Decreased Haemoglobin Level Measured at Admission Predicts Long Term Mortality after the First Episode of Acute Pulmonary Embolism
Abstract
1. Introduction
2. Material and Methods
3. Statistics
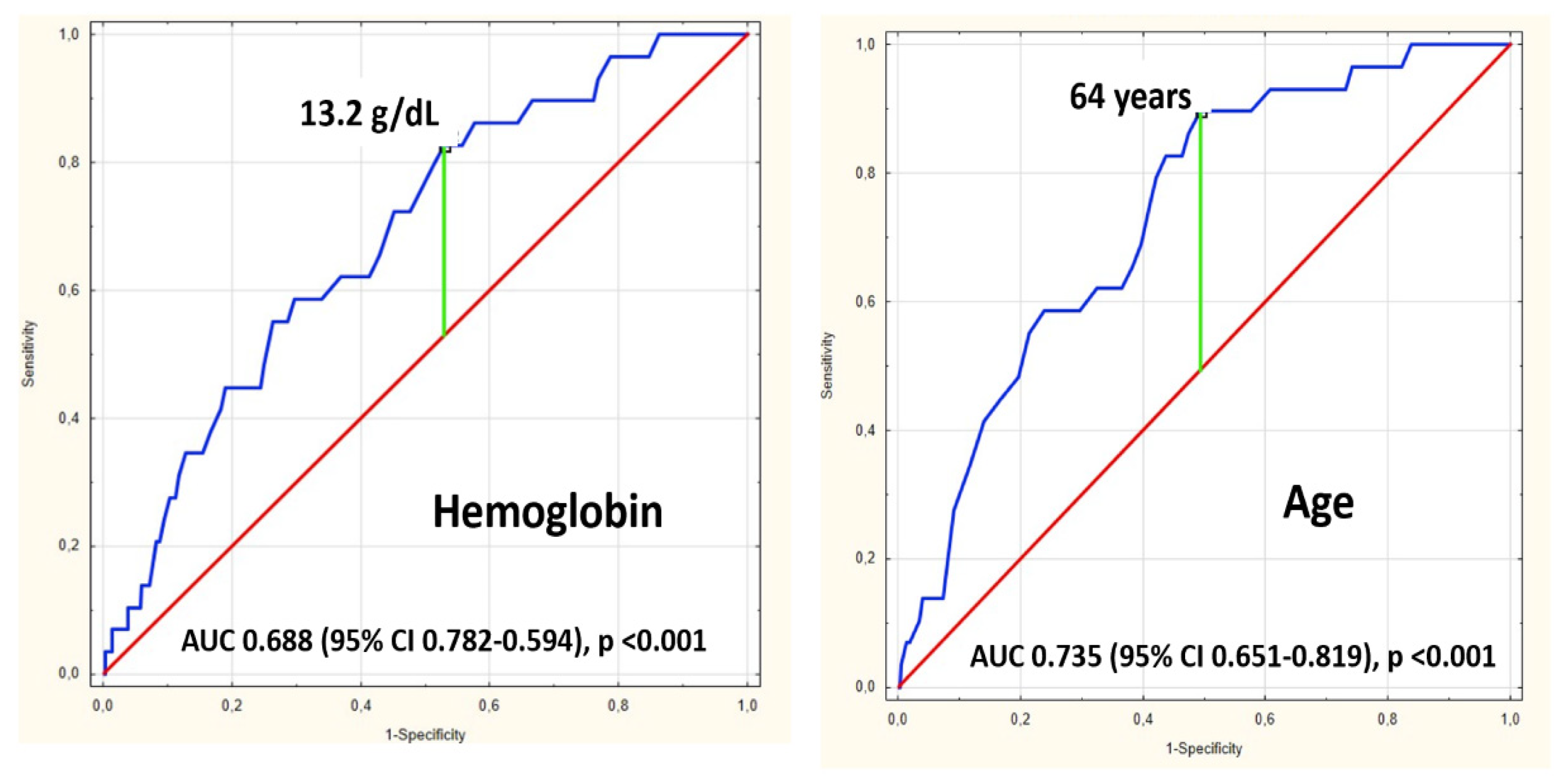
4. Results
Patients Characteristics and Management
5. Discussion
6. Limitations of the Current Study
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | area under the ROC curve |
| CTEPH | chronic thromboembolic pulmonary hypertension |
| CI | confidence intervals |
| ESC | European Society of Cardiology |
| HR | heart rate |
| ISTH | International Society on Thrombosis and Haemostasis |
| MD | mean/median differences |
| NPV | negative predictive value |
| NT-proBNP | N-terminal pro-brain natriuretic peptide |
| OR | odds ratio |
| PE | pulmonary embolism |
| PH | pulmonary hypertension |
| PPV | positive predictive value |
| RHC | right heart catheterisation |
| sPESI | simplified Pulmonary Embolism Severity Index |
| ROC | receiver operating characteristic |
| VTE | venous thromboembolism |
References
- Klok, F.A.; Ageno, W.; Ay, C.; Bäck, M.; Barco, S.; Bertoletti, L.; Becattini, C.; Carlsen, J.; Delcroix, M.; van Es, N.; et al. Optimal follow-up after acute pulmonary embolism: A position paper of the European Society of Cardiology Working Group on Pulmonary Circulation and Right Ventricular Function, in collaboration with the European Society of Cardiology Working Group on Atherosclerosis and Vascular Biology, endorsed by the European Respiratory Society. Eur. Heart J. 2021, 43, 183–189. [Google Scholar] [CrossRef]
- Carson, J.L.; Kelley, M.A.; Duff, A.; Weg, J.G.; Fulkerson, W.J.; Palevsky, H.I.; Schwartz, J.S.; Thompson, B.T.; Popovich, J.; Hobbins, T.E.; et al. The Clinical Course of Pulmonary Embolism. N. Engl. J. Med. 1992, 326, 1240–1245. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed]
- Dentali, F.; Riva, N.; Turato, S.; Grazioli, S.; Squizzato, A.; Steidl, L.; Guasti, L.; Grandi, A.M.; Ageno, W. Pulmonary embolism severity index accurately predicts long-term mortality rate in patients hospitalized for acute pulmonary embolism. J. Thromb. Haemost. 2013, 11, 2103–2110. [Google Scholar] [CrossRef]
- Buchan, T.A.; Ching, C.; Foroutan, F.; Malik, A.; Daza, J.F.; Hing, N.N.F.; Siemieniuk, R.; Evaniew, N.; Orchanian-Cheff, A.; Ross, H.J.; et al. Prognostic value of natriuretic peptides in heart failure: Systematic review and meta-analysis. Heart Fail. Rev. 2021, 27, 645–654. [Google Scholar] [CrossRef]
- Gardner, R.S.; Özalp, F.; Murday, A.J.; Robb, S.D.; McDonagh, T.A. N-terminal pro-brain natriuretic peptide A new gold standard in predicting mortality in patients with advanced heart failure. Eur. Heart J. 2003, 24, 1735–1743. [Google Scholar] [CrossRef]
- Bibbins-Domingo, K.; Gupta, R.; Na, B.; Wu, A.H.; Schiller, N.B.; Whooley, M.A. N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP), cardiovascular events, and mortality in patients with stable coronary heart disease. JAMA 2007, 297, 169–176. [Google Scholar] [CrossRef]
- Hoiseth, A.D.; Omland, T.; Hagve, T.A.; Brekke, P.H.; Soyseth, V. NT-proBNP independently predicts long term mortality after acute exacerbation of COPD—A prospective cohort study. Respir. Res. 2012, 13, 97. [Google Scholar] [CrossRef]
- Malezieux-Picard, A.; Azurmendi, L.; Pagano, S.; Vuilleumier, N.; Sanchez, J.C.; Zekry, D.; Reny, J.L.; Stirnemann, J.; Garin, N.; Prendki, V. Role of Clinical Characteristics and Biomarkers at Admission to Predict One-Year Mor-tality in Elderly Patients with Pneumonia. J. Clin. Med. 2021, 11, 105. [Google Scholar] [CrossRef]
- Jiménez, D.; Aujesky, D.; Moores, L.; Gómez, V.; Lobo, J.L.; Uresandi, F.; Otero, R.; Monreal, M.; Muriel, A.; Yusen, R.D. Simplification of the Pulmonary Embolism Severity Index for Prognostication in Patients with Acute Symptomatic Pulmonary Embolism. Arch. Intern. Med. 2010, 170, 1383–1389. [Google Scholar] [CrossRef]
- Kaatz, S.; Ahmad, D.; Spyropoulos, A.C.; Schulman, S. Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2015, 13, 2119–2126. [Google Scholar] [CrossRef]
- Ng, A.C.C.; Yong, A.S.C.; Chow, V.; Chung, T.; Freedman, S.B.; Kritharides, L. Cardiac troponin-T and the prediction of acute and long-term mortality after acute pulmonary embolism. Int. J. Cardiol. 2013, 165, 126–133. [Google Scholar] [CrossRef]
- Nutritional Anaemias. Report of a WHO Scientific Group. Geneva, World Health Organization. 1968. (WHO Technical Report Series, No. 405). Available online: http://whqlibdoc.who.int/trs/WHO_TRS_405.pdf (accessed on 3 September 2022).
- Chopra, V.K.; Anker, S.D. Anaemia, iron deficiency and heart failure in 2020: Facts and numbers. ESC Heart Fail. 2020, 7, 2007–2011. [Google Scholar] [CrossRef]
- Komajda, M.; Anker, S.D.; Charlesworth, A.; Okonko, D.; Metra, M.; Di Lenarda, A.; Remme, W.; Moullet, C.; Swedberg, K.; Cleland, J.G.; et al. The impact of new onset anaemia on morbidity and mortality in chronic heart failure: Results from COMET. Eur. Heart J. 2006, 27, 1440–1446. [Google Scholar] [CrossRef]
- Von Haehling, S.; Schefold, J.C.; Hodoscek, L.M.; Doehner, W.; Mannaa, M.; Anker, S.D.; Lainscak, M. Anaemia is an independent predictor of death in patients hospitalized for acute heart failure. Clin. Res. Cardiol. 2010, 99, 107–113. [Google Scholar] [CrossRef]
- McCullough, P.A.; Barnard, D.; Clare, R.; Ellis, S.J.; Fleg, J.L.; Fonarow, G.C.; Franklin, B.A.; Kilpatrick, R.D.; Kitzman, D.W.; O’Connor, C.M.; et al. Anemia and Associated Clinical Outcomes in Patients with Heart Failure Due to Reduced Left Ventricular Systolic Function. Clin. Cardiol. 2013, 36, 611–620. [Google Scholar] [CrossRef]
- Padda, J.; Khalid, K.; Hitawala, G.; Batra, N.; Pokhriyal, S.; Mohan, A.; Cooper, A.C.; Jean-Charles, G. Acute Anemia and Myocardial Infarction. Cureus 2021, 13, e17096. [Google Scholar] [CrossRef]
- Lee, W.-C.; Fang, H.-Y.; Chen, H.-C.; Chen, C.-J.; Yang, C.-H.; Hang, C.-L.; Wu, C.-J.; Fang, C.-Y. Anemia: A significant cardiovascular mortality risk after ST-segment elevation myocardial infarction complicated by the comorbidities of hypertension and kidney disease. PLoS ONE 2017, 12, e0180165. [Google Scholar] [CrossRef]
- Akgüllü, Ç.; Ömürlü, I.K.; Eryılmaz, U.; Avcil, M.; Dağtekin, E.; Akdeniz, M.; Güngör, H.; Zencir, C. Predictors of early death in patients with acute pulmonary embolism. Am. J. Emerg. Med. 2015, 33, 214–221. [Google Scholar] [CrossRef]
- Vuilleumier, N.; Le Gal, G.; Verschuren, F.; Perrier, A.; Bounameaux, H.; Turck, N.; Sanchez, J.-C.; Mensi, N.; Perneger, T.; Hochstrasser, D.; et al. Cardiac biomarkers for risk stratification in non-massive pulmonary embolism: A multicenter prospective study. J. Thromb. Haemost. 2009, 7, 391–398. [Google Scholar] [CrossRef]
- Hendricks, S.; Dykun, I.; Balcer, B.; Totzeck, M.; Rassaf, T.; Mahabadi, A.A. Higher BNP/NT-pro BNP levels stratify prognosis equally well in patients with and without heart failure: A meta-analysis. ESC Heart Fail. 2022. [Google Scholar] [CrossRef] [PubMed]
- Boucly, A.; Weatherald, J.; Savale, L.; Jaïs, X.; Cottin, V.; Prevot, G.; Picard, F.; de Groote, P.; Jevnikar, M.; Bergot, E.; et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur. Respir. J. 2017, 50, 1700889. [Google Scholar] [CrossRef] [PubMed]
- Bassan, F.; Bassan, R.; Esporcatte, R.; Santos, B.; Tura, B. Very Long-Term Prognostic Role of Admission BNP in Non-ST Segment Elevation Acute Coronary Syndrome. Arq. Bras. Cardiol. 2016, 106, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.R.; Houweling, A.H.; Granton, J.; Rudski, L.; Dennie, C.; Hirsch, A. Long-term outcomes after pulmonary embolism: Current knowledge and future research. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 2014, 25, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Barco, S.; Rosenkranz, S.; Lankeit, M.; Held, M.; Gerhardt, F.; Bruch, L.; Ewert, R.; Faehling, M.; Freise, J.; et al. Late outcomes after acute pulmonary embolism: Rationale and design of FOCUS, a prospective observational multicenter cohort study. J. Thromb. Thrombolysis 2016, 42, 600–609. [Google Scholar] [CrossRef]
- Heit, J.A.; Silverstein, M.D.; Mohr, D.N.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J., III. Predictors of survival after deep vein thrombosis and pulmonary embolism: A population-based, cohort study. Arch. Intern. Med. 1999, 159, 445–453. [Google Scholar] [CrossRef]
- Klok, F.; Zondag, W.; Van Kralingen, K. Patient Outcomes after Acute Pulmonary Embolism: A Pooled Survival Analysis of Different Adverse Events. Am. J. Respir. Crit. Care Med. 2010, 181, 501–506. [Google Scholar] [CrossRef]
| All Patients n = 402 | Survivors n = 373 | Nonsurvivors n = 29 | p-Value | ||
|---|---|---|---|---|---|
| Female/Male, n | 216/186 | 197/176 | 19/10 | 0.19 | |
| Age, years | 57.5 (18–97) | 63 (18–97) | 81 (40–93) | <0.001 | |
| Chronic heart failure, n (%) | 71 (17.7%) | 63 (16.9%) | 8 (27.5%) | 0.15 | |
| Coronary artery disease, n (%) | 23 (5.7%) | 20 (5.4%) | 3 (10.4%) | 0.25 | |
| Chronic lung disease, n (%) | 45 (11.2%) | 40 (10.7%) | 5 (17.3%) | 0.28 | |
| Active neoplasm at PE diagnosis, n (%) | 73 (18.2%) | 62 (16.6%) | 11 (37.9%) | 0.004 | |
| Neoplasm diagnosed during follow-up, n (%) | 13 (3.2%) | 9 (2.4%) | 4 (13.8%) | <0.001 | |
| Unprovoked PE, n (%) | 190 (47%) | 180 (48%) | 10 (34%) | 0.21 | |
| Major surgery, n (%) | 35 (8.7%) | 33 (8.8%) | 2 (6.9%) | Ns | |
| sPESI, points | 1.5 (0–5) | 1 (0–5) | 2 (0–4) | <0.001 | |
| Low | 86 (21.4%) | 84 (22.5%) | 2 (6.9%) | 0.13 | |
| PE severity, n (%) | Intermediate | 299 (74.4%) | 273 (73.2%) | 26 (89.7%) | |
| High | 17 (4.2%) | 16 (4.3%) | 1 (3.4%) | ||
| right to left ventricular ratio > 1 in echo 4 chamber view, n (%) | 101 (32.4%) | 94 (32.7%) | 7 (29.2%) | 0.72 | |
| LV EF (%) | 60 (15–70) | 60 (15–70) | 60 (20–65) | 0.08 | |
| Troponin (μg/L) | 0.0175 (0.003–1.59) | 0.038 (0.003–1.59) | 0.074 (0.01–0.8) | 0.17 | |
| NT-proBNP (pg/mL) | 3710.5 (2–28,879) | 344.5 (5–28,879) | 1440 (74–12,330) | 0.004 | |
| D-dimer (µg/L) | 19,050 (2–111,459) | 4558 (2–111,459) | 4613 (580–26,945) | 0.79 | |
| Hemoglobin at admission (g/dL) | 10 (3.1–19.3) | 13.1 (3.1–19.3) | 11.7 (6–14.8) | <0.001 | |
| Plasma creatinine (mg/dL) | 1.04 (0.33–6.5) | 0.9 (0.33–5.4) | 1 (0.48–6.5) | 0.36 | |
| estimated glomerular filtration rate (CockroftGault, mL/min) | 89.18 (9.2 ≥ 100) | 80.11 (10.9 ≥ 100) | 59.52 (9.2 ≥ 100) | 0.03 | |
| n | Deaths | Mortality | OR | OR When Group with Age < 64 Years and Hb > 13.2 g/dL as Reference | |
|---|---|---|---|---|---|
| Age < 64 years and Hb > 13.2 g/dL | 97 | 1 | 1.03% | 2.1 95% CI 0.2–23.7, p = 0.54 | 1 as reference |
| Age < 64 years and Hb < 13.2 g/dL | 93 | 2 | 2.15% | 2.1 95% CI 0.2–23.7, p = 0.54 | |
| Age > 64 years and Hb > 13.2 g/dL | 89 | 5 | 5.62% | 3.6 95% CI 1.3–10.1 p = 0.012 | 5.7 95% CI 0.7–49.9, p = 0.11 |
| Age > 64 years and Hb < 13.2 g/dL | 118 | 21 | 17.80% | 20.8 95% CI 2.7–157.6, p = 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Justyna, A.; Dzikowska-Diduch, O.; Pacho, S.; Ciurzyński, M.; Skowrońska, M.; Wyzgał-Chojecka, A.; Piotrowska-Kownacka, D.; Pruszczyk, K.; Pucyło, S.; Sikora, A.; et al. Decreased Haemoglobin Level Measured at Admission Predicts Long Term Mortality after the First Episode of Acute Pulmonary Embolism. J. Clin. Med. 2022, 11, 7100. https://doi.org/10.3390/jcm11237100
Justyna A, Dzikowska-Diduch O, Pacho S, Ciurzyński M, Skowrońska M, Wyzgał-Chojecka A, Piotrowska-Kownacka D, Pruszczyk K, Pucyło S, Sikora A, et al. Decreased Haemoglobin Level Measured at Admission Predicts Long Term Mortality after the First Episode of Acute Pulmonary Embolism. Journal of Clinical Medicine. 2022; 11(23):7100. https://doi.org/10.3390/jcm11237100
Chicago/Turabian StyleJustyna, Aleksandra, Olga Dzikowska-Diduch, Szymon Pacho, Michał Ciurzyński, Marta Skowrońska, Anna Wyzgał-Chojecka, Dorota Piotrowska-Kownacka, Katarzyna Pruszczyk, Szymon Pucyło, Aleksandra Sikora, and et al. 2022. "Decreased Haemoglobin Level Measured at Admission Predicts Long Term Mortality after the First Episode of Acute Pulmonary Embolism" Journal of Clinical Medicine 11, no. 23: 7100. https://doi.org/10.3390/jcm11237100
APA StyleJustyna, A., Dzikowska-Diduch, O., Pacho, S., Ciurzyński, M., Skowrońska, M., Wyzgał-Chojecka, A., Piotrowska-Kownacka, D., Pruszczyk, K., Pucyło, S., Sikora, A., & Pruszczyk, P. (2022). Decreased Haemoglobin Level Measured at Admission Predicts Long Term Mortality after the First Episode of Acute Pulmonary Embolism. Journal of Clinical Medicine, 11(23), 7100. https://doi.org/10.3390/jcm11237100






