Electrocardiogram, Echocardiogram and NT-proBNP in Screening for Thromboembolism Pulmonary Hypertension in Patients after Pulmonary Embolism
Abstract
1. Introduction
2. Methods
2.1. Echocardiography
2.2. Electrocardiogram
2.3. NT-proBNP
2.4. Diagnoses
2.5. Statisitcal Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AcT | acceleration time of pulmonary ejection |
| AUC | area under the ROC curve |
| CTEPD | chronic thromboembolic pulmonary disease |
| CTEPH | chronic thromboembolic pulmonary hypertension |
| EACVI | European Association of Cardiovascular Imaging |
| ECG | electrocardiogram |
| ESC | European Society of Cardiology |
| HR | heart rate |
| IVC | inferior vena cava |
| LAA | left atrium area |
| LV | left ventricle |
| 4ch | four chamber view |
| MD | mean/median differences |
| NPV | negative predictive value |
| NT-proBNP | N-terminal pro-brain natriuretic peptide |
| OR | odds ratio |
| PE | pulmonary embolism |
| PH | pulmonary hypertension |
| PPV | positive predictive value |
| RAA | right atrium area |
| RAD | right axis deviation |
| RBBB | right bundle branch block |
| RHC | right heart catheterization |
| ROC | receiver operating characteristic |
| RV | right ventricle |
| RVSP | right ventricle systolic pressure |
| TRPG | tricuspid regurgitation peak gradient |
| TAPSE | tricuspid annular plane systolic excursion |
References
- Ende-Verhaar, Y.M.; Cannegieter, S.C.; Vonk Noordegraaf, A.; Delcroix, M.; Pruszczyk, P.; Mairuhu, A.T.; Huisman, M.V.; Klok, F.A. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: A contempo-rary view of the published literature. Eur. Respir. J. 2017, 49, 1601792. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Humbert, M.; Vachiéry, J.-L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk-Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmo-nary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplanta-tion (ISHLT). Eur. Respir. J. 2015, 46, 903–975. [Google Scholar] [PubMed]
- Lewczuk, J.; Piszko, P.; Jagas, J.; Porada, A.; Sobkowicz, B.; Wrabec, K.; Wójciak, S. Prognostic Factors in Medically Treated Patients With Chronic Pulmonary Embolism. Chest 2001, 119, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Barco, S.; Konstantinides, S.; Dartevelle, P.; Fadel, E.; Jenkins, D.; Kim, N.H.; Madani, M.; Matsubara, H.; Mayer, E.; et al. Determinants of diagnostic delay in chronic thromboembolic pulmonary hypertension: Results from the European CTEPH Registry. Eur. Respir. J. 2018, 52, 1801687. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.-J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jimenez, D.; et al. The Task Force for the diagnosis and management of acute pulmonary em-bolism of the European Society of Cardiology (ESC). 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur. Respir. J. 2019, 54, 543–603. [Google Scholar]
- Gopalan, D.; Delcroix, M.; Held, M. Diagnosis of chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2017, 26, 160108. [Google Scholar] [CrossRef]
- Delcroix, M.; Torbicki, A.; Gopalan, D.; Sitbon, O.; Klok, F.A.; Lang, I.; Jenkins, D.; Kim, N.H.; Humbert, M.; Jais, X.; et al. ERS statement on chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2020, 57, 2002828. [Google Scholar] [CrossRef]
- Boon, G.J.A.M.; Ende-Verhaar, Y.M.; Bavalia, R.; El Bouazzaoui, L.H.; Delcroix, M.; Dzikowska-Diduch, O.; Huisman, M.V.; Kurnicka, K.; Mairuhu, A.T.A.; Middeldorp, S.; et al. Non-invasive early exclusion of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: The InShape II study. Thorax 2021, 76, 1002–1009. [Google Scholar] [CrossRef]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations:an expert consen-sus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Gold-stein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Im-aging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Held, M.; Hesse, A. A symptom-related monitoring program following pulmonary embolism for the early detection of CTEPH: A prospective observational registry study. BMC Pulm. Med. 2014, 14, 141. [Google Scholar]
- Habib, G.; Torbicki, A. The role of echocardiography in the diagnosis and management of patients with pulmo-nary hypertension. Eur. Respir. Rev. Eur. Respir. Soc. 2010, 19, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, B.; Weyer, G. Pulmonary Hypertension: Diagnosis and Treatment. Am. Fam. Physician 2016, 94, 463–469. [Google Scholar]
- Janda, S.; Shahidi, N.; Gin, K.; Swiston, J. Diagnostic accuracy of echocardiography for pulmonary hypertension: A systematic review and meta-analysis. Heart 2011, 97, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Kitabatake, A.; Inoue, M.; Asao, M.; Masuyama, T.; Tanouchi, J.; Morita, T.; Mishima, M.; Uematsu, M.; Shimazu, T.; Hori, M.; et al. Noninvasive evaluation of pulmonary hypertension by a pulsed Doppler technique. Circulation 1983, 68, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Dzikowska-Diduch, O.; Kostrubiec, M.; Kurnicka, K.; Lichodziejewska, B.; Pacho, S.; Miroszewska, A.; Bródka, K.; Skowrońska, M.; Łabyk, A.; Roik, M.; et al. “The post-pulmonary syndrome-results of echocardiographic driven follow up after acute pulmonary embolism”. Thromb. Res. 2019, 186, 30–35. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd BF 3rd Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gil-lebert, T.C.; Klein, A.L.; Lancellotti, P.; Marino, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Kerr, B.; Brandon, L. Atrial Fibrillation, thromboembolic risk, and the potential role of the natriuretic peptides, a focus on BNP and NT-proBNP–A narrative review. IJC Heart Vasc. 2022, 43, 101132. [Google Scholar] [CrossRef]
- Henkens, I.R.; Mouchaers, K.T.B.; Vonk-Noordegraaf, A.; Boonstra, A.; Swenne, C.A.; Maan, A.C.; Man, S.-C.; Twisk, J.W.R.; Van Der Wall, E.E.; Schalij, M.J.; et al. Improved ECG detection of presence and severity of right ventricular pressure load validated with cardiac magnetic resonance imaging. Am. J. Physiol. Circ. Physiol. 2008, 294, H2150–H2157. [Google Scholar] [CrossRef]
- Thomson, D.; Kourounis, G.; Trenear, R.; Messow, C.-M.; Hrobar, P.; Mackay, A.; Isles, C. ECG in suspected pulmonary embolism. Postgrad. Med. J. 2019, 95, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; van Kralingen, K.W.; van Dijk, A.P.; Heyning, F.H.; Vliegen, H.W. Prospective cardiopulmonary screening program to detect chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. Haematologica 2010, 95, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Coquoz, N.; Weilenmann, D.; Stolz, D.; Popov, V.; Azzola, A.; Fellrath, J.-M.; Stricker, H.; Pagnamenta, A.; Ott, S.; Ulrich, S.; et al. Multicentre observational screening survey for the detection of CTEPH following pulmonary embolism. Eur. Respir. J. 2018, 51, 1702505. [Google Scholar] [CrossRef]
- Surie, S.; Gibson, N.S.; Gerdes, V.E.; Bouma, B.J.; Smit, B.L.V.E.; Buller, H.R.; Bresser, P. Active search for chronic thromboembolic pulmonary hypertension does not appear indicated after acute pulmonary embolism. Thromb. Res. 2010, 125, e202–e205. [Google Scholar] [CrossRef]
- Caravita, S.; Faini, A.; Deboeck, G.; Bondue, A.; Naeije, R.; Parati, G.; Vachiéry, J.-L. Pulmonary hypertension and ventilation during exercise: Role of the pre-capillary component. J. Heart Lung Transplant. 2017, 36, 754–762. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | n | Patients with Symptoms | n | Patients without Symptoms | MD/OR (95% CI) | p |
|---|---|---|---|---|---|---|
| n | 155 | 106 | ||||
| Sex, female, n (%) | 155 | 85 (54.8) | 106 | 53 (50.0) | 1.21 (0.72,2.05) | 0.52 |
| Age, years, mean ± SD | 155 | 61.07 ± 17.10 | 106 | 49.75 ± 18.36 | 11.32 (7.4,16.21) | <0.001 2 |
| CTEPH/CTED, n (%) | 155 | 20 (12.9) | 106 | 0 (0.0) | - | <0.001 1 |
| Drug, n (%) | ||||||
| Acenocumarol | 152 | 52 (34.2) | 86 | 37 (43.0) | - | 0.0651 |
| Dabigatran | 10 (6.6) | 4 (4.7) | ||||
| Dalteparin | 1 (0.7) | 1 (1.2) | ||||
| Enoxaparin | 8 (5.3) | 5 (5.8) | ||||
| Nadroparin | 2 (1.3) | 0 (0.0) | ||||
| Riwaroxaban | 64 (42.1) | 22 (25.6) | ||||
| Warfarin | 15 (9.9) | 17 (19.8) | ||||
| IVC, mean ± SD | 151 | 15.36 ± 4.60 | 104 | 13.96 ± 3.76 | 1.40 (0.37,2.44) | 0.0082 |
| LV4ch, mean ± SD | 131 | 45.67 ± 5.50 | 73 | 43.50 ± 4.24 | 2.17 (−1.23,1.87) | 0.6832 |
| RV4ch, mean ± SD | 130 | 33.50 ± 6.60 | 74 | 31.88 ± 2.59 | 1.63 (0.85,3.86) | 0.0022 |
| RV/LV, mean ± SD | 130 | 0.80 ± 0.12 | 73 | 0.75 ± 0.12 | 0.04 (0.01,0.08) | 0.0062 |
| LV EF, mean ± SD | 155 | 60.77 ± 5.43 | 106 | 62.91 ± 3.22 | −2.14 (−3.19,01.07) | <0.001 2 |
| LAA, cm2, mean ± SD | 140 | 19.89 ± 4.07 | 81 | 16.96 ± 3.31 | 2.92 (1.89,3.88) | <0.001 2 |
| RAA, cm2, mean ± SD | 138 | 18.12 ± 4.14 | 78 | 15.42 ± 3.28 | 2.71 (1.67,3.68) | <0.001 2 |
| Heart rhythm, n (%) | ||||||
| Atrial fibrillation | 154 | 10 (6.5) | 106 | 0 (0.0) | - | 0.0051 |
| Stimulation | 1 (0.6) | 0 (0.0) | ||||
| Tachycardia | 0 (0.0) | 1 (0.9) | ||||
| Sinus rhythm | 143 (92.7) | 105 (99.1) | ||||
| TRPG, median (Q1,Q3) | 150 | 25.50 (20.00,32.75) | 103 | 23.00 (17.00,27.50) | 2.50 (1.00,6.00) | 0.0013 |
| RVSP, median (Q1,Q3) | 146 | 31.00 (25.00,40.75) | 102 | 28.00 (23.00,33.75) | 3.00 (2.00,7.00) | 0.0013 |
| AcT, mean ± SD | 148 | 112.08 ± 28.07 | 101 | 123.70 ± 24.03 | −11.62 (−18.17,−5.07) | 0.0012 |
| TAPSE, mean ± SD | 134 | 23.34 ± 3.79 | 80 | 23.66 ± 3.21 | −0.33 (−1.29,0.63) | 0.5032 |
| HR, mean ± SD | 137 | 70.58 ± 10.66 | 58 | 69.45 ± 9.71 | 1.14 (−1.97,4.24) | 0.4702 |
| RAD, n (%) | 131 | 4 (3.1) | 57 | 0 (0.0) | - | 0.3161 |
| RBBB, n (%) | 121 | 10 (8.3) | 53 | 1 (1.9) | 4.65 (0.63,206.97) | 0.1761 |
| S1Q3T3, n (%) | 117 | 26 (22.2) | 53 | 7 (13.2) | 1.87 (0.72,5.50) | 0.243 |
| NTproBNP, median (Q1,Q3) (pg/mL) | 155 | 108.00 (45.00,339.50) | 106 | 29.00 (20.00,96.25) | 79.00 (31.00,85.00) | <0.001 3 |
| NTproBNP > 125 pg/mL, n (%) | 155 | 68 (43.9) | 106 | 20 (18.9) | 3.35 (1.82,6.34) | <0.001 |
| D-dimer,(ng/mL) median (Q1,Q3) | 95 | 300.00 (205.50,488.50) | 95 | 239.00 (170.00,420.00) | 61.00 (0.01,93.00) | 0.0493 |
| Characteristics. | n | Patients with Symptoms with CTEPH/CTEPD | n | Patients with Symptoms without CTEPH/CTEPD | MD/OR (95% CI) | p |
|---|---|---|---|---|---|---|
| n | 20 | 135 | ||||
| RV4ch, mean ± SD | 20 | 36.50 ± 7.78 | 110 | 32.00 ± 6.58 | 4.50 (1.61,6.99) | 0.003 2 |
| LAA, cm2, mean ± SD | 19 | 21.58 ± 4.68 | 121 | 19.62 ± 3.93 | 1.96 (−0.39,4.31) | 0.097 2 |
| RAA, cm2, mean ± SD | 19 | 21.53 ± 5.08 | 119 | 17.58 ± 3.71 | 3.95 (1.42,6.47) | 0.004 2 |
| TRPG, median (Q1,Q3) | 20 | 45.00 (30.75,62.00) | 130 | 24.00 (20.00,30.00) | 21.00 (13.00,33.00) | <0.001 3 |
| AcT, mean ± SD | 19 | 88.42 ± 39.21 | 129 | 115.57 ± 24.36 | −27.15 (−46.43,−7.86) | 0.008 2 |
| RAD, n (%) | 19 | 1 (5.3) | 112 | 3 (2.7) | 2.01 (0.04,26.60) | 0.469 1 |
| RBBB, n (%) | 17 | 4 (23.5) | 104 | 6 (5.8) | 4.92 (1.90,24.18) | 0.034 1 |
| S1Q3T3, n (%) | 16 | 6 (37.5) | 101 | 20 (19.8) | 2.41 (0.64,8.40) | 0.191 |
| NTproBNP, median (Q1,Q3) | 20 | 151.00 (85.25,843.00) | 135 | 99.00 (43.00,300.50) | 52.00 (12.00,372.00) | 0.022 3 |
| NTproBNP > 125, n (%) | 20 | 12 (60.0) | 135 | 56 (41.5) | 2.11 (0.74,6.36) | 0.149 |
| Characteristics | n | Patients with CTEPH | n | Patients with CTED | MD / OR (95% CI) | p |
|---|---|---|---|---|---|---|
| n | 13 | 7 | ||||
| RV4ch, mean ± SD | 13 | 42.00 (42.00,42.00) | 7 | 31.00 (31.00,31.00) | 11.00 (−4.00,18.00) | 0.249 3 |
| LAA, cm2, mean ± SD | 13 | 21.92 ± 4.70 | 6 | 20.83 ± 5.00 | 1.09 (−4.36,6.54) | 0.663 2 |
| RAA, cm2, mean ± SD | 13 | 22.77 ± 4.90 | 6 | 18.83 ± 4.75 | 3.94 (−1.33,9.21) | 0.127 2 |
| TRPG, median (Q1,Q3) | 13 | 59.23 ±23.81 | 7 | 34.71 ± 12.66 | 24.52 (7.39,41.65) | 0.008 2 |
| AcT, mean ± SD | 12 | 78.50 (67.50,86.25) | 7 | 106.00 (78.50,140.00) | −27.50 (−72.00,10.00) | 0.162 3 |
| RAD, n (%) | 12 | 1 (8.3) | 7 | 0 (0.0) | - | >0.999 1 |
| RBBB, n (%) | 10 | 3 (30.0) | 7 | 1 (14.3) | 2.44 (0.15,156.95) | 0.603 1 |
| S1Q3T3, n (%) | 10 | 3 (30.0) | 6 | 3 (50.0) | 0.45 (0.03,5.51) | 0.607 1 |
| NTproBNP, median (Q1,Q3) | 13 | 435.00 (132.00,1494.00) | 7 | 107.00 (76.50,313.50) | 328.00 (−33.00,1387.00) | 0.115 3 |
| NTproBNP > 125, n (%) | 13 | 10 (76.9) | 7 | 2 (28.6) | 7.32 (0.74,117.26) | 0.062 1 |
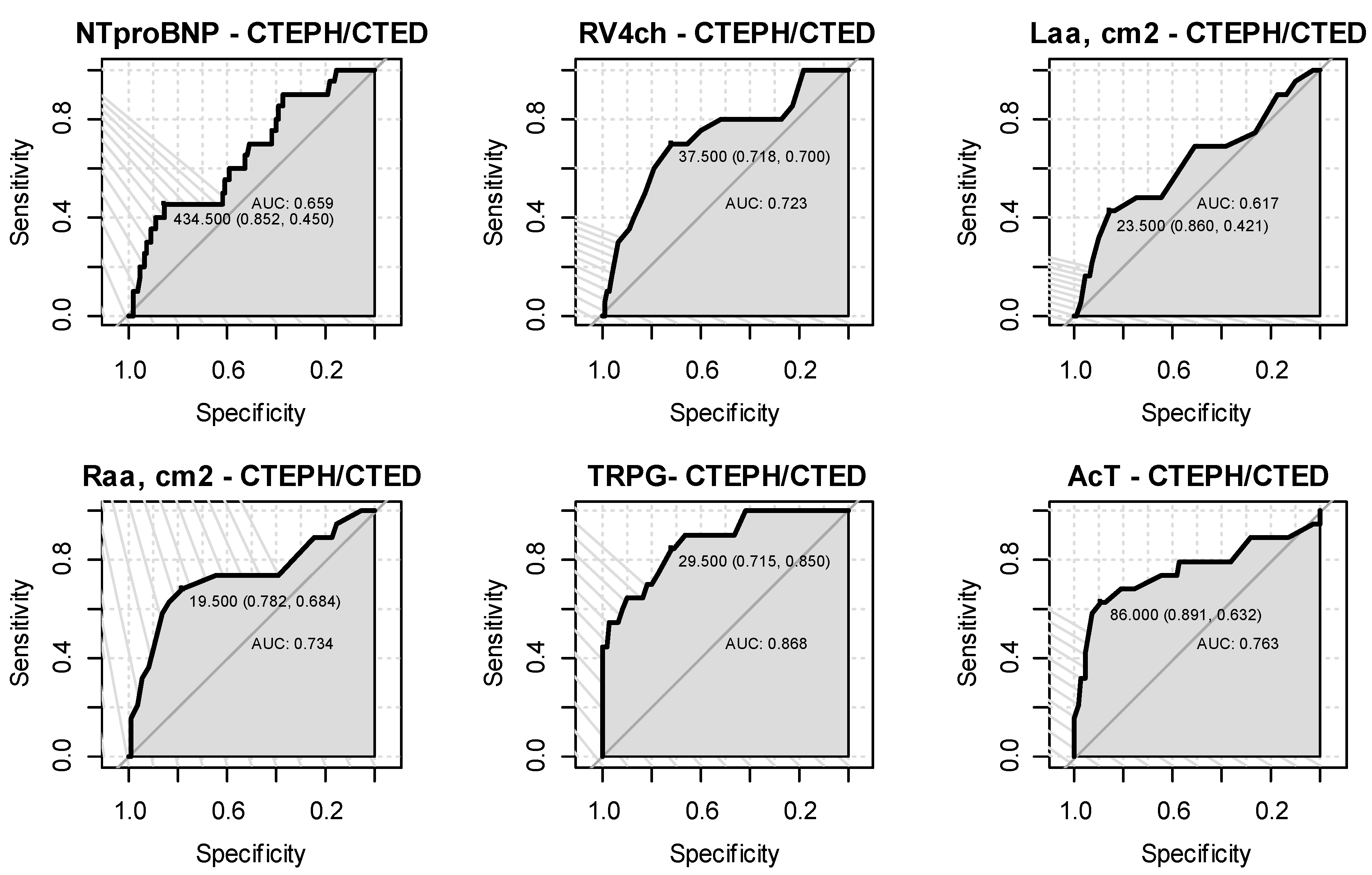
| Characteristics | AUC (95% CI) | Cut-off Point | Sensitivity | Specificity | Accuracy | NPV | PPV | p |
|---|---|---|---|---|---|---|---|---|
| RV4ch | 0.723 (0.591,0.855) | 37.5 | 0.7 | 0.72 | 0.72 | 0.93 | 0.31 | 0.002 |
| LAA, cm2 | 0.617 (0.464,0.771) | 23.5 | 0.42 | 0.86 | 0.8 | 0.9 | 0.32 | 0.056 |
| RAA, cm2 | 0.734 (0.589,0.879) | 19.5 | 0.68 | 0.78 | 0.77 | 0.94 | 0.33 | 0.001 |
| TRPG | 0.868 (0.785,0.952) | 29.5 | 0.85 | 0.72 | 0.73 | 0.97 | 0.31 | <0.001 |
| AcT | 0.763 (0.611,0.914) | 86 | 0.63 | 0.89 | 0.86 | 0.94 | 0.46 | 0.001 |
| NTproBNP | 0.659 (0.528,0.789) | 434.5 | 0.45 | 0.85 | 0.8 | 0.91 | 0.31 | 0.297 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzikowska-Diduch, O.; Kurnicka, K.; Lichodziejewska, B.; Dudzik-Niewiadomska, I.; Machowski, M.; Roik, M.; Wiśniewska, M.; Siwiec, J.; Staniszewska, I.M.; Pruszczyk, P. Electrocardiogram, Echocardiogram and NT-proBNP in Screening for Thromboembolism Pulmonary Hypertension in Patients after Pulmonary Embolism. J. Clin. Med. 2022, 11, 7369. https://doi.org/10.3390/jcm11247369
Dzikowska-Diduch O, Kurnicka K, Lichodziejewska B, Dudzik-Niewiadomska I, Machowski M, Roik M, Wiśniewska M, Siwiec J, Staniszewska IM, Pruszczyk P. Electrocardiogram, Echocardiogram and NT-proBNP in Screening for Thromboembolism Pulmonary Hypertension in Patients after Pulmonary Embolism. Journal of Clinical Medicine. 2022; 11(24):7369. https://doi.org/10.3390/jcm11247369
Chicago/Turabian StyleDzikowska-Diduch, Olga, Katarzyna Kurnicka, Barbara Lichodziejewska, Iwona Dudzik-Niewiadomska, Michał Machowski, Marek Roik, Małgorzata Wiśniewska, Jan Siwiec, Izabela Magdalena Staniszewska, and Piotr Pruszczyk. 2022. "Electrocardiogram, Echocardiogram and NT-proBNP in Screening for Thromboembolism Pulmonary Hypertension in Patients after Pulmonary Embolism" Journal of Clinical Medicine 11, no. 24: 7369. https://doi.org/10.3390/jcm11247369
APA StyleDzikowska-Diduch, O., Kurnicka, K., Lichodziejewska, B., Dudzik-Niewiadomska, I., Machowski, M., Roik, M., Wiśniewska, M., Siwiec, J., Staniszewska, I. M., & Pruszczyk, P. (2022). Electrocardiogram, Echocardiogram and NT-proBNP in Screening for Thromboembolism Pulmonary Hypertension in Patients after Pulmonary Embolism. Journal of Clinical Medicine, 11(24), 7369. https://doi.org/10.3390/jcm11247369






