Comparative Visual Outcome Analysis of a Diffractive Multifocal Intraocular Lens and a New Diffractive Multifocal Lens with Extended Depth of Focus
Abstract
1. Introduction
2. Materials and Methods
2.1. Statistical Analysis
2.2. Surgical Technique
2.3. IOL Calculation
3. Results
3.1. Patient Demographics
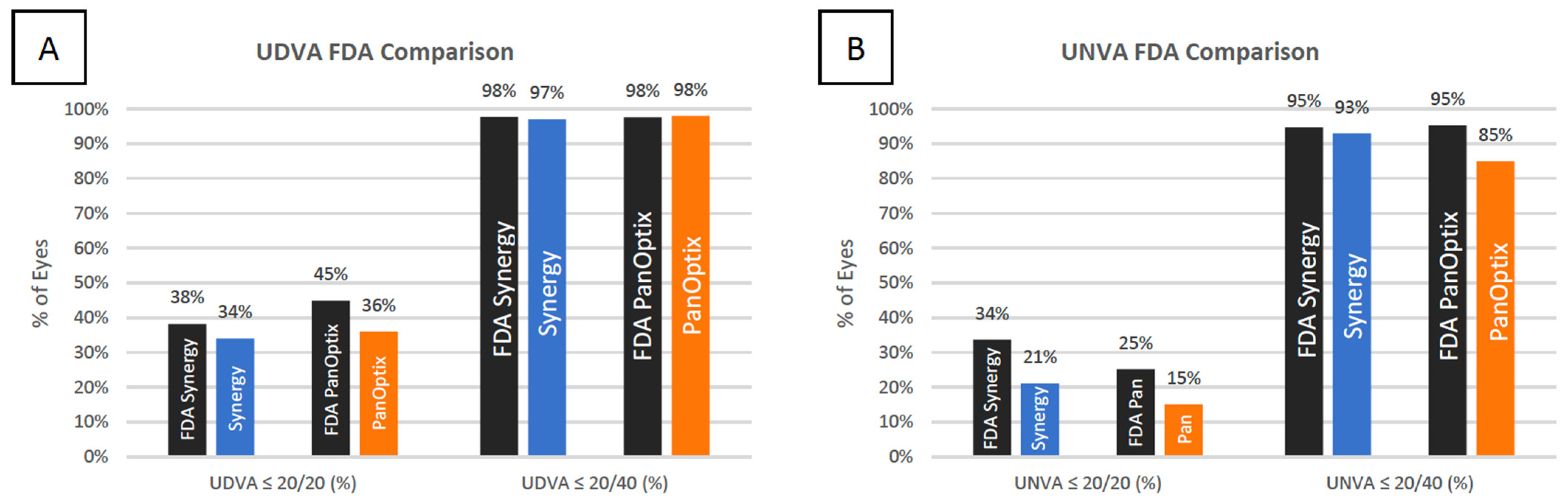
3.2. Refractive Outcomes
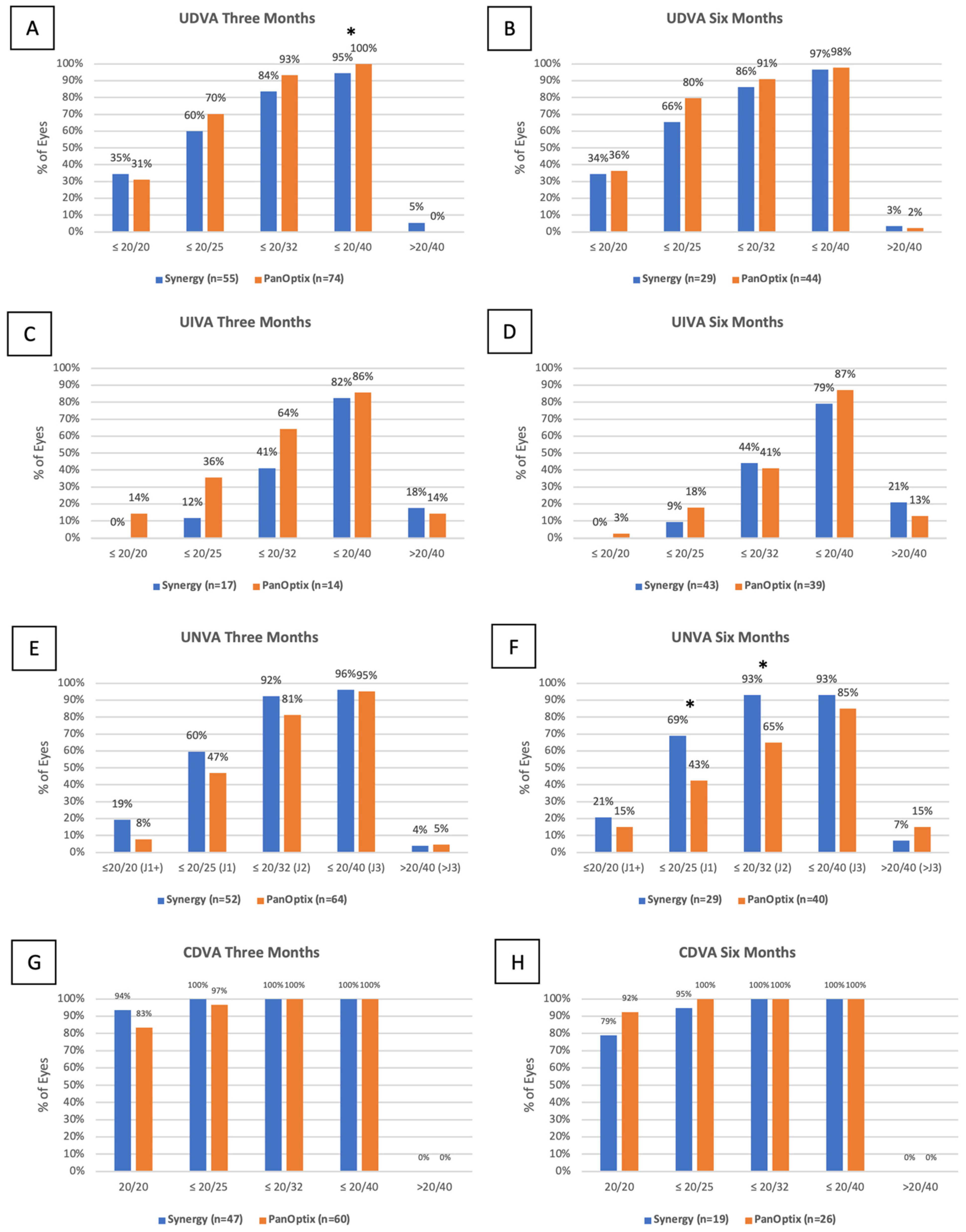
3.3. Uncorrected Distance Visual Acuity
3.4. Uncorrected Intermediate Visual Acuity
3.5. Uncorrected near Visual Acuity
3.6. Corrected Distance Visual Acuity
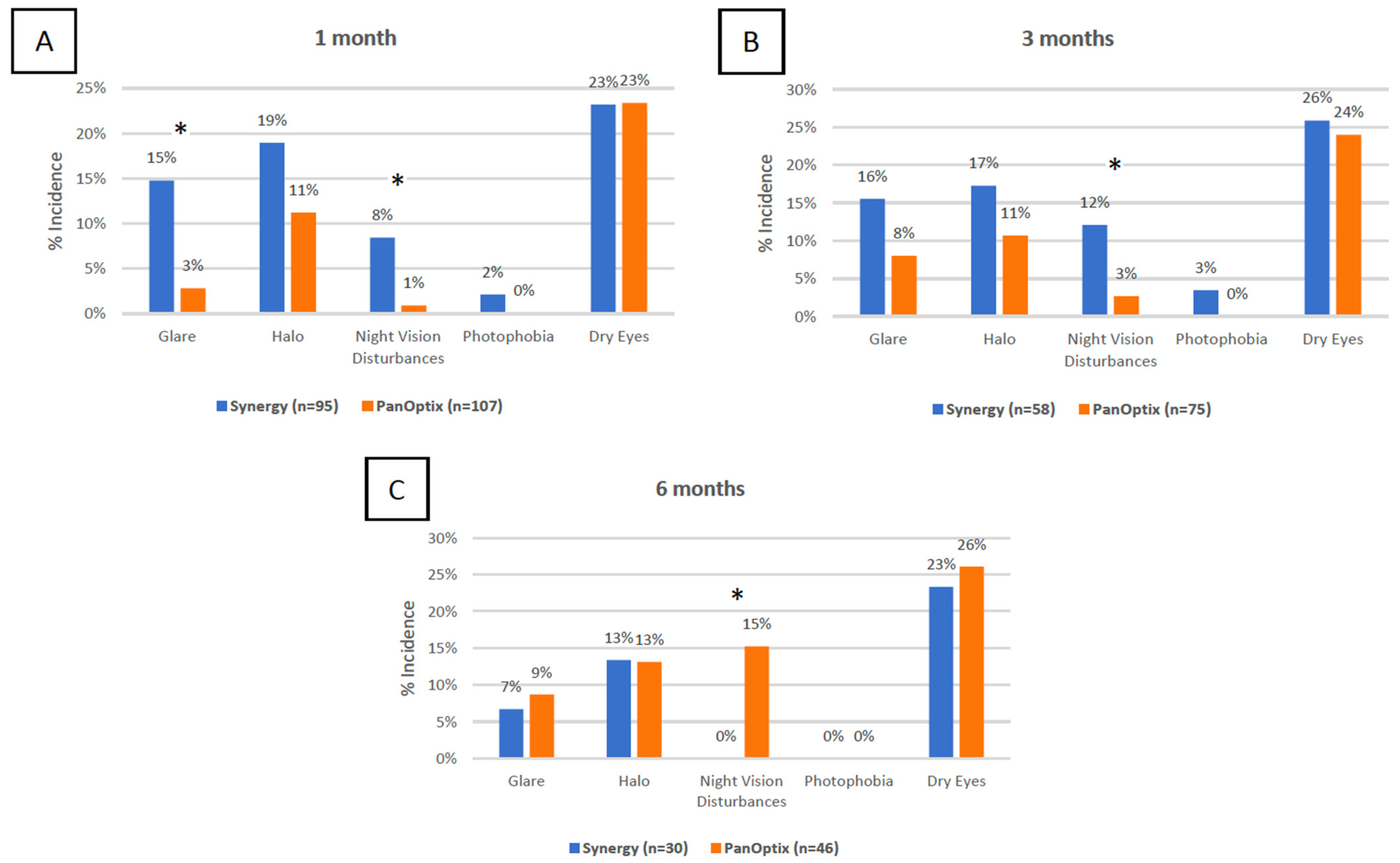
3.7. Subjective Measures
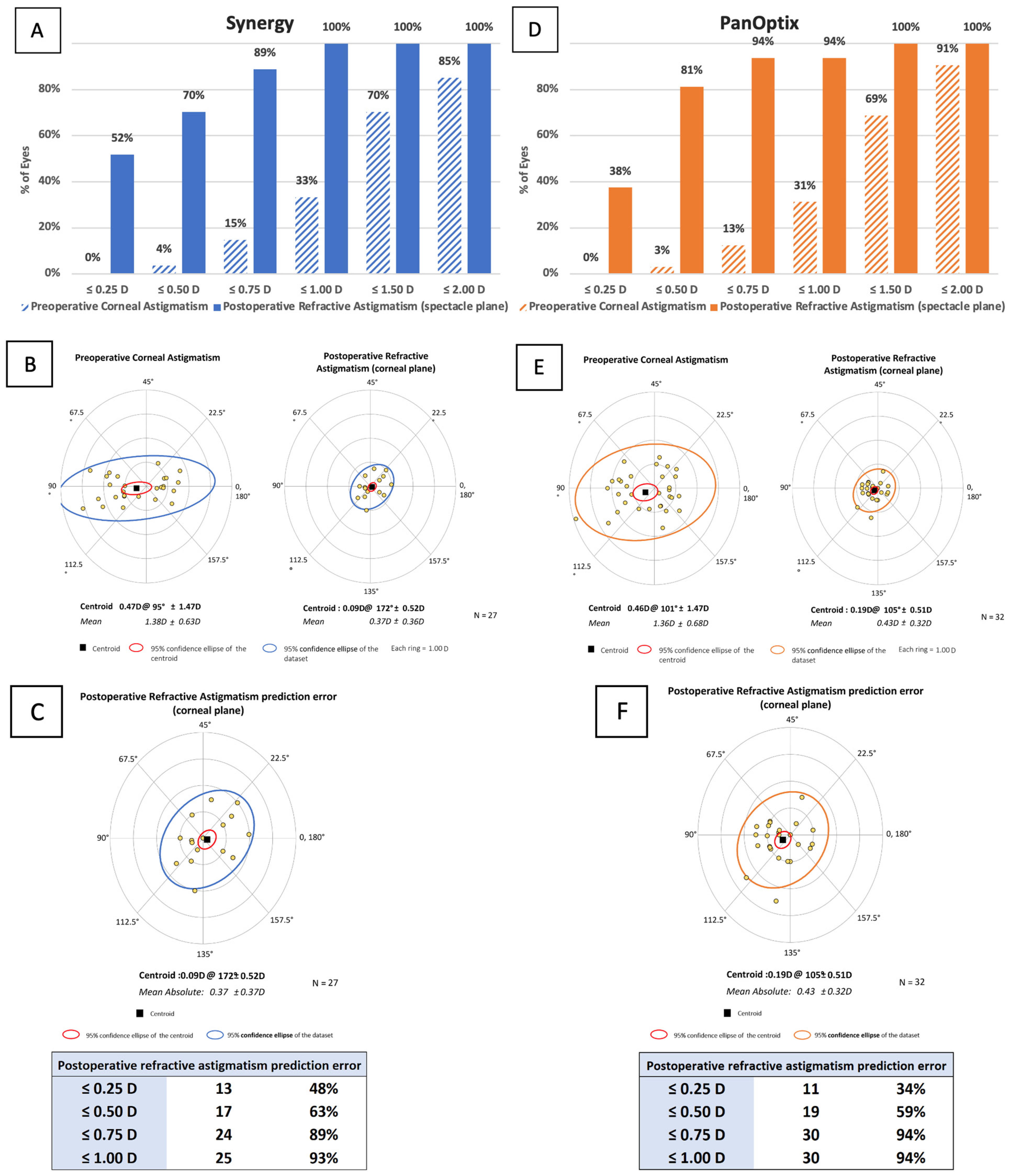
3.8. Vector Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Synergy Study | Eyes (n) | Int (mo) | UDVA (logMar) | UIVA (logMar) | UNVA (logMar) | UDVA ≤ 20/20 (%) | UDVA ≤ 20/40 (%) | UIVA ≤ 20/20 (%) | UIVA ≤ 20/40 (%) | UNVA ≤ 20/20 (%) | UNVA ≤ 20/40 (%) | Funding |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Palomino-Bautista [21], 2021 | 25 | 3 | - | - | - | - | - | - | - | - | - | Y |
| Ribeiro [9], 2021 | 54 | 3 | 0.04 ± 0.10 | 0.04 ± 0.09 | 0.05 ± 0.13 | 51.9 | 98.1 | - | - | - | - | Y |
| Ozturkmen [13], 2021 | 60 | 6 | −0.01 ± 0.04 * | 0.05 ± 0.03 * | 0.03 ± 0.05 * | 90 * | - | - | - | 72 * | 100 * | N |
| Gabrić [22], 2021 | 206 | 3 | 0.00 ± 0.03 * | - | 0.04 ± 0.02 * | 96.1 * | 100 * | - | - | 91.3 * | 100 * | Y |
| Dick [4], 2022 | 100 | 3 | - | - | - | - | - | - | - | - | - | Y |
| Shin [8], 2022 | 17 | 3 | 0.04 ± 0.07 | 0.01 ± 0.04 | 0.32 ± 0.09 | - | - | - | - | - | - | N |
| Ferreira [10], 2022 | 60 | 3 | 0.04 ± 0.10 | - | - | 80 * | 100 * | 77 * | 100 * | 73 * | 100 * | Y |
| FDA Trial [5], 2021 | 135 | 6 | 0.09 | 0.02 * | 0.06 * | 38.2 | 97.7 | 55.0 | 98.5 | 33.6 | 94.7 | Y |
| Current Study | 105 | 6 | 0.13 ± 0.16 | 0.01 ± 0.12 | 0.10 ± 0.07 | 34 | 97 | 0 | 79 | 21 | 93 | N |
Appendix B
| PanOptix Study | Eyes (n) | Int (mo) | UDVA (logMar) | UIVA (logMar) | UNVA (logMar) | UDVA ≤ 20/20 (%) | UDVA ≤ 20/40 (%) | UIVA ≤ 20/20 (%) | UIVA ≤ 20/40 (%) | UNVA ≤ 20/20 (%) | UNVA ≤ 20/40 (%) | Funding |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monaco [23], 2017 | 60 | 4 | 0.00 ± 0.04 | 0.23 ± 0.07 | 0.02 ± 0.06 | - | - | - | - | - | - | N |
| Garcia-Perez [24], 2017 | 58 | 1 | 0.03 ± 0.05 | 0.12 ± 0.14 | 0.02 ± 0.10 | - | - | - | - | - | - | N |
| Kohnen [25], 2017 | 54 | 3 | 0.04 ± 0.13 | 0.06 ± 0.12 | 0.06 ± 0.107 | 57 | 96 | 40 | 100 | 50 | 100 | N |
| Lawless [26], 2017 | 66 | 1, 2 | 0.01 ± 0.1 | 0.3 ± 0.14 | 0.18 ± 0.1 | 78.8 | 100 | - | - | 50 | - | N |
| De Medeiros [3], 2017 | 20 | 1–6 | 0.01 ± 0.04 * | 0.14 ± 0.05 * | −0.03 ± 0.04 * | - | - | - | - | - | - | N |
| Gundersen and Potvin [27], 2017 | 120 | 6–24 | −0.05 ± 0.07 * | - | 0.07 ± 0.07 | - | - | - | - | - | - | Y |
| Vilar [28], 2017 | 40 | 1 | 0.01 ± 0.04 | 0.14 ± 0.05 | −0.03 ± 0.04 | - | - | - | - | - | - | N |
| Böhm [29], 2018 | 27 | 3 | 0.04 ± 0.13 | 0.08 ± 0.12 | 0.06 ± 0.11 | - | - | - | - | - | - | Y |
| Escandón-García [30], 2018 | 90 | 1–2 | 0.07 ± 0.10 * | - | - | - | - | - | - | - | - | Y |
| Mencucci [31], 2018 | 120 | 3 | 0.01 ± 0.09 | 0.11 ± 0.04 | 0.17 ± 0.05 | 50 | 100 | 2 | 100 | 0 | 100 | N |
| Alió [32], 2018 | 52 | 6 | 0.07 ± 0.10 * | 0.12 ± 0.13 * | 0.16 ± 0.09 * | - | - | - | - | - | - | Y |
| Cochener [33], 2018 | 120 | 6 | 0.87 ± 0.16 d | 0.55 ± 0.12 d | 0.61 ± 0.11 d | 50 | 95 | - | - | - | - | N |
| Martínez de Carneros-Llorente [34], 2019 | 40 | 6 | 0.07 ± 0.10 | - | - | - | - | - | - | - | - | N |
| Donmez [35], 2019 | 138 | 6 | 0.02 ± 0.05 | 0.06 ± 0.07 | 0.05 ± 0.07 | - | 100 | - | 100 | - | 100 | N |
| Yesilirmak [36], 2019 | 20 | 6 | −0.14 ± 0.05 * | 0.03 ± 0.05 * | 0.00 ± 0.00 * | 100 * | 100 * | 75 * | 100 * | 100 * | 100 * | N |
| Středová [37], 2019 | 32 | 1, 27 | 0.94 ± 0.10 d | - | 1.00 ± 0.0 d | - | - | - | - | - | 96.3 | N |
| Rementería-Capelo [18], 2019 (spherical) | 166 | 3 | 0.06 ± 0.07 | 0.2 ± 0.1 | 0.05 ± 0.07 | - | - | - | - | - | - | N |
| Rementería-Capelo [18], 2019 (toric) | 84 | 3 | 0.07 ± 0.1 | 0.23 ± 0.2 | 0.07 ± 0.12 | - | - | - | - | - | - | N |
| De Medeiros [38], 2019 | 26 | 6–12 | 0.09 ± 0.00 * | 0.39 ± 0.2 * | −0.01 ± 0.16 * | - | - | - | - | - | - | Y |
| Ribeiro [39], 2020 | 60 | 3 | 0.06 ± 0.11 | 0.05 ± 0.08 | 0.05 ± 0.10 | - | - | - | - | - | - | Y |
| Nicula [40], 2020 | 128 | 12 | 0.07 ± 0.14 | 0.08 ± 0.14 | 0.07 ± 0.14 | 62.8 | - | 62.8 | - | 62 | - | N |
| Kim [41], 2020 | 88 | 3 | 0.08 ± 0.12 | 0.05 ± 0.13 | 0.09 ± 0.13 | 52 | 100 | 66 | 100 | 57 | 96 | Y |
| Kohnen [42], 2020 | 50 | 3 | 0.02 ± 0.12 | 0.12 ± 0.11 | 0.10 ± 0.11 | 68 | 100 | 29 | 100 | 32 | 100 | Y |
| Kohnen [43], 2020 | 290 | 12 | 0.02 ± 0.11 * | 0.04 ± 0.12 * | 0.07 ± 0.11 * | 70 | 99 | 51 | 98 | 44 | 97 | Y |
| Song [44], 2020 | 50 | 6 | 0.03 ± 0.08 | 0.05 ± 0.05 | 0.08 ± 0.09 | - | 100 * | - | 100 * | - | 100 * | Y |
| Lapid-Gortzak [45], 2020 | 93 | 4–6 | 0.01 ± 0.01 * | 0.05 ± 0.13 * | 0.08 ± 0.10 * | - | - | - | - | - | - | Y |
| Carreño [46], 2020 | 200 | 1 | −0.08 * | 0.03 * | −0.04 * | - | - | - | - | - | - | Y |
| Ribeiro [47], 2020 | 30 | 3 | 0.05 ± 0.09 | 0.11 ± 0.13 | 0.05 ± 0.11 | - | - | - | - | - | - | N |
| Serdiuk [48], 2020 | 28 | 6 | 0.33 ± 0.29 d | - | - | 64 | 100 | 0 | 96 | 11 | 96 | N |
| Alfonso [49], 2020 | 80 | 6 | 0.85 ± 0.19 | - | 0.71 ± 0.10 | 70 * | 100 * | 0 * | 100 * | 35 * | 100 * | N |
| Pedrotti [50], 2020 | 50 | 3 | −0.02 ± 0.09 | 0.12 ± 0.04 | 0.14 ± 0.045 | - | - | - | - | - | - | Y |
| Doroodgar [51], 2020 | 62 | 24, 48 | 0.02 ± 0.04 * | 0.04 ± 0.06 * | 0.03 ± 0.05 * | - | - | - | - | - | - | N |
| Rementería-Capelo [52], 2021 | 72 | 3 | 0.04 ± 0.06 | 0.18 ± 0.13 | 0.06 ± 0.07 | - | - | - | - | - | - | N |
| Blaylock [53], 2020 | 137 | 3 | 0.04 ± 0.09 | −0.07 ± 0.08 | 0.04 ± 0.07 | 64.7 | 100 | 94.9 | 99.3 | 73 | 100 | Y |
| Moshirfar [14], 2021 | 113 | 3 | 0.09 ± 0.13 | - | 0.16 ± 0.14 | 21 | 100 | - | - | 23 | 96 | N |
| Choi [54], 2021 | 50 | 6 | 0.04 ± 0.07 | - | 0.03 ± 0.06 | 70 | 100 | - | - | - | - | Y |
| Ramamurthy [55], 2021 | 141 | 3 | 0.09 ± 0.14 | 0.12 ± 0.15 | 0.14 ± 0.15 | 53.7 * | 100 * | 34.8 * | 98.5 * | 31.8 * | 92.4 * | Y |
| Ison [56], 2021 | 134 | 1 | 0.01 ± 0.10 * | - | 0.14 ± 0.06 * | 72 * | 100 * | - | - | - | - | N |
| Yoo [57], 2021 | 25 | 3 | 0.08 ± 0.14 | - | 0.05 ± 0.08 | - | - | - | - | - | - | Y |
| Galvis [58], 2022 | 130 | 1.5+ | 0.04 ± 0.06 * | 0.07 ± 0.08 * | 0.05 ± 0.08 * | 63.1 | 100 | - | - | - | 100 | N |
| Sandoval [59], 2022 | 56 | 3 | 0.04 ± 0.09 | 0.13 ± 0.10 | 0.13 ± 0.07 | - | - | - | - | - | - | Y |
| Blaylock [60], 2022 | 35 | 3 | 0.09 ± 0.08 | 0.01 ± 0.12 | 0.05 ± 0.10 | 28.6 | 100 | 77.8 | 100 | 65.6 | 100 | Y |
| Imburgia [61], 2022 | 32 | 12 | 0.01 ± 0.06 | 0.09 ± 0.05 | 0.04 ± 0.05 | 75 | - | 19 | 100 | 56 | 100 | N |
| FDA Trial [20], 2019 | 129 | 6 | - | - | - | 44.9 | 97.6 | 37 | 96.9 | 25.2 | 95.3 | Y |
| Current Study, 2022 | 119 | 6 | 0.09 ± 0.16 | 0.02 ± 0.12 | 0.02 ± 0.07 | 36 | 98 | 4 | 87 | 15 | 85 | N |
References
- Lee, C.M.; Afshari, N.A. The global state of cataract blindness. Curr. Opin. Ophthalmol. 2017, 28, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Wilkins, M.; Kim, T.; Malyugin, B.; Mehta, J.S. Cataracts. Lancet 2017, 390, 600–612. [Google Scholar] [CrossRef] [PubMed]
- De Medeiros, A.L.; de Araújo Rolim, A.G.; Motta, A.F.P.; Ventura, B.; Vilar, C.; Chaves, M.; Carricondo, P.; Hida, W. Comparison of visual outcomes after bilateral implantation of a diffractive trifocal intraocular lens and blended implantation of an extended depth of focus intraocular lens with a diffractive bifocal intraocular lens. Clin. Ophthalmol. 2017, 11, 1911–1916. [Google Scholar] [CrossRef] [PubMed]
- Dick, H.B.; Ang, R.; Corbett, D.; Hoffmann, P.; Tetz, M.; Villarrubia, A.; Palomino, C.; Castillo-Gomez, A.; Tsai, L.; Thomas, E.K.; et al. Comparison of 3-month visual outcomes of a new multifocal intraocular lens versus a trifocal intraocular lens. J. Cataract. Refract. Surg. 2022, 48, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Summary of Safety and Effectiveness Data (SSED) TECNIS SynergyTM IOLs SUMMARY OF SAFETY AND EFFECTIVENESS DATA (SSED). Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf/P980040S039b.pdf (accessed on 10 July 2022).
- Sudhir, R.R.; Dey, A.; Bhattacharrya, S.; Bahulayan, A. Acrysof IQ panoptix intraocular lens versus extended depth of focus intraocular lens and trifocal intraocular lens: A clinical overview. Asia-Pac. J. Ophthalmol. 2019, 8, 335–349. [Google Scholar] [CrossRef]
- Son, H.S.; Labuz, G.; Khoramnia, R.; Merz, P.; Yildirim, T.M.; Auffarth, G.U. Ray propagation imaging and optical quality evaluation of different intraocular lens models. PLoS ONE 2020, 15, e0228342. [Google Scholar] [CrossRef]
- Shin, D.E.; Lee, H.; Kim, T.I.; Koh, K. Comparison of visual results and optical quality of two presbyopia-correcting intraocular lenses: TECNIS symfony versus TECNIS synergy. Eur. J. Ophthalmol. 2022, 32, 3461–3469. [Google Scholar] [CrossRef]
- Ribeiro, F.J.; Ferreira, T.B.; Silva, D.; Matos, A.C.; Gaspar, S. Visual outcomes and patient satisfaction after implantation of a presbyopia-correcting intraocular lens that combines extended depth-of-focus and multifocal profiles. J. Cataract Refract. Surg. 2021, 47, 1448–1453. [Google Scholar] [CrossRef]
- Ferreira, T.B.; Ribeiro, F.J.; Silva, D.; Matos, A.C.; Gaspar, S.; Almeida, S. Comparison of refractive and visual outcomes of 3 presbyopia-correcting intraocular lenses. J Cataract Refract. Surg. 2022, 48, 280–287. [Google Scholar] [CrossRef]
- Schwiegerling, J. Field Guide to Visual and Ophthalmic Optics; SPIE Press: Bellingham, WA, USA, 2004. [Google Scholar]
- Abulafia, A.; Koch, D.D.; Holladay, J.T.; Wang, L.; Hill, W. Pursuing perfection in intraocular lens calculations: IV. Rethinking astigmatism analysis for intraocular lens-based surgery: Suggested terminology, analysis, and standards for outcome reports. J. Cataract Refract. Surg. 2018, 44, 1169–1174. [Google Scholar] [CrossRef]
- Ozturkmen, C.; Kesim, C.; Karadeniz, P.G.; Sahin, A. Visual acuity, defocus curve and patient satisfaction of a new hybrid EDOF-multifocal diffractive intraocular lens. Eur. J. Ophthalmol. 2022, 32, 2988–2993. [Google Scholar] [CrossRef] [PubMed]
- Moshirfar, M.; Ellis, J.; Beesley, D.; McCabe, S.; Lewis, A.; West, W.; Ronquillo, Y.; Hoopes, P. Comparison of the Visual Outcomes of an Extended Depth-of-Focus Lens and a Trifocal Lens. Clin. Ophthalmol. 2021, 15, 3051–3063. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, D.; Wang, Y.; Chen, W.; Xiao, W.; Xiang, Y.; Zhu, Y.; Chen, C.; Dong, X.; Liu, Y.; et al. Comparison of Visual Neuroadaptations After Multifocal and Monofocal Intraocular Lens Implantation. Front. Neurosci. 2021, 15, 648863. [Google Scholar] [CrossRef] [PubMed]
- Kretz, F.T.A.; Tandogan, T.; Khoramnia, R.; Auffarth, G.U. High order aberration and straylight evaluation after cataract surgery with implantation of an aspheric, aberration correcting monofocal intraocular lens. Int. J. Ophthalmol. 2015, 8, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Miret, J.J.; Camps, V.J.; García, C.; Caballero, M.; Gonzalez-Leal, J.; San, C.; Del Raspieg, V.; Vicente, S.; Raspeig, D.; Alicante, S. Analysis and comparison of monofocal, extended depth of focus and trifocal intraocular lens profiles. Sci. Rep. 2022, 12, 8654. [Google Scholar] [CrossRef] [PubMed]
- Rementería-Capelo, L.A.; Contreras, I.; García-Pérez, J.L.; Blázquez, V.; Ruiz-Alcocer, J. Visual quality and patient satisfaction with a trifocal intraocular lens and its new toric version. J. Cataract Refract. Surg. 2019, 45, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- Schneck, M.E.; Haegerstöm-Portnoy, G.; Lott, L.A.; Brabyn, J.A. Monocular vs. binocular measurement of spatial vision in elders. Optom. Vis. Sci. 2010, 87, 526–531. [Google Scholar] [CrossRef]
- PMA P040020/S087: FDA Summary of Safety and Effectiveness Data Summary of Safety and Effectiveness DATA (SSED). Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf4/P040020S049B.pdf (accessed on 10 July 2022).
- Palomino-Bautista, C.; Sánchez-Jean, R.; Carmona-Gonzalez, D.; Piñero, D.P.; Molina-Martín, A. Depth of field measures in pseudophakic eyes implanted with different type of presbyopia-correcting IOLS. Sci. Rep. 2021, 11, 12081. [Google Scholar] [CrossRef]
- Gabrić, N.; Gabrić, I.; Gabrić, K.; Biščević, A.; Piñero, D.P.; Bohač, M. Clinical outcomes with a new continuous range of vision presbyopia-correcting intraocular lens. J. Refract. Surg. 2021, 37, 256–262. [Google Scholar] [CrossRef]
- Monaco, G.; Gari, M.; di Censo, F.; Poscia, A.; Ruggi, G.; Scialdone, A. Visual performance after bilateral implantation of 2 new presbyopia-correcting intraocular lenses: Trifocal versus extended range of vision. J. Cataract Refract. Surg. 2017, 43, 737–747. [Google Scholar] [CrossRef]
- García-Pérez, J.L.; Gros-Otero, J.; Sánchez-Ramos, C.; Blázquez, V.; Contreras, I. Short term visual outcomes of a new trifocal intraocular lens. BMC Ophthalmol. 2017, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Kohnen, T.; Herzog, M.; Hemkeppler, E.; Schönbrunn, S.; De Lorenzo, N.; Petermann, K.; Böhm, M. Visual Performance of a Quadrifocal (Trifocal) Intraocular Lens Following Removal of the Crystalline Lens. Am. J. Ophthalmol. 2017, 184, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Lawless, M.; Hodge, C.; Reich, J.; Levitz, L.; Bhatt, U.; McAlinden, C.; Roberts, K.; Roberts, T. Visual and refractive outcomes following implantation of a new trifocal intraocular lens. Eye Vis. 2017, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, K.G.; Potvin, R. Trifocal intraocular lenses: A comparison of the visual performance and quality of vision provided by two different lens designs. Clin. Ophthalmol. 2017, 11, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Vilar, C.; Hida, W.T.; de Medeiros, A.L.; Magalhães, K.R.; Tzelikis, P.F.; Chaves, M.; Pimenta Motta, A.F.; Carricondo, P.C.; Alves, M.R.; Nosé, W. Comparison between bilateral implantation of a trifocal intraocular lens and blended implantation of two bifocal intraocular lenses. Clin. Ophthalmol. 2017, 11, 1393–1397. [Google Scholar] [CrossRef]
- Böhm, M.; Hemkeppler, E.; Herzog, M.; Schönbrunn, S.; de’Lorenzo, N.; Petermann, K.; Kohnen, T. Comparison of a panfocal and trifocal diffractive intraocular lens after femtosecond laser–assisted lens surgery. J. Cataract. Refract. Surg. 2018, 44, 1454–1462. [Google Scholar] [CrossRef]
- Escandón-García, S.; Ribeiro, F.J.; McAlinden, C.; Queirós, A.; González-Méijome, J.M. Through-Focus Vision Performance and Light Disturbances of 3 New Intraocular Lenses for Presbyopia Correction. J. Ophthalmol. 2018, 2018, 6165493. [Google Scholar] [CrossRef]
- Mencucci, R.; Favuzza, E.; Caporossi, O.; Savastano, A.; Rizzo, S. Comparative analysis of visual outcomes, reading skills, contrast sensitivity, and patient satisfaction with two models of trifocal diffractive intraocular lenses and an extended range of vision intraocular lens. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 1913–1922. [Google Scholar] [CrossRef]
- Alió, J.L.; Plaza-Puche, A.B.; Alió del Barrio, J.L.; Amat-Peral, P.; Ortuño, V.; Yébana, P.; Al-Shymali, O.; Vega-Estrada, A. Clinical outcomes with a diffractive trifocal intraocular lens. Eur. J. Ophthalmol. 2018, 28, 419–424. [Google Scholar] [CrossRef]
- Cochener, B.; Boutillier, G.; Lamard, M.; Auberger-Zagnoli, C. A Comparative Evaluation of a New Generation of Diffractive Trifocal and Extended Depth of Focus Intraocular Lenses. J. Refract. Surg. 2018, 34, 507–514. [Google Scholar] [CrossRef]
- Martínez de Carneros-Llorente, A.; Martínez de Carneros, A.; Martínez de Carneros-Llorente, P.; Jiménez-Alfaro, I. Comparison of visual quality and subjective outcomes among 3 trifocal intraocular lenses and 1 bifocal intraocular lens. J. Cataract Refract. Surg. 2019, 45, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Donmez, O.; Asena, B.S.; Kaskaloglu, M.; Akova, Y.A. Patients satisfaction and clinical outcomes of binocular implantation of a new trifocal intraocular lens. Int. Ophthalmol. 2020, 40, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Yesilirmak, N.; Akova, Y.A.; Donmez, O. Comparison of mix-and-match implanted bifocal IOLs and bilateral implanted trifocal IOLs after femtosecond laser-assisted cataract surgery. J. Refract. Surg. 2019, 35, 559–564. [Google Scholar] [CrossRef]
- Středová, M.; Řeháková, T.; Veliká, V.; Rozsíval, P.; Hejsek, L.; Jirásková, N. Evaluation of retinal light scattering, visual acuity, refraction and subjective satisfaction in patients after Acrysof IQ PanOptix intraocular lens implantation. Czech Slovak Ophthalmol. 2020, 75, 316–322. [Google Scholar] [CrossRef] [PubMed]
- De Medeiros, A.L.; Saraiva, F.J.; Iguma, C.I.; Kniggendorf, D.; Alves, G.; Pereira, M.; Chaves, D.; Vilar, C.; Motta, A.; Carricondo, P.; et al. Comparison of visual outcomes after bilateral implantation of two intraocular lenses with distinct diffractive optics. Clin. Ophthalmol. 2019, 13, 1657–1663. [Google Scholar] [CrossRef]
- Ribeiro, F.J.; Ferreira, T.B. Comparison of visual and refractive outcomes of 2 trifocal intraocular lenses. J. Cataract Refract. Surg. 2020, 46, 694–699. [Google Scholar] [CrossRef]
- Nicula, C.A.; Popescu, R.; Rednik, A.M.; Nicula, D.; Bulboaca, A.E.; Stanescu, I. Refractive Lens Exchange in Hyperopic Presbyopes with the Acrysof IQ Panoptix Intraocular Lens: One-Year Results and Analysis of the Literature. Ther. Clin. Risk Manag. 2020, 16, 1125–1137. [Google Scholar] [CrossRef]
- Kim, T.I.; Chung, T.Y.; Kim, M.J.; Lee, K.; Hyon, J.Y. Visual outcomes and safety after bilateral implantation of a trifocal presbyopia correcting intraocular lens in a Korean population: A prospective single-arm study. BMC Ophthalmol. 2020, 20, 288. [Google Scholar] [CrossRef]
- Kohnen, T.; Lwowski, C.; Hinzelmann, L.; Ahmad, W.; Petermann, K.; Hemkeppler, E.; Pawlowicz, K.; Böhm, M. Presbyopia correction in astigmatic eyes using a toric trifocal intraocular lens with quadrifocal technology. J. Refract. Surg. 2020, 36, 638–644. [Google Scholar] [CrossRef]
- Kohnen, T.; Marchini, G.; Alfonso, J.F.; Bala, C.; Cochener, B.; Martinez, A.; Carreño, E. Innovative trifocal (quadrifocal) presbyopia-correcting IOLs: 1-year outcomes from an international multicenter study. J. Cataract Refract. Surg. 2020, 46, 1142–1148. [Google Scholar] [CrossRef]
- Song, J.E.; Khoramnia, R.; Son, H.S.; Knorz, M.C.; Choi, C.Y. Comparison between Bilateral Implantation of a Trifocal IOL and Mix-and-Match Implantation of a Bifocal IOL and an Extended Depth of Focus IOL. J. Refract. Surg. 2020, 36, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Lapid-Gortzak, R.; Bhatt, U.; Sanchez, J.G.; Guarro, M.; Hida, W.; Bala, C.; Nosé, R.; Rodriguez Alvira, F.; Martinez, A. Multicenter visual outcomes comparison of 2 trifocal presbyopia-correcting IOLs: 6-month postoperative results. J. Cataract Refract. Surg. 2020, 46, 1534–1542. [Google Scholar] [CrossRef] [PubMed]
- Carreño, E.; Carreño, E.A.; Carreño, R.; Carreño, M.; López, V.; Potvin, R. Refractive and Visual Outcomes after Bilateral Implantation of a Trifocal Intraocular Lens in a Large Population. Clin. Ophthalmol. 2020, 14, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.; Ferreira, T.B. Comparison of clinical outcomes of 3 trifocal IOLs. J. Cataract Refract. Surg. 2020, 46, 1247–1252. [Google Scholar] [CrossRef]
- Serdiuk, V.; Ustymenko, S.; Fokina, S.; Ivantsov, I. Comparison of three different presbyopia-correcting intraocular lenses. Rom. J. Ophthalmol. 2020, 64, 364–379. [Google Scholar] [CrossRef]
- Alfonso, J.F.; Fernández-Vega-Cueto, L.; Fernández-Vega, L.; Montés-Micó, R. Visual Function after Implantation of a Presbyopia-Correcting Trifocal Intraocular Lens. Ophthalmic Res. 2020, 63, 152–164. [Google Scholar] [CrossRef]
- Pedrotti, E.; Carones, F.; Talli, P.; Bonacci, E.; Selvi, F.; Galzignato, A.; Besutti, A.; De Gregorio, A.; Marchini, G. Comparative analysis of objective and subjective outcomes of two different intraocular lenses: Trifocal and extended range of vision. BMJ Open Ophthalmol. 2020, 5, e000497. [Google Scholar] [CrossRef]
- Doroodgar, F.; Niazi, F.; Sanginabadi, A.; Karimian, F.; Niazi, S.; Alinia, C.; Ali Javadi, M. Visual performance of four types of diffractive multifocal intraocular lenses and a review of articles. Int. J. Ophthalmol. 2021, 14, 356–365. [Google Scholar] [CrossRef]
- Rementería-Capelo, L.A.; García-Pérez, J.L.; Gros-Otero, J.; Carrillo, V.; Pérez-Lanzac, J.; Contreras, I. Real-world evaluation of visual results and patient satisfaction for extended range of focus intraocular lenses compared to trifocal lenses. Int. Ophthalmol. 2021, 41, 163–172. [Google Scholar] [CrossRef]
- Blaylock, J.F.; Hall, B. Astigmatic Results of a Diffractive Trifocal Toric IOL Following Intraoperative Aberrometry Guidance. Clin. Ophthalmol. 2020, 14, 4373–4378. [Google Scholar] [CrossRef]
- Choi, J.Y.; Choi, A.; Kwon, H.; Jeon, S. Accuracy of IOL Power Calculation Formulas for Quadrifocal Acrysof IQ PanOptix TFNT Implantation in Patients with Previous Corneal Refractive Surgery: Comparison of SS-OCT-Based Biometers. J. Refract. Surg. 2021, 37, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, D.; Vasavada, A.; Padmanabhan, P.; Reddy, J.; Shetty, N.; Dey, A.; Sudhir, R. Clinical Outcomes after Bilateral Implantation of a Trifocal Presbyopia-Correcting Intraocular Lens in an Indian Population. Clin. Ophthalmol. 2021, 15, 213–225. [Google Scholar] [CrossRef]
- Ison, M.; Scott, J.; Apel, J.; Apel, A. Patient Expectation, Satisfaction and Clinical Outcomes with a New Multifocal Intraocular Lens. Clin. Ophthalmol. 2021, 15, 4131–4140. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.S.; Kang, M.C.; Park, J.; Kwon, H.; Chung, E.; Lim, D.; Chung, T. Factors affecting prediction error after cataract surgery with implantation of various multifocal IOLs in patients with previous refractive laser surgery. Ann. Transl. Med. 2021, 9, 1720. [Google Scholar] [CrossRef] [PubMed]
- Galvis, V.; Escaf, L.C.; Escaf, L.J.; Tello, A.; Rodríguez, L.; Lapid-Gortzak, R.; Carreño, N.; Berrospi, R.; Niño, C.; Viberg, A.; et al. Visual and satisfaction results with implantation of the trifocal Panoptix® intraocular lens in cataract surgery. J. Optom. 2022, 15, 219–227. [Google Scholar] [CrossRef]
- Sandoval, H.P.; Potvin, R.; Solomon, K.D. The Effects of Angle Kappa on Clinical Results and Patient-Reported Outcomes after Implantation of a Trifocal Intraocular Lens. Clin. Ophthalmol. 2022, 16, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Blaylock, J.F.; Hall, B.J. Refractive Outcomes Following Trifocal Intraocular Lens Implantation in Post-Myopic LASIK and PRK Eyes. Clin. Ophthalmol. 2022, 16, 2129–2136. [Google Scholar] [CrossRef]
- Imburgia, A.; Gaudenzi, F.; Mularoni, K.; Mussoni, G.; Mularoni, A. Comparison of clinical performance and subjective outcomes between two diffractive trifocal intraocular lenses (IOLs) and one monofocal IOL in bilateral cataract surgery. Front. Biosci.—Landmark 2022, 27, 41. [Google Scholar] [CrossRef]
| Preoperative Parameters | Synergy | PanOptix | Non-Toric Synergy | Non-Toric PanOptix | Toric Synergy | Toric PanOptix | p Value |
|---|---|---|---|---|---|---|---|
| Eyes (n) | 105 | 119 | 74 | 85 | 31 | 34 | - |
| Sex (n, %) | |||||||
| Males | 27 (39.1) | 38 (53.5) | 19 (38.0) | 30 (54.5) | 12 (52.2) | 12 (46.2) | - |
| Females | 42 (61.0) | 33 (46.5) | 31 (62.0) | 25 (45.5) | 11 (47.8) | 14 (53.8) | |
| Age (years) | |||||||
| Mean ± SD | 67 ± 8.56 | 66.83 ± 7.43 | 66.47 ± 7.57 | 67.27 ± 7.30 | 68.29 ± 10.58 | 65.74 ± 7.73 | 0.83 |
| Range | (39, 83) | (45, 81) | (39, 78) | (53, 81) | (47, 83) | (45, 77) | |
| IOP (mmHg) | |||||||
| Mean ± SD | 13.9 ± 3.1 | 14.7 ± 3.6 | 13.9 ± 3.1 | 15.1 ± 3.7 | 13.7 ± 3.1 | 13.7 ± 3.3 | 0.07 |
| Range | (4, 21) | (8, 30) | (4, 21) | (8, 30) | (6, 20) | (9, 26) | |
| AL (mm) | |||||||
| Mean ± SD | 23.99 ± 1.15 | 23.75 ± 1.07 | 23.94 ± 1.23 | 23.85 ± 0.92 | 24.10 ± 0.97 | 23.51 ± 1.34 | 0.18 |
| Range | (20.63, 27.22) | (20.29, 27.16) | (20.63, 27.22) | (22.02, 27.16) | (22.63, 26.39) | (20.29, 27.0) | |
| ACD (mm) | |||||||
| Mean ± SD | 3.25 ± 0.36 | 3.19 ± 0.35 | 3.24 ± 0.35 | 3.23 ± 0.35 | 3.27 ± 0.40 | 3.09 ± 0.33 | 0.24 |
| Range | (2.24, 4.11) | (2.44, 4.43) | (2.3, 4.02) | (2.61, 4.43) | (2.24, 4.11) | (2.44, 3.97) | |
| AD (mm) | |||||||
| Mean ± SD | 2.71 ± 0.38 | 2.64 ± 0.33 | 2.71 ± 0.36 | 2.67 ± 0.33 | 2.70 ± 0.42 | 2.56 ± 0.32 | 0.3 |
| Range | (1.7, 3.61) | (1.91, 3.56) | (1.8, 3.45) | (2.07, 3.56) | (1.7, 3.61) | (1.91, 3.42) | |
| Km (D) | |||||||
| Mean ± SD | 43.67 ± 1.26 | 43.72 ± 1.40 | 43.79 ± 1.25 | 43.54 ± 1.38 | 43.40 ± 1.27 | 44.15 ± 1.38 | 0.21 |
| Range | (39.1, 47.0) | (39.8, 47.2) | (40.95, 47.0) | (39.8, 46.6) | (39.1, 45.75) | (41.15, 47.2) | |
| LT (mm) | |||||||
| Mean ± SD | 4.44 ± 3.76 | 4.50 ± 0.40 | 4.43 ± 0.39 | 4.49 ± 0.39 | 4.46 ± 0.35 | 4.53 ± 0.44 | 0.69 |
| Range | (3.09, 5.21) | (2.98, 5.75) | (3.09, 5.16) | (2.98, 5.51) | (3.74, 5.21) | (3.89, 5.75) | |
| WTW (mm) | |||||||
| Mean ± SD | 12.09 ± 0.47 | 12.01 ± 0.42 | 12.13 ± 0.42 | 12.01 ± 0.43 | 12.01 ± 0.56 | 12.03 ± 0.40 | 0.43 |
| Range | (10.7, 13.62) | (10.98, 12.84) | (10.7, 13.16) | (10.98, 12.84) | (10.79, 13.62) | (11.14, 12.76) | |
| Sphere (D) | |||||||
| Mean ± SD | −0.46 ± 2.92 | −0.16 ± 2.86 | −0.57 ± 3.04 | 0.03 ± 2.57 | −0.17 ± 2.65 | −0.62 ± 3.49 | 0.73 |
| Range | (−8.25, 4.75) | (−9.75, 7.75) | (−8.25, 4.75) | (−9.75, 3.0) | (−7.25, 3.75) | (−8.25, 7.75) | |
| SEQ (D) | |||||||
| Mean ± SD | −0.88 ± 2.98 | −0.61 ± 2.99 | −0.90 ± 3.11 | −0.30 ± 2.64 | −0.76 ± 2.65 | −1.39 ± 3.65 | 0.53 |
| Range | (−8.63, 3.88) | (−10.5, 6.88) | (−8.63, 3.88) | (−10.5, 2.75) | (−7.75, 2.38) | (−9.38, 6.88) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moshirfar, M.; Stapley, S.R.; Corbin, W.M.; Bundogji, N.; Conley, M.; Darquea, I.M.; Ronquillo, Y.C.; Hoopes, P.C. Comparative Visual Outcome Analysis of a Diffractive Multifocal Intraocular Lens and a New Diffractive Multifocal Lens with Extended Depth of Focus. J. Clin. Med. 2022, 11, 7374. https://doi.org/10.3390/jcm11247374
Moshirfar M, Stapley SR, Corbin WM, Bundogji N, Conley M, Darquea IM, Ronquillo YC, Hoopes PC. Comparative Visual Outcome Analysis of a Diffractive Multifocal Intraocular Lens and a New Diffractive Multifocal Lens with Extended Depth of Focus. Journal of Clinical Medicine. 2022; 11(24):7374. https://doi.org/10.3390/jcm11247374
Chicago/Turabian StyleMoshirfar, Majid, Seth R. Stapley, Wyatt M. Corbin, Nour Bundogji, Matthew Conley, Ines M. Darquea, Yasmyne C. Ronquillo, and Phillip C. Hoopes. 2022. "Comparative Visual Outcome Analysis of a Diffractive Multifocal Intraocular Lens and a New Diffractive Multifocal Lens with Extended Depth of Focus" Journal of Clinical Medicine 11, no. 24: 7374. https://doi.org/10.3390/jcm11247374
APA StyleMoshirfar, M., Stapley, S. R., Corbin, W. M., Bundogji, N., Conley, M., Darquea, I. M., Ronquillo, Y. C., & Hoopes, P. C. (2022). Comparative Visual Outcome Analysis of a Diffractive Multifocal Intraocular Lens and a New Diffractive Multifocal Lens with Extended Depth of Focus. Journal of Clinical Medicine, 11(24), 7374. https://doi.org/10.3390/jcm11247374







