The Assessment of the Socioemotional Disorder in Neurodegenerative Diseases with the Revised Self-Monitoring Scale (RSMS)
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Neuropsychological Procedures
2.3. Social Cognition Scale, RSMS
3. Statistical Analyses
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | Anterior Cingulate Cortex |
| ACE-R (Revised) | Addenbrooke’s Cognitive Examination-Revised |
| AD | Alzheimer’s Disease |
| BPSD | Behavioral and Psychological Problems |
| bvFTD | behavioral variant Frontotemporal Dementia |
| dACC | dorsal Anterior Cingulate Cortex |
| EF | Executive Functions |
| FTD | Frontotemporal Dementia |
| GRN | Progranulin |
| lvPPA | logopenic Primary Progressive Aphasia |
| MAPT | Microtubule-Associated Protein Tau genes |
| MMSE | Mini Mental State Examination |
| MPFC | Ventral Medial Prefrontal Cortex |
| NPI | Neuropsychiatric Inventory |
| OFC | Orbitofrontal Cortex |
| PD | Parkinson’s Disease |
| PDD | Parkinson’s Dementia |
| PPA | Primary Progressive Aphasia |
| RSMS | Revised Self-Monitoring Scale |
| svPPA | semantic Primary Progressive Aphasia |
| ToM | Theory of Mind |
| vACC | ventral Anterior Cingulate Cortex |
| VTA | Ventral Tegmental Area |
References
- Greenwald, A.G.; Lai, C.K. Implicit Social Cognition. Annu. Rev. Psychol. 2020, 71, 419–445. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, C.; Beauchamp, M.H. Social cognition. Handb. Clin. Neurol. 2020, 173, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Quesque, F.; Rossetti, Y. What Do Theory-of-Mind Tasks Actually Measure? Theory and Practice. Perspect. Psychol. Sci. 2020, 15, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Schurz, M.; Radua, J.; Aichhorn, M.; Richlan, F.; Perner, J. Fractionating theory of mind: A meta-analysis of functional brain imaging studies. Neurosci. Biobehav. Rev. 2014, 42, 9–34. [Google Scholar] [CrossRef]
- Van Overwalle, F.; Baetens, K.; Mariën, P.; Vandekerckhove, M. Social cognition and the cerebellum: A meta-analysis of over 350 fMRI studies. Neuroimage 2014, 86, 554–572. [Google Scholar] [CrossRef]
- Christidi, F.; Migliaccio, R.; Santamaría-García, H.; Santangelo, G.; Trojsi, F. Social Cognition Dysfunctions in Neurodegenerative Diseases: Neuroanatomical Correlates and Clinical Implications. Behav. Neurol. 2018, 2018, 1849794. [Google Scholar] [CrossRef]
- Spitzer, N.; Shafir, T.; Lerman, Y.; Werner, P. The Relationship between Caregiver Burden and Emotion Recognition Deficits in Persons with MCI and Early AD: The Mediating Role of Caregivers’ Subjective Evaluations. Alzheimer Dis. Assoc. Disord. 2019, 33, 266–271. [Google Scholar] [CrossRef]
- Kessels, R.P.C.; Elferink, M.W.; van Tilborg, I. Social cognition and social functioning in patients with amnestic mild cognitive impairment or Alzheimer’s dementia. J. Neuropsychol. 2020, 15, 186–203. [Google Scholar] [CrossRef]
- Belfort, T.; Simões, J.P.; Santos, R.L.; Lacerda, I.; Dourado, M.C.N. Social cognition: Patterns of impairments in mild and moderate Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2020, 35, 1385–1392. [Google Scholar] [CrossRef]
- Dos Santos, T.T.; De Carvalho, R.L.; Nogueira, M.; Baptista, M.A.; Kimura, N.; Lacerda, I.B.; Dourado, M.C. The Relationship between Social Cognition and Executive Functions in Alzheimer’s Disease: A Systematic Review. Curr. Alzheimer Res. 2020, 17, 487–497. [Google Scholar] [CrossRef]
- Bonner, M.F.; Ash, S.; Grossman, M. The New Classification of Primary Progressive Aphasia into Semantic, Logopenic, or Nonfluent/Agrammatic Variants. Curr. Neurol. Neurosci. Rep. 2010, 10, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Shany-Ur, T.; Poorzand, P.; Grossman, S.N.; Growdon, M.E.; Jang, J.Y.; Ketelle, R.S.; Miller, B.L.; Rankin, K.P. Comprehension of insincere communication in neurodegenerative disease: Lies, sarcasm, and theory of mind. Cortex 2012, 48, 1329–1341. [Google Scholar] [CrossRef] [PubMed]
- Toller, G.; Ranasinghe, K.; Cobigo, Y.; Staffaroni, A.; Appleby, B.; Brushaber, D.; Coppola, G.; Dickerson, B.; Domoto-Reilly, K.; Fields, J.; et al. Revised Self-Monitoring Scale: A potential endpoint for frontotemporal dementia clinical trials. Neurology 2020, 94, e2384–e2395. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, R.; Buono, V.L.; Corallo, F.; Foti, M.; Di Lorenzo, G.; Bramanti, P.; Marino, S. Nonmotor Symptoms in Parkinson Disease: A Descriptive Review on Social Cognition Ability. J. Geriatr. Psychiatry Neurol. 2017, 30, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Lennox, R.D.; Wolfe, R.N. Revision of the self-monitoring scale. J. Pers. Soc. Psychol. 1984, 46, 1349–1364. [Google Scholar] [CrossRef]
- Cramer, K.M.; Gruman, J.A. The Lennox and Wolfe Revised Self-Monitoring Scale: Latent structure and gender invariance. Pers. Individ. Differ. 2002, 32, 627–637. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2011, 7, 263–269. [Google Scholar] [CrossRef]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; Van Swieten, J.C.; Seelaar, H.; Dopper, E.G.P.; Onyike, C.U.; et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011, 134 Pt 9, 2456–2477. [Google Scholar] [CrossRef]
- Gorno-Tempini, M.L.; Hillis, A.E.; Weintraub, S.; Kertesz, A.; Mendez, M.; Cappa, S.F.; Ogar, J.M.; Rohrer, J.D.; Black, S.; Boeve, B.F.; et al. Classification of primary progressive aphasia and its variants. Neurology 2011, 76, 1006–1014. [Google Scholar] [CrossRef]
- Berg, D.; Postuma, R.B.; Adler, C.H.; Bloem, B.R.; Chan, P.; Dubois, B.; Gasser, T.; Goetz, C.G.; Halliday, G.; Joseph, L.; et al. MDS research criteria for prodromal Parkinson’s disease. Mov. Disord. 2015, 30, 1600–1611. [Google Scholar] [CrossRef]
- Narme, P.; Mouras, H.; Roussel, M.; Duru, C.; Krystkowiak, P.; Godefroy, O. Emotional and cognitive social processes are impaired in Parkinson’s disease and are related to behavioral disorders. Neuropsychology 2013, 27, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Mioshi, E.; Dawson, K.; Mitchell, J.; Arnold, R.; Hodges, J.R. The Addenbrooke’s Cognitive Examination Revised (ACE-R): A brief cognitive test battery for dementia screening. Int. J. Geriatr. Psychiatry 2006, 21, 1078–1085. [Google Scholar] [CrossRef]
- Cummings, J.L. The Neuropsychiatric Inventory: Assessing psychopathology in dementia patients. Neurology 1997, 48 (Suppl. S6), 10S–16S. [Google Scholar] [CrossRef] [PubMed]
- Parthimos, T.P.; Karavasilis, E.; Rankin, K.P.; Seimenis, I.; Leftherioti, K.; Papanicolaou, A.C.; Miller, B.; Papageorgiou, S.G.; Papatriantafyllou, J.D. The Neural Correlates of Impaired Self-Monitoring Among Individuals with Neurodegenerative Dementias. J. Neuropsychiatry Clin. Neurosci. 2019, 31, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Franklin, H.D.; Russell, L.L.; Peakman, G. The Revised Self-Monitoring Scale Detects Early Impairment of Social Cognition in Genetic Frontotemporal Dementia within the GENFI Cohort. Alzheimer’s Res. Ther. 2021, 13, 127. [Google Scholar] [CrossRef]
- Parkinson, C.; Liu, S.; Wheatley, T. A common cortical metric for spatial, temporal, and social distance. J. Neurosci. 2014, 34, 1979–1987. [Google Scholar] [CrossRef]
- Smallwood, J.; Brown, K.; Baird, B.; Schooler, J.W. Cooperation between the default mode network and the frontal-parietal network in the production of an internal train of thought. Brain Res. 2012, 1428, 60–70. [Google Scholar] [CrossRef]
- Le Heron, C.; Apps, M.; Husain, M. The anatomy of apathy: A neurocognitive framework for amotivated behaviour. Neuropsychologia 2018, 118, 54–67. [Google Scholar] [CrossRef]
- Murley, A.G.; Rouse, M.A.; Jones, P.S.; Ye, R.; Hezemans, F.H.; O’Callaghan, C.; Frangou, P.; Kourtzi, Z.; Rua, C.; Carpenter, T.A.; et al. GABA and glutamate deficits from frontotemporal lobar degeneration are associated with disinhibition. Brain 2020, 143, 3449–3462. [Google Scholar] [CrossRef]
- Fostinelli, S.; De Amicis, R.; Leone, A.; Giustizieri, V.; Binetti, G.; Bertoli, S.; Battezzati, A.; Cappa, S.F. Eating Behavior in Aging and Dementia: The Need for a Comprehensive Assessment. Front. Nutr. 2020, 7, 604488. [Google Scholar] [CrossRef] [PubMed]
| Total Sample N = 331; 100% | Healthy Group a N = 81; 24.5% | AD b N = 127; 38.4% | bvFTD c N = 47; 14.2% | svPPA or FTD/SD d N = 37; 11.2% | PD e N = 21; 6.3% | PDD f N = 18; 5.4% | p | |
|---|---|---|---|---|---|---|---|---|
| Gender, N (%) | ||||||||
| Males | 141 (42.6) | 27 (33.3) | 53 (41.7) | 17 (36.2) | 22 (59.5) | 15 (71.4) | 7 (38.9) | 0.009 ‡ |
| Females | 190 (57.4) | 54 (66.7) | 74 (58.3) | 30 (63.8) | 15 (40.5) | 6 (28.6) | 11 (61.1) | |
| Age (years), mean (SD) | 69.1 (9.6) | 62.5 (9.4) b,d,e,f | 72.8 (8.4) a,c | 65.9 (9.4) b,e,f | 68.7 (7.6) a,f | 73.6 (6.3) a,c | 76.7 (6.9) a,c,d | <0.001 + |
| Years of education, mean (SD) | 11.4 (5.2) | 13.7 (2.9) b,c,d,f | 10.7 (6.4)a | 10.5 (4.2) a | 10.2 (4.4) a | 12.7 (4.1) | 9 (4.6) a | <0.001 + |
| Years of disease, median (IQR) | 2 (0.6–4) | - | 3 (2–4) | 3 (1.6–5) | 4 (2–5) | 3 (2–6) | 3 (2–5) | 0.354 ++ |
| MMSE, mean (SD) | 23.4 (6.2) | 29.4 (0.8) b,c,d,f | 22 (4.8) a,c,d,e | 19.4 (7) a,b,e | 18.8 (7.8) a,b,e | 27.2 (1.3) b,c,d,f | 21.7 (3.6) a,e | <0.001 + |
| NPI delusions, median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) c,d | 0 (0–3) b | 0 (0–2) b | 0 (0–0) | 0 (0–2) | <0.001 ++ |
| NPI hallucinations, median (IQR) | 0 (0–0) | 0 (0–0) f | 0 (0–0) f | 0 (0–0) f | 0 (0–0) | 0 (0–0) | 0 (0–1) a,b,c | <0.001 ++ |
| NPI agitation/aggressive behavior, median (IQR) | 0 (0–2) | 0 (0–0) d | 0 (0–1) d | 0 (0–2) | 2.5 (0–4) a,b | 1 (0–3) | 0 (0–4) | <0.001 ++ |
| NPI depression, median (IQR) | 0 (0–3) | 0 (0–2) | 0 (0–3) | 0 (0–3) | 1.5 (0–3.5) | 1 (0–4) | 3 (0–4) | 0.091 ++ |
| NPI anxiety, median (IQR) | 0 (0–3) | 0 (0–4) | 0 (0–3) | 0 (0–3) | 1 (0–4) | 2 (0–4) | 3 (0–6) | 0.383 ++ |
| NPI euphoria, median (IQR) | 0 (0–0) | 0 (0–0) c,d | 0 (0–0) c | 0 (0–3) a,b | 0 (0–1.5) a | 0 (0–0) | 0 (0–0) | <0.001 ++ |
| NPI apathy, median (IQR) | 3 (0–8) | 0 (0–0) b,c,d,e,f | 3 (0–4) a,c | 6 (3–12) a,b | 3 (2–8) a | 3 (2–8) a | 3 (3–6) a | <0.001 ++ |
| NPI disinhibition, median (IQR) | 0 (0–1) | 0 (0–0) c,d | 0 (0–0) d | 0 (0–3) a | 0.5 (0–4) a,b | 0 (0–0) | 0 (0–0) | 0.001 ++ |
| NPI irritability, median (IQR) | 0 (0–3) | 0 (0–3) | 0 (0–3) | 0 (0–2) | 2 (0–5) | 2 (0–3) | 3 (0–6) | 0.093 ++ |
| NPI wandering, median (IQR) | 0 (0–0) | 0 (0–0) c,d | 0 (0–0) c | 1 (0–8) a,b,f | 0 (0–3) a | 0 (0–0) | 0 (0–0) c | <0.001 ++ |
| NPI sleeping problems, median (IQR) | 0 (0–3) | 0 (0–0) d,e,f | 0 (0–3) | 0 (0–4) | 1.5 (0–4) a | 3 (0–4) a | 2 (0–6) a | 0.004 ++ |
| NPI eating disorders, median (IQR) | 0 (0–4) | 0 (0–0) c,d,e,f | 0 (0–3) c | 4 (0–8) a,b | 0 (0–5) a | 0 (0–4) a | 1 (0–4) a | <0.001 ++ |
| NPI TOTAL, median (IQR) | 16 (8–27) | 7 (3–12) b,c,d,e,f | 13 (6–19) a,c,d | 24.5 (15–38) a,b | 25.5 (12–43.5) a,b | 15.5 (10–28) a | 25 (15–31) a | <0.001 ++ |
| ACE R, mean (SD) | 68.9 (21.7) | 94.4 (3.8)b,c,d,e,f | 63.4 (15.3) a,c,d,e | 54.2 (22.1) a,b,e | 51.1 (21.8) a,b,e | 78.6 (8) a,b,c,d,f | 56.6 (9.2) a,e | <0.001 + |
| ACE R ORIENT, mean (SD) | 8.1 (2.3) | 10 (0.2) b,c,d,f | 7.5 (2.1) a,e | 6.9 (2.4) a,e | 6.9 (3.3) a,e | 9.7 (0.6)b,c,d,f | 7.7 (1.7) a,e | <0.001 + |
| ACE R ATTENT, mean (SD) | 6.8 (1.8) | 8 (0.1) b,c,d,f | 6.4 (1.8) a,e | 5.6 (2.2) a,e | 6.1 (2.2) a,e | 7.8 (0.4) b,c,d | 6.4 (1.6) a | <0.001 + |
| ACE R MEM, mean (SD) | 15.5 (7.2) | 24.2 (2.2) b,c,d,e,f | 12.1 (5) a,e | 12.2 (7.1) a,e | 11.1 (5.5) a,e | 18.4 (4.6) a,b,c,d,f | 11.6 (4.6) a,e | <0.001 + |
| ACE R VERFLUEN, mean (SD) | 6.5 (3.8) | 11.3 (1.7) b,c,d,e,f | 5.3 (2.5) a,c,d,e | 3.7 (2.6) a,b,e | 3.7 (2.4) a,b,e | 7.1 (2.7) a,b,c,d,f | 4.3 (2.1) a,e | <0.001 + |
| ACE_R_VERFLUEN_PHON, mean (SD) | 3.4 (2.0) | 5.3 (0.1) b,c,d,e,f | 3.1 (0.2)a,c,d | 1,9 (0.2) a,b,e | 1.6 (0.2) a,b,e | 3.7 (0.5) a | 2.7 (0.4) a,c,d | <0.008 ++ |
| ACE_R_VERFLUEN_CAT, mean (SD) | 3.4 (2.1) | 5.9 (0.1) b,c,d,e,f | 2.7 (0.1) a,c,d,f | 1.7 (0.2) a,b,e | 1.6 (0.3) a,b,e | 4.1 (0.6) a | 2.3 (0.5) a,b,c,d | <0.004 ++ |
| ACE R LANG, mean (SD) | 21.2 (5.5) | 25.7 (0.9) b,c,d,f | 20.6 (4.9) a,d | 18.7 (5.6) a,d,e | 14.8 (7.1) a,b,c,e | 23.2 (2.1) c,d,f | 18.5 (4.4) a,f | <0.001 + |
| ACE R VS, mean (SD) | 12 (3.9) | 15.3 (1) b,c,d,e,f | 11.6 (3.2) a,c,f | 9.8 (3.9) a,b | 10.3 (5.1) a | 12.1 (2.9) a,f | 8.1 (4.2) a,b,e | <0.001 + |
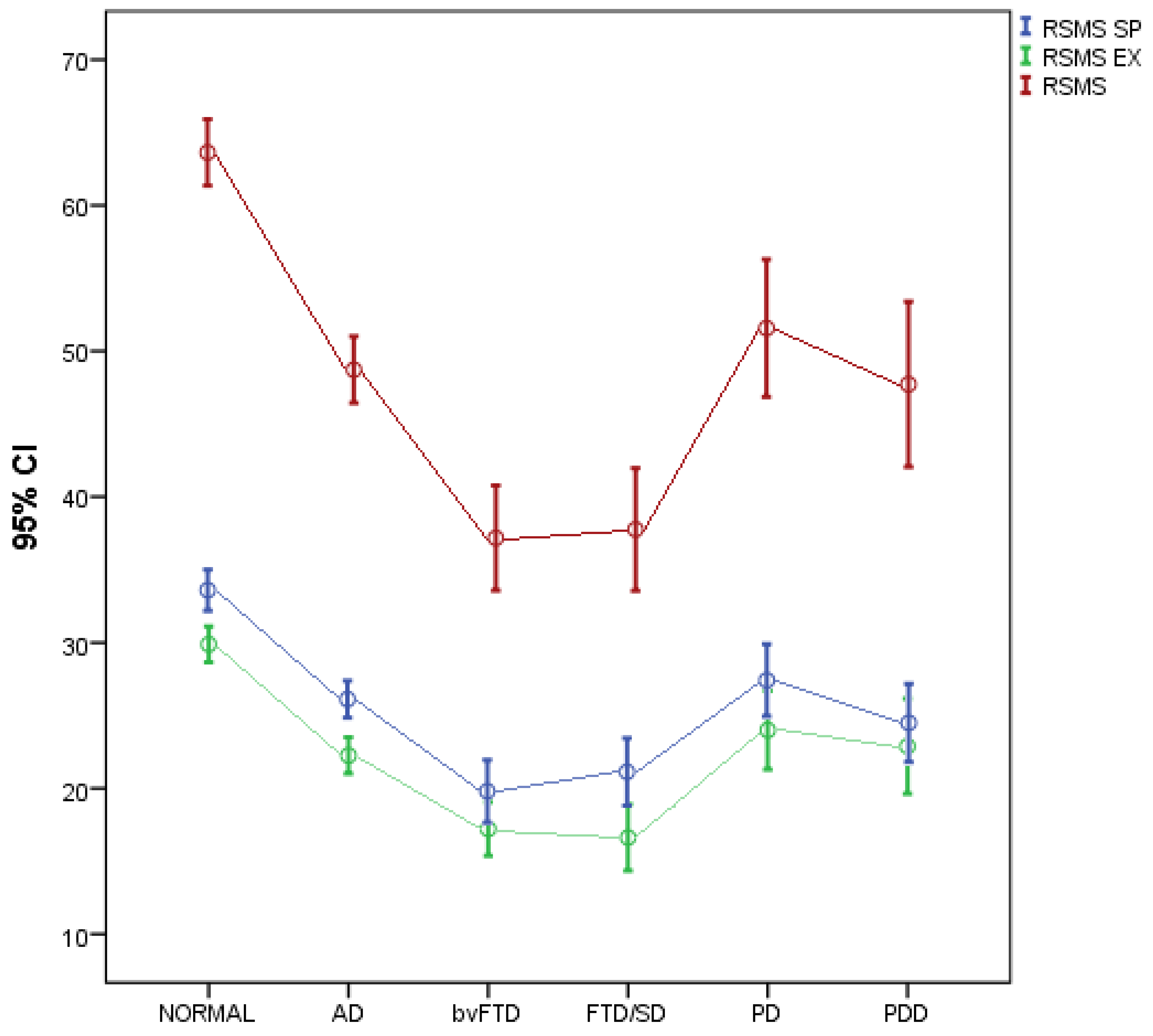
| RSMS SP | RSMS EX | RSMS | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Healthy | 33.7 (6.4) | 29.9 (5.6) | 63.6 (10.3) |
| AD | 26.4 (7.2) | 22.3 (7.1) | 48.7 (13.1) |
| bvFTD | 20.0 (7.3) | 17. 2 (6.3) | 37.2 (12.3) |
| FTD/SD | 21.1 (6.9) | 16.6 (6.8) | 37.8 (12.6) |
| PD | 27.6 (5.4) | 24.0 (5.9) | 51.6 (10.4) |
| PDD | 24.5 (5.4) | 22.9 (6.6) | 47.7 (11.4) |
| Pa | < 0.001 | <0.001 | <0.001 |
| Pb | |||
| AD vs. bvFTD | <0.001 | <0.001 | <0.001 |
| AD vs. FTD/SD | 0.007 | 0.005 | |
| AD vs. HEALTHY | 0.002 | 0.001 | <0.001 |
| bvFTD vs. HEALTHY | <0.001 | <0.001 | <0.001 |
| bvFTD vs. PD | 0.011 | 0.005 | 0.002 |
| bvFTD vs. PDD | 0.018 | 0.030 | |
| FTD/SD vs. HEALTHY | <0.001 | <0.001 | <0.001 |
| FTD/SD vs. PD | 0.019 | 0.031 | |
| FTD/SD vs. PDD | 0.050 | ||
| HEALTHY vs. PDD | 0.019 | ||
| AD vs. bvFTD | <0.001 | <0.001 | <0.001 |
| AD vs. FTD/SD | 0.007 | 0.005 |
| RSMS SP | RSMS EX | RSMS | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| ACE R | 0.48a *** | 0.48a *** | 0.51a *** |
| ACE R ORIENT | 0.34a *** | 0.32a *** | 0.35a *** |
| ACE R ATTENT | 0.32a *** | 0.31a *** | 0.34a *** |
| ACE R MEM | 0.43a *** | 0.42a *** | 0.45a *** |
| ACE R VERFLUEN | 0.52a *** | 0.51a *** | 0.55a *** |
| ACE-R VERFLUEN_PHON | 0.49b *** | 0.50b *** | 0.53b *** |
| ACE-R VERFUEN_CATEG | 0.52b *** | 0.54b *** | 0.57b *** |
| ACE-R LANG | 0.41a *** | 0.43a *** | 0.45a *** |
| ACE R VS | 0.40a *** | 0.37a *** | 0.41a *** |
| RSMS SP | RSMS EX | RSMS | |
|---|---|---|---|
| NPI delusions | −0.14b * | −0.10b | |
| NPI hallucinations | −0.08b | 0.04b | |
| NPI aggressive beh/agitation | −0.28b *** | −0.20b ** | |
| NPI depression | −0.08b | −0.06b | |
| NPI anxiety | −0.04b | −0.05b | |
| NPI euphoria | −0.26b *** | −0.20b ** | |
| NPI apathy | −0.49b *** | −0.47b *** | |
| NPI disinhibition | −0.38b *** | −0.33b *** | |
| NPI irritability | −0.11b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitriou, T.; Parthimos, T.; Kamtsadeli, V.; Tsinia, N.; Hatzopoulou, M.; Lykou, E.; Chatziantoniou, L.; Papatriantafyllou, O.; Tzavara, C.; Zikos, P.; et al. The Assessment of the Socioemotional Disorder in Neurodegenerative Diseases with the Revised Self-Monitoring Scale (RSMS). J. Clin. Med. 2022, 11, 7375. https://doi.org/10.3390/jcm11247375
Dimitriou T, Parthimos T, Kamtsadeli V, Tsinia N, Hatzopoulou M, Lykou E, Chatziantoniou L, Papatriantafyllou O, Tzavara C, Zikos P, et al. The Assessment of the Socioemotional Disorder in Neurodegenerative Diseases with the Revised Self-Monitoring Scale (RSMS). Journal of Clinical Medicine. 2022; 11(24):7375. https://doi.org/10.3390/jcm11247375
Chicago/Turabian StyleDimitriou, Tatiana, Theodore Parthimos, Vasiliki Kamtsadeli, Niki Tsinia, Maria Hatzopoulou, Evi Lykou, Lina Chatziantoniou, Olga Papatriantafyllou, Chara Tzavara, Panagiotis Zikos, and et al. 2022. "The Assessment of the Socioemotional Disorder in Neurodegenerative Diseases with the Revised Self-Monitoring Scale (RSMS)" Journal of Clinical Medicine 11, no. 24: 7375. https://doi.org/10.3390/jcm11247375
APA StyleDimitriou, T., Parthimos, T., Kamtsadeli, V., Tsinia, N., Hatzopoulou, M., Lykou, E., Chatziantoniou, L., Papatriantafyllou, O., Tzavara, C., Zikos, P., Papageorgiou, S., Miller, B., Rankin, K., & Papatriantafyllou, J. (2022). The Assessment of the Socioemotional Disorder in Neurodegenerative Diseases with the Revised Self-Monitoring Scale (RSMS). Journal of Clinical Medicine, 11(24), 7375. https://doi.org/10.3390/jcm11247375







