Compromised Cranio-Spinal Suspension in Chiari Malformation Type 1: A Potential Role as Secondary Pathophysiology
Abstract
1. Introduction
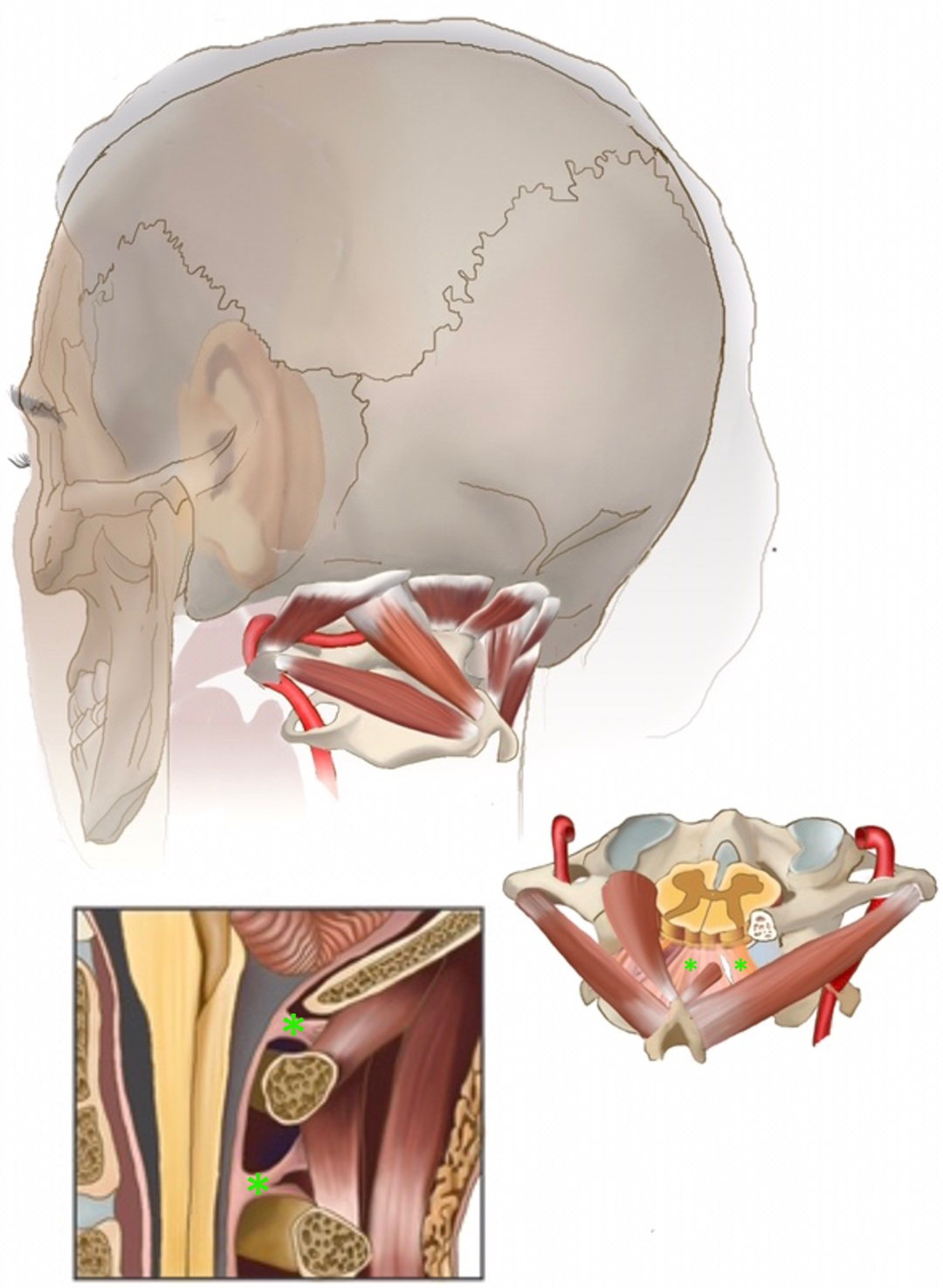
1.1. Intra- and Extradural Cranio-Spinal Suspension and the Cisterna Magna (CM)
1.2. Cranio-Spinal Suspension at the CM in Chiari Malformation Type I
1.3. Viscoelastic Suspension Structures in CMI
2. Materials and Methods
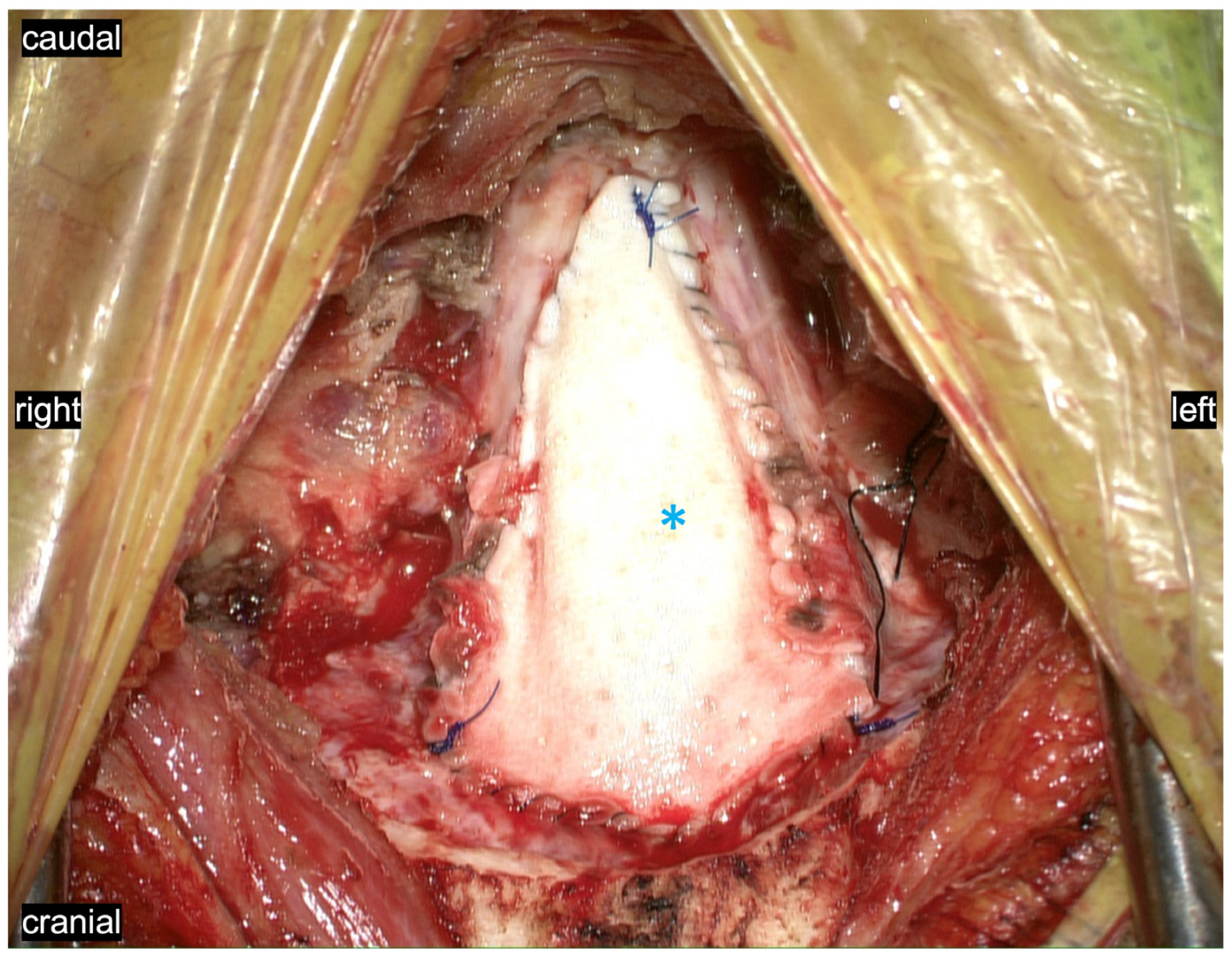
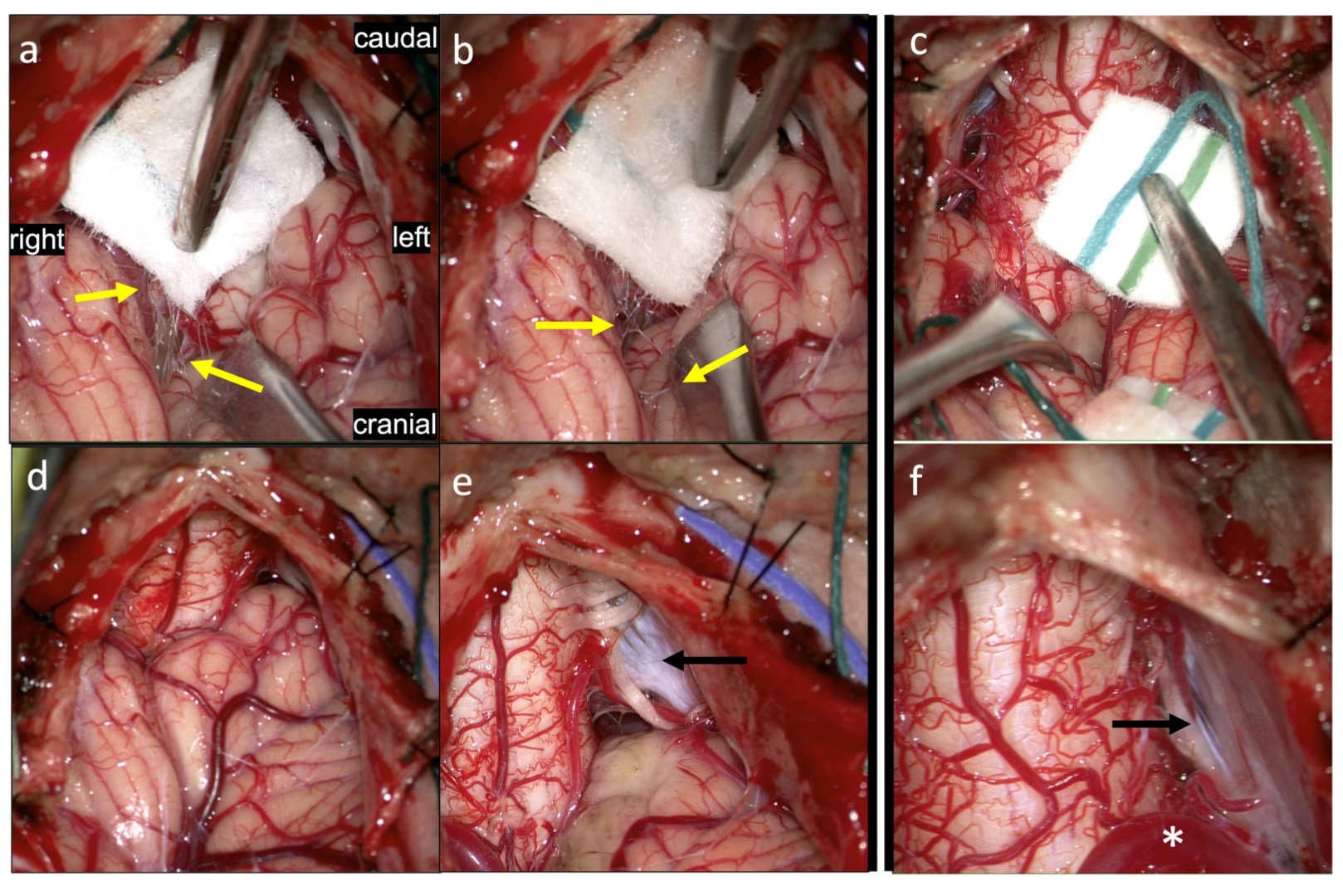
3. Results: Illustrations, Descriptions and Intraoperative Findings of Spinal Cord Movement and Suspension
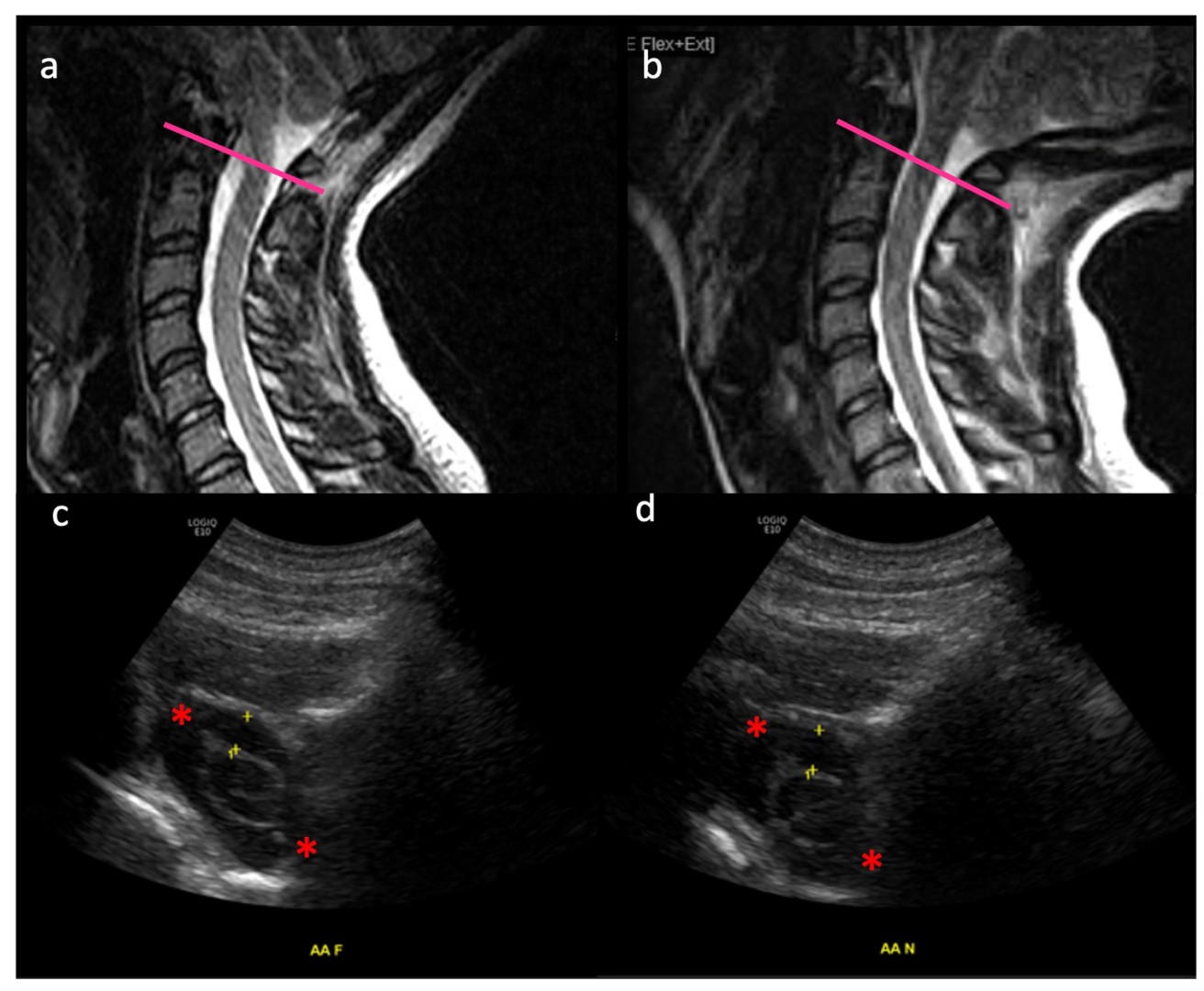
3.1. Movement of the Spinal Cord in Healthy Subjects
3.2. Myodural Bridges
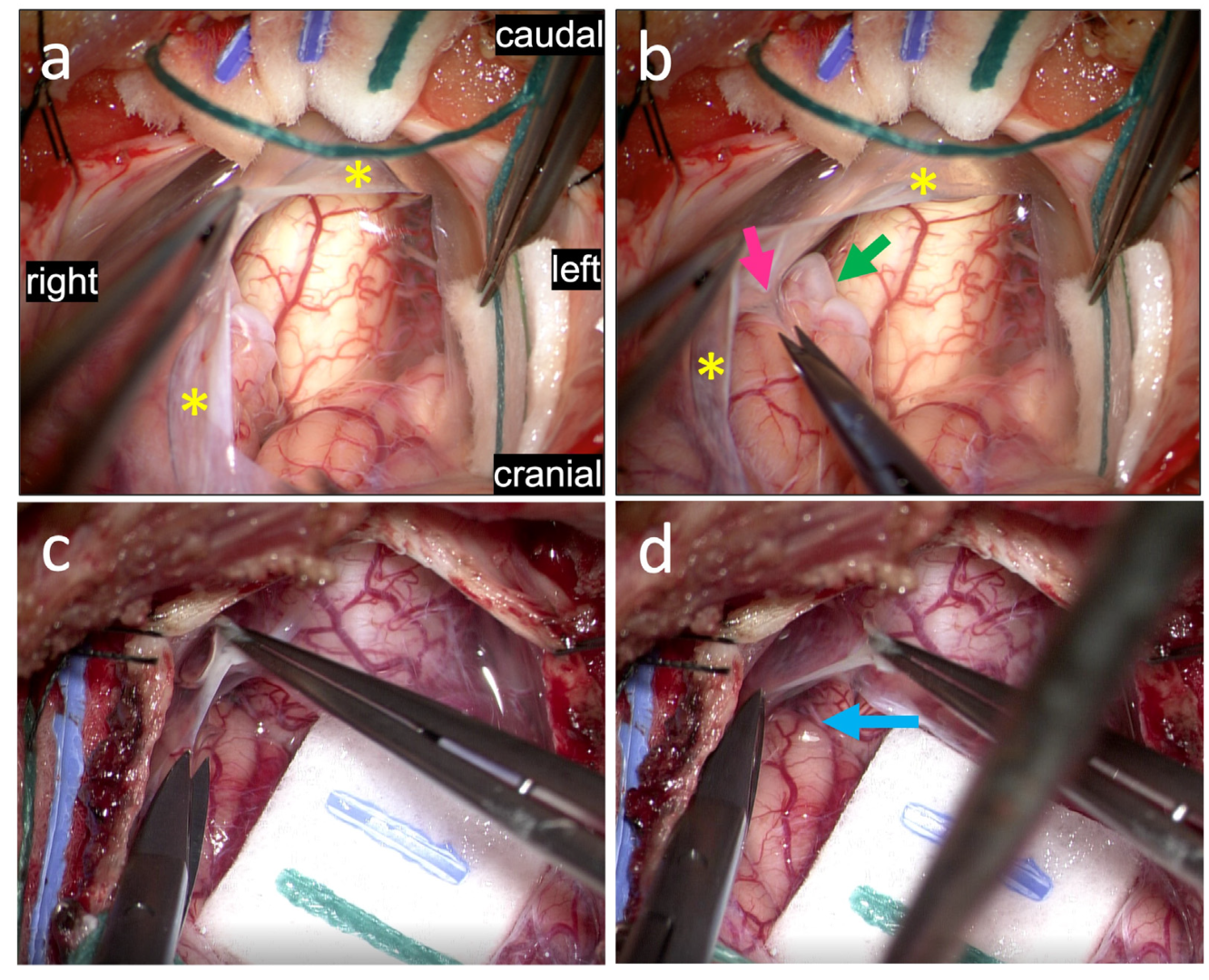
3.3. Pathological Arachnoid Structures
3.4. Tonsils
3.5. Dentate Ligaments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klinge, P.M.; McElroy, A.; Donahue, J.E.; Brinker, T.; Gokaslan, Z.L.; Beland, M.D. Abnormal spinal cord motion at the craniocervical junction in hypermobile Ehlers-Danlos patients. J. Neurosurg. Spine 2021, 35, 8–24. [Google Scholar] [CrossRef]
- Labuda, R.; Nwotchouang, B.S.T.; Ibrahimy, A.; Allen, P.A.; Oshinski, J.N.; Klinge, P.; Loth, F. A new hypothesis for the pathophysiology of symptomatic adult Chiari malformation Type I. Med. Hypotheses 2022, 158, 110740. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Tang, W.; Gong, D.Z.; Li, X.-Y.; Zhang, J.-H.; Sun, J.-H.; Wang, B.; Zhang, Y.; Chen, Y.-X.; Zhang, Z.-H.; et al. The morphology, biomechanics, and physiological function of the suboccipital myodural connections. Sci. Rep. 2021, 11, 8064. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Shao, C.X.; Zhang, Y.; Liu, C.; Chen, Y.-X.; Wang, X.-M.; Chi, Y.-Y.; Yu, S.-B.; Sui, H.-J. Head-nodding: A driving force for the circulation of cerebrospinal fluid. Sci. Rep. 2021, 11, 14233. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Gong, J.; Yu, S.B.; Yang, H.; Song, Y.; Zhang, X.; Zhang, J.; Hack, G.D.; Li, T.; Chi, Y.; et al. The relationship between compensatory hyperplasia of the myodural bridge complex and reduced compliance of the various structures within the cranio-cervical junction. Anat. Rec. 2022, in press. [CrossRef]
- Zheng, N.; Chung, B.S.; Li, Y.L.; Liu, T.-Y.; Zhang, L.-X.; Ge, Y.-Y.; Wang, N.-X.; Zhang, Z.-H.; Cai, L.; Chi, Y.-Y.; et al. The myodural bridge complex defined as a new functional structure. Surg. Radiol. Anat. 2020, 42, 143–153. [Google Scholar] [CrossRef]
- Ito, K.; Yamada, M.; Horiuchi, T.; Hongo, K. Microanatomy of the dura mater at the craniovertebral junction and spinal region for safe and effective surgical treatment. J. Neurosurg. Spine 2020, 33, 165–171. [Google Scholar] [CrossRef]
- Beland, M.D.; McElroy, A.W.; Brinker, T.; Gokaslan, Z.L.; Klinge, P.M. In Vivo Ultrasound Imaging of the Spinal Cord and Subarachnoid Space at the C1-C2 Level in Healthy Adult Subjects. Ultrasound Q. 2020, 38, 49–52. [Google Scholar] [CrossRef]
- Cousins, J.; Haughton, V. Motion of the cerebellar tonsils in the foramen magnum during the cardiac cycle. Am. J. Neuroradiol. 2009, 30, 1587–1588. [Google Scholar] [CrossRef]
- Wang, C.S.; Wang, X.; Fu, C.H.; Wei, L.Q.; Zhou, D.Q.; Lin, J.K. Analysis of cerebrospinal fluid flow dynamics and morphology in Chiari I malformation with cine phase-contrast magnetic resonance imaging. Acta Neurochir. 2014, 156, 707–713. [Google Scholar] [CrossRef]
- Lawrence, B.J.; Luciano, M.; Tew, J.; Ellenbogen, R.G.; Oshinski, J.N.; Loth, F.; Culley, A.P.; Martin, B.A. Cardiac-Related Spinal Cord Tissue Motion at the Foramen Magnum is Increased in Patients with Type I Chiari Malformation and Decreases Postdecompression Surgery. World Neurosurg. 2018, 116, e298–e307. [Google Scholar] [CrossRef] [PubMed]
- Bolognese, P.A.; Brodbelt, A.; Bloom, A.B.; Kula, R.W. Chiari I Malformation: Opinions on Diagnostic Trends and Controversies from a Panel of 63 International Experts. World Neurosurg. 2019, 130, e9–e16. [Google Scholar] [CrossRef] [PubMed]
- Akbari, S.H.A.; Yahanda, A.T.; Ackerman, L.L.; Adelson, P.D.; Ahmed, R.; Albert, G.W.; Aldana, P.R.; Alden, T.D.; Anderson, R.C.E.; Bauer, D.F.; et al. Complications and outcomes of posterior fossa decompression with duraplasty versus without duraplasty for pediatric patients with Chiari malformation type I and syringomyelia: A study from the Park-Reeves Syringomyelia Research Consortium. J. Neurosurg. Pediatr. 2022, 30, 39–51. [Google Scholar] [CrossRef]
- Ohnishi, Y.; Fujiwara, S.; Takenaka, T.; Kawamoto, S.; Iwatsuki, K.; Kishima, H. An increase in the posterior subarachnoid space accelerates the timing of syrinx resolution after foramen magnum decompression of type I Chiari malformation. Sci. Rep. 2021, 11, 19152. [Google Scholar] [CrossRef]
- Guan, J.; Yuan, C.; Zhang, C.; Ma, L.; Yao, Q.; Cheng, L.; Liu, Z.; Wang, K.; Duan, W.; Wang, X.; et al. Intradural Pathology Causing Cerebrospinal Fluid Obstruction in Syringomyelia and Effectiveness of Foramen Magnum and Foramen of Magendie Dredging Treatment. World Neurosurg. 2020, 144, e178–e188. [Google Scholar] [CrossRef]
- Nishikawa, M.; Yamagata, T.; Naito, K.; Kunihiro, N.; Sakamoto, H.; Hara, M.; Ohata, K.; Goto, T. Surgical Management of Chiari Malformation Type I Associated with Syringomyelia: Outcome of Surgeries Based on the New Classification and Study of Cerebrospinal Fluid Dynamics. J. Clin. Med. 2022, 11, 4556. [Google Scholar] [CrossRef] [PubMed]
- Pujol, J.; Roig, C.; Capdevila, A.; Pou, A.; Marti-Vilalta, J.; Kulisevsky, J.; Escartin, A.; Zannoli, G. Motion of the cerebellar tonsils in Chiari type I malformation studied by cine phase-contrast MRI. Neurology. 1995, 45, 1746–1753. [Google Scholar] [CrossRef]
- Leung, V.; Magnussen, J.S.; Stoodley, M.A.; Bilston, L.E. Cerebellar and hindbrain motion in Chiari malformation with and without syringomyelia. J. Neurosurg. Spine 2016, 24, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Dlouhy, B.J.; Dawson, J.D.; Menezes, A.H. Intradural pathology and pathophysiology associated with Chiari I malformation in children and adults with and without syringomyelia. J. Neurosurg. Pediatr. 2017, 20, 526–541. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.B.; Zhang, Z.H.; Yu, S.B.; Sun, J.-X.; Ding, S.-W.; Ma, G.-J.; Zheng, N.; Sui, H.-J. Scanning Electron Microscopic Observation of Myodural Bridge in the Human Suboccipital Region. Spine 2020, 45, E1296–E1301. [Google Scholar] [CrossRef] [PubMed]
- Meadows, J.; Kraut, M.; Guarnieri, M.; Haroun, R.I.; Carson, B.S. Asymptomatic Chiari Type I malformations identified on magnetic resonance imaging. J. Neurosurg. 2000, 92, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Milhorat, T.H.; Bolognese, P.A.; Nishikawa, M.; McDonnell, N.B.; Francomano, C.A. Syndrome of occipitoatlantoaxial hypermobility, cranial settling, and chiari malformation type I in patients with hereditary disorders of connective tissue. J. Neurosurg. Spine 2007, 7, 601–609. [Google Scholar] [CrossRef]
- Wan, M.J.; Nomura, H.; Tator, C.H. Conversion to symptomatic Chiari I malformation after minor head or neck trauma. Neurosurgery 2008, 63, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Bunc, G.; Vorsic, M. Presentation of a previously asymptomatic Chiari I malformation by a flexion injury to the neck. J. Neurotrauma 2001, 18, 645–648. [Google Scholar] [CrossRef]
- Goel, A. Is atlantoaxial instability the cause of Chiari malformation? Outcome analysis of 65 patients treated by atlantoaxial fixation. J. Neurosurg. Spine 2015, 22, 116–127. [Google Scholar] [CrossRef]
- Goel, A.; Kaswa, A.; Shah, A. Atlantoaxial Fixation for Treatment of Chiari Formation and Syringomyelia with No Craniovertebral Bone Anomaly: Report of an Experience with 57 Cases. Acta Neurochir Suppl. 2019, 125, 101–110. [Google Scholar] [CrossRef]
- Klinge, P.M.; McElroy, A.; Leary, O.P.; Donahue, J.E.; Mumford, A.; Brinker, T.; Gokaslan, Z.L. Not Just an Anchor: The Human Filum Terminale Contains Stretch Sensitive and Nociceptive Nerve Endings and Responds to Electrical Stimulation with Paraspinal Muscle Activation. Neurosurgery 2022, 91, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Enix, D.E.; Scali, F.; Pontell, M.E. The cervical myodural bridge, a review of literature and clinical implications. J. Can. Chiropr. Assoc. 2014, 58, 184–192. [Google Scholar]

| Figure/ Video | Age, Sex | Pre-Operative Symptoms | Duration of Symptoms | Preop MRI | Syrinx Y/N | Post-Operative Follow-Up |
|---|---|---|---|---|---|---|
| Figure 2 | 47F | Valsalva induced headaches, blurry vision and episodic tinnitus and hearing loss, cognitive slowing and brain fog | >3 months | 3.8 mm tonsillar herniation | N | Complete resolution of preopeartive symptoms (3 m post surgery) |
| Figure 4, Figure 5, Video S1 | 10F | Mix of suboccipital headaches and “migraine” type frontal headaches refractory to medical management and physical therapy, light sensitivity | 1 year | 7.5 mm tonsillar herniation | N | Gradual resolution of preoperative headaches over the course of 1year post surgery |
| Figure 5 | 47F | Valsalva induced headaches, neck pain, episodic vergigo and dizzines, difficulty swallowing, fatigue and cognitive slowing with brain fog, co-morbiditie of hypermobile Ehlers-Danlos-Syndrome and tethered cord syndrome | 3 years | 5.8 mm tonsillar herniation | Y | Headaches and preoperative dizziness gradually improved, residul back and leg pain required tethered cord release 6 months post Chiari decompression, stable syrinx |
| Video S2 | 35F | Suboccipital and frontal headaches with co-morbdity of known left sided migraines refractory to medical management, episodic vertigo/dizziness | 3 years | 6 mm tonsillar herniation | N | Resolution of headaches including improved frequency and intensity of left sided mgraines 1y post surgery |
| Video S4 | 27F | Valsalva induced headaches and neck pain, subjective weakness in the right hand, brain fog, episodic vertigo/dizziness with drop attacks, brain fog, difficulties with proprioception | >3 years | 22 mm tonsillar herniation, reduced clivoaxial angle in supine position <125) no radiographic evidence of craniocervical instability | Y | Residual neck pain and neck spasm, occasional headaches and vertigo, resolution of the remainder symptoms at the 1y post surgery, near complete resolution of the syrinx |
| Video S3 | 6M | Global headaches with “seizure-like” episodes, seizures ruled out, neck spasms, inconsolible and aggressive behaviour during pain episodes, symptoms refractory to intensive medical and physical therapy | >12 months | 11 mm tonsillar herniation | N | Significantly improved pain and symptom episodes compared ot preop 1 months post surgery sustained at 6 months post decompression |
| Figure 5c,d; Figure 6c,f (non-Chiari “control”) | 11M | progressive papilledema, visual acuity decline, scoliosis | 4 years | absent tonsillar herniation. progressive thoracic syrinx and scoliosis | Y | Continuosly Improved papilledema and visual acuity per ophthalmological follow up assessment in the year following decompression, stable syrinx |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, B.; Poggi, J.A.; Amaral-Nieves, N.; Wojcik, D.; Ma, K.L.; Leary, O.P.; Klinge, P.M. Compromised Cranio-Spinal Suspension in Chiari Malformation Type 1: A Potential Role as Secondary Pathophysiology. J. Clin. Med. 2022, 11, 7437. https://doi.org/10.3390/jcm11247437
Shao B, Poggi JA, Amaral-Nieves N, Wojcik D, Ma KL, Leary OP, Klinge PM. Compromised Cranio-Spinal Suspension in Chiari Malformation Type 1: A Potential Role as Secondary Pathophysiology. Journal of Clinical Medicine. 2022; 11(24):7437. https://doi.org/10.3390/jcm11247437
Chicago/Turabian StyleShao, Belinda, Jonathan A. Poggi, Natalie Amaral-Nieves, Daniel Wojcik, Kevin L. Ma, Owen P. Leary, and Petra M. Klinge. 2022. "Compromised Cranio-Spinal Suspension in Chiari Malformation Type 1: A Potential Role as Secondary Pathophysiology" Journal of Clinical Medicine 11, no. 24: 7437. https://doi.org/10.3390/jcm11247437
APA StyleShao, B., Poggi, J. A., Amaral-Nieves, N., Wojcik, D., Ma, K. L., Leary, O. P., & Klinge, P. M. (2022). Compromised Cranio-Spinal Suspension in Chiari Malformation Type 1: A Potential Role as Secondary Pathophysiology. Journal of Clinical Medicine, 11(24), 7437. https://doi.org/10.3390/jcm11247437






