| Vitamin D [ng/mL] | 18.5 [14.8–23.5] | 37.9 [20.3–40.1] | 0.011 * |
| Calcium [mmol/L] | 2.5 [2.4–2.6] | 2.5 [2.5–2.6] | 0.793 |
| Ferritin [ng/mL] | 94.2 [64.0–224.6] | 133.6 [63.5–175.7] | 0.988 |
| antyHCV Positive n = 28 | antyHCV Negative n = 29 | p-Value | |
|---|---|---|---|
| M [Q1–Q3] | M [Q1–Q3] | ||
| IL-6 [pg/mL] | 228.3 [175.4–345.2] | 189.5 [155.0–260.7] | 0.367 |
| CH3 [pg/mL] | 952.0 [860.5–1076.0] | 1054.6 [939.4–1146.3] | 0.034 * |
| CICP [ng/mL] | 131.6 [119.8–199.1] | 191.2 [161.6–220.0] | 0.020 * |
| PINP [ng/mL] | 974.8 [886.1–1175.1] | 1090.8 [927.5–1212.0] | 0.271 |
| BALP [U/L] | 281.1 [250.2–330.5] | 322.5 [288.1–353.0] | 0.046 * |
| Osteocalcin [ng/mL] | 34.9 [32.0–38.2] | 28.0 [25.3–32.9] | 0.022 * |
| Vitamin D [ng/mL] | 19.2 [15.7–31.4] | 19.4 [15.3–23.2] | 0.640 |
| Calcium [mmol/L] | 2.5 [2.5–2.6] | 2.5 [2.5–2.6] | 0.581 |
| Ferritin [ng/mL] | 119.7 [88.0–254.7] | 66.1 [44.1–103.7] | 0.003 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czajkowska, S.; Rupa-Matysek, J.; Wojtasińska, E.; Nijakowski, K.; Surdacka, A.; Gil, L. The Profile of Markers of Bone Turnover, Inflammation and Extracellular Neutrophil Traps on Bone Mass in Haemophilia and the Development of Haemophilic Arthropathy. J. Clin. Med. 2022, 11, 4711. https://doi.org/10.3390/jcm11164711
Czajkowska S, Rupa-Matysek J, Wojtasińska E, Nijakowski K, Surdacka A, Gil L. The Profile of Markers of Bone Turnover, Inflammation and Extracellular Neutrophil Traps on Bone Mass in Haemophilia and the Development of Haemophilic Arthropathy. Journal of Clinical Medicine. 2022; 11(16):4711. https://doi.org/10.3390/jcm11164711
Chicago/Turabian StyleCzajkowska, Sylwia, Joanna Rupa-Matysek, Ewelina Wojtasińska, Kacper Nijakowski, Anna Surdacka, and Lidia Gil. 2022. "The Profile of Markers of Bone Turnover, Inflammation and Extracellular Neutrophil Traps on Bone Mass in Haemophilia and the Development of Haemophilic Arthropathy" Journal of Clinical Medicine 11, no. 16: 4711. https://doi.org/10.3390/jcm11164711
APA StyleCzajkowska, S., Rupa-Matysek, J., Wojtasińska, E., Nijakowski, K., Surdacka, A., & Gil, L. (2022). The Profile of Markers of Bone Turnover, Inflammation and Extracellular Neutrophil Traps on Bone Mass in Haemophilia and the Development of Haemophilic Arthropathy. Journal of Clinical Medicine, 11(16), 4711. https://doi.org/10.3390/jcm11164711








