Persistent Inflammation in Cerebral Palsy: Pathogenic Mediator or Comorbidity? A Scoping Review
Abstract
1. Introduction
- (1)
- Inflammation persists well after the original brain injury with long-term consequence in CP. If inflammation persists, other commonly characterized symptoms of CP, including pain and cognition may be confounded or exacerbated. For example, systemic immune disturbances in innate and cellular immunity increase brain glial cell responsiveness, which may worsen neurological deficits [12]. In this context, inflammation may be viewed as a contributing factor to the symptoms experienced by people with CP, and present with the motor and movement impairments, in other terms be a “comorbidity” of CP.
- (2)
- Inflammation persists in CP without long-term consequence or indeed, does not persist at all. If inflammation is not a comorbidity of CP, it may instead standalone as a pathogenic feature of CP; meaning that the original inflammatory insult that causes the motor and postural impairment exists with no other long-term implications on health or outcomes. Alternatively, that inflammation does not persist.
2. Materials and Methods
2.1. Study Eligibility Criteria
2.2. Search Strategy
2.3. Study Selection
2.4. Data Extraction
2.5. Defining Reportable Inflammatory Biomarkers
2.6. Data Synthesis
3. Results
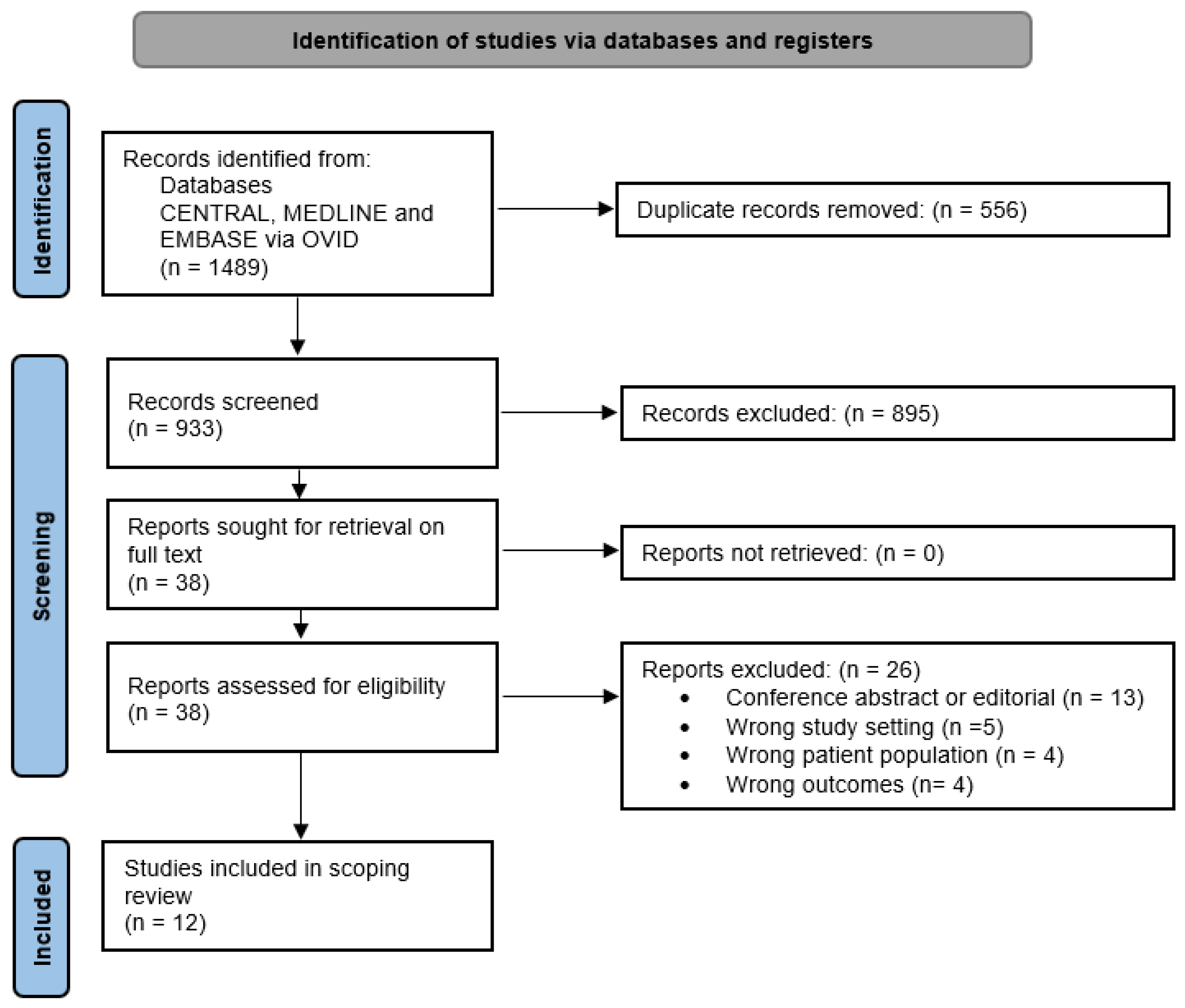
3.1. Search Results
3.2. Study Characteristics
3.2.1. Study Design
3.2.2. Participant Features
3.2.3. CP Cohort Etiology
3.2.4. Samples Analyzed, Method and Biomarker Details
3.3. Results of Inflammatory Biomarkers in CP
3.3.1. Stimulation Assays of Immune Function from Case-Control Studies
3.3.2. Cross-Sectional Cytokine Analysis from Case-Control Studies
3.3.3. Proportions of Immune Cell Types in Case-Controls
3.3.4. Genetic Changes and Gene Expression in Case-Controls
3.4. Additional Findings from Single Arm Studies
3.5. Subgroup Findings of Biomakers in CP across Included Studies
3.5.1. Participant Age Subgroup Analysis
3.5.2. Type and Topography of CP Subgroup Analysis
3.5.3. Severity of CP Subgroup Analysis
3.5.4. Etiology of CP Subgroup Analysis
4. Discussion
4.1. The Evidence of Persistent Inflammation in CP
4.2. Differences in Persistent Inflammation in CP and within Subgroups
4.3. Targeting Inflammation as a Comorbidity of CP
4.4. Outstanding Unknowns of Inflammation in CP
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M.; Damiano, D.; Dan, B.; Jacobsson, B. A report: The definition and classification of cerebral palsy April 2006. Dev. Med. Child Neurol. Suppl. 2007, 109, 8–14. [Google Scholar] [PubMed]
- Girard, S.; Kadhim, H.; Roy, M.; Lavoie, K.; Brochu, M.E.; Larouche, A.; Sébire, G. Role of perinatal inflammation in cerebral palsy. Pediatr. Neurol. 2009, 40, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, H.; Gressens, P.; Mallard, C. Inflammation during fetal and neonatal life: Implications for neurologic and neuropsychiatric disease in children and adults. Ann. Neurol. 2012, 71, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, R.C.; Moreira, J.M.; Lauar, A.O.; da Silva, A.A.S.; Teixeira, A.L.; ACS, E.S. Inflammatory biomarkers in children with cerebral palsy: A systematic review. Res. Dev. Disabil. 2019, 95, 103508. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Chang, Y.C.; Wang, S.T.; Lee, T.Y.; Lin, C.F.; Huang, C.C. Altered inflammatory responses in preterm children with cerebral palsy. Ann. Neurol. 2010, 68, 204–212. [Google Scholar] [CrossRef]
- Taher, N.A.B.; Kelly, L.A.; Al-Harbi, A.I.; O’Dea, M.I.; Zareen, Z.; Ryan, E.; Molloy, E.J.; Doherty, D.G. Altered distributions and functions of natural killer T cells and γδ T cells in neonates with neonatal encephalopathy, in school-age children at follow-up, and in children with cerebral palsy. J. Neuroimmunol. 2021, 356, 577597. [Google Scholar] [CrossRef]
- Upadhyay, J.; Ansari, M.N.; Samad, A.; Sayana, A. Dysregulation of multiple signaling pathways: A possible cause of cerebral palsy. Exp. Biol. Med. 2022, 247, 779–787. [Google Scholar] [CrossRef]
- Hagberg, H.; Mallard, C.; Ferriero, D.M.; Vannucci, S.J.; Levison, S.W.; Vexler, Z.S.; Gressens, P. The role of inflammation in perinatal brain injury. Nat. Rev. Neurol. 2015, 11, 192–208. [Google Scholar] [CrossRef]
- Zareen, Z.; Strickland, T.; Fallah, L.; McEneaney, V.; Kelly, L.; McDonald, D.; Molloy, E.J. Cytokine dysregulation in children with cerebral palsy. Dev. Med. Child. Neurol. 2021, 63, 407–412. [Google Scholar] [CrossRef]
- Fleiss, B.; Gressens, P. Tertiary mechanisms of brain damage: A new hope for treatment of cerebral palsy? Lancet Neurol. 2012, 11, 556–566. [Google Scholar] [CrossRef]
- Schleiss, M.R. Altered cytokine responses in children with cerebral palsy: Pathogenesis and novel therapies. Dev. Med. Child Neurol. 2021, 63, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Sankowski, R.; Mader, S.; Valdés-Ferrer, S.I. Systemic inflammation and the brain: Novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration. Front. Cell. Neurosci. 2015, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Romero, B.; Robinson, K.G.; Batish, M.; Akins, R.E. An Emerging Role for Epigenetics in Cerebral Palsy. J. Pers. Med. 2021, 11, 1187. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.B.; Grether, J.K.; Dambrosia, J.M.; Walsh, E.; Kohler, S.; Satyanarayana, G.; Nelson, P.G.; Dickens, B.F.; Phillips, T.M. Neonatal cytokines and cerebral palsy in very preterm infants. Pediatr. Res. 2003, 53, 600–607. [Google Scholar] [CrossRef]
- Wang, B.; Wang, F.; Wu, D.; Xu, X.; Yang, L.; Zhu, J.; Yuan, J.; Tang, J. Relationship Between TNF-alpha and the Risk of Cerebral Palsy: A Systematic Review and Meta-Analysis. Front. Neurol. 2022, 13, 929280. [Google Scholar] [CrossRef]
- Peters, M.D.; Godfrey, C.; McInerney, P.; Munn, Z.; Tricco, A.C.; Khalil, H. Chapter 11: Scoping Reviews (2020 Version). Available online: https://doi.org/10.46658/JBIMES-20-12 (accessed on 1 December 2022). [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ng, T.K.S.; Coughlan, C.; Heyn, P.C.; Tagawa, A.; Carollo, J.J.; Kua, E.H.; Mahendran, R. Increased plasma brain-derived neurotrophic factor (BDNF) as a potential biomarker for and compensatory mechanism in mild cognitive impairment: A case-control study. Aging 2021, 13, 22666–22689. [Google Scholar] [CrossRef]
- Tao, W.; Wen, F.; Yao, H.; Sun, Y. The Influence of Erythropoietin and Proinflammatory Cytokines in the Development of Cerebral Palsy. Vasc. Dis. Prev. 2008, 5, 29–32. [Google Scholar]
- Goracke-Postle, C.J.; Burkitt, C.C.; Panoskaltsis-Mortari, A.; Ehrhardt, M.; Wilcox, G.L.; Graupman, P.; Partington, M.; Symons, F.J. Expression of and correlational patterns among neuroinflammatory, neuropeptide, and neuroendocrine molecules from cerebrospinal fluid in cerebral palsy. BMC Neurol. 2021, 21, 384. [Google Scholar] [CrossRef]
- Koh, H.; Hwang, K.; Lim, H.Y.; Kim, Y.J.; Lee, Y.H. Mononuclear cells from the cord blood and granulocytecolony stimulating factor-mobilized peripheral blood: Is there a potential for treatment of cerebral palsy? Neural. Regen Res. 2015, 10, 2018–2024. [Google Scholar] [CrossRef]
- Pingel, J.; Barber, L.; Andersen, I.T.; Walden, F.V.; Wong, C.; Døssing, S.; Nielsen, J.B. Systemic inflammatory markers in individuals with cerebral palsy. Eur. J. Inflamm. 2019, 17, 2058739218823474. [Google Scholar] [CrossRef]
- Riewruja, K.; Amarase, C.; Osateerakun, P.; Weerasopone, S.; Limpaphayom, N.; Honsawek, S. Neutrophil-to-lymphocyte ratio predicts the severity of motor impairment in cerebral palsy children living at home and the rehabilitation center: A comparative study. Biomed. Rep. 2020, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, X. Plasma Tumor Necrosis Factor-alpha (TNF-alpha) Levels Correlate with Disease Severity in Spastic Diplegia, Triplegia, and Quadriplegia in Children with Cerebral Palsy. Med. Sci. Monit. 2015, 21, 3868–3874. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Chen, M.; Bi, D.; Song, J.; Zhang, X.; Wang, Y.; Zhu, D.; Shang, Q.; Xu, F.; Wang, X.; et al. Combined Analysis of Interleukin-10 Gene Polymorphisms and Protein Expression in Children With Cerebral Palsy. Front. Neurol. 2018, 9, 182. [Google Scholar] [CrossRef]
- Von Walden, F.; Gantelius, S.; Liu, C.; Borgstrom, H.; Bjork, L.; Gremark, O.; Stal, P.; Nader, G.A.; Ponte, N.E. Muscle contractures in patients with cerebral palsy and acquired brain injury are associated with extracellular matrix expansion, pro-inflammatory gene expression, and reduced rRNA synthesis. Muscle Nerve 2018, 58, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Koh, H.; Hwang, K. Intracellular Expression of Neurotrophic Factors in Mononuclear Cells of Cord Blood and G-CSF-Mobilized Peripheral Blood As a Potential Source for Cellular Therapy. Blood 2014, 124, 5121. [Google Scholar] [CrossRef]
- Kadhim, H.; Sebire, G. Immune mechanisms in the pathogenesis of cerebral palsy: Implication of proinflammatory cytokines and T lymphocytes. Eur. J. Paediatr. Neurol. 2002, 6, 139–142. [Google Scholar] [CrossRef]
- Djukic, M.; Gibson, C.S.; Maclennan, A.H.; Goldwater, P.N.; Haan, E.A.; McMichael, G.; Priest, K.; Dekker, G.A.; Hague, W.M.; Chan, A.; et al. Genetic susceptibility to viral exposure may increase the risk of cerebral palsy. Aust. N. Z. J. Obstet. Gynaecol. 2009, 49, 247–253. [Google Scholar] [CrossRef]
- Kitase, Y.; Chin, E.M.; Ramachandra, S.; Burkhardt, C.; Madurai, N.K.; Lenz, C.; Hoon, A.H., Jr.; Robinson, S.; Jantzie, L.L. Sustained peripheral immune hyper-reactivity (SPIHR): An enduring biomarker of altered inflammatory responses in adult rats after perinatal brain injury. J. Neuroinflammation 2021, 18, 242. [Google Scholar] [CrossRef]
- Sadowska, M.; Sarecka-Hujar, B.; Kopyta, I. Cerebral Palsy: Current Opinions on Definition, Epidemiology, Risk Factors, Classification and Treatment Options. Neuropsychiatr Dis. Treat. 2020, 16, 1505–1518. [Google Scholar] [CrossRef]
- Hollung, S.J.; Bakken, I.J.; Vik, T.; Lydersen, S.; Wiik, R.; Aaberg, K.M.; Andersen, G.L. Comorbidities in cerebral palsy: A patient registry study. Dev. Med. Child. Neurol. 2020, 62, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Badawi, N.; Hunt, R.; Wallace, E.; Walker, K.; Fahey, M. Stem cell intervention for cerebral palsy: Systematic review with meta-analysis. Dev. Med. Child Neurol. 2016, 58 (Suppl. S3), 40. [Google Scholar] [CrossRef]
- Sun, J.M.; Case, L.E.; McLaughlin, C.; Burgess, A.; Skergan, N.; Crane, S.; Jasien, J.M.; Mikati, M.A.; Troy, J.; Kurtzberg, J. Motor function and safety after allogeneic cord blood and cord tissue-derived mesenchymal stromal cells in cerebral palsy: An open-label, randomized trial. Dev. Med. Child Neurol. 2022, 64, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Stockx, E.M.; Camilleri, P.; Skuza, E.M.; Churchward, T.; Howes, J.M.; Ho, M.; McDonald, T.; Freezer, N.; Hamilton, G.; Wilkinson, M.H.; et al. New acoustic method for detecting upper airway obstruction in patients with sleep apnoea. Respirology 2010, 15, 326–335. [Google Scholar] [CrossRef] [PubMed]
- McGrath-Morrow, S.A.; Ryan, T.; Riekert, K.; Lefton-Greif, M.A.; Eakin, M.; Collaco, J.M. The impact of bronchopulmonary dysplasia on caregiver health related quality of life during the first 2 years of life. Pediatr. Pulmonol. 2013, 48, 579–586. [Google Scholar] [CrossRef]
- Luo, Y.; Cai, X.; Liu, S.; Wang, S.; Nold-Petry, C.A.; Nold, M.F.; Bufler, P.; Norris, D.; Dinarello, C.A.; Fujita, M. Suppression of antigen-specific adaptive immunity by IL-37 via induction of tolerogenic dendritic cells. Proc. Natl. Acad. Sci. USA 2014, 111, 15178–15183. [Google Scholar] [CrossRef]
- Nold, M.F.; Nold-Petry, C.A.; Zepp, J.A.; Palmer, B.E.; Bufler, P.; Dinarello, C.A. IL-37 is a fundamental inhibitor of innate immunity. Nat. Immunol. 2010, 11, 1014–1022. [Google Scholar] [CrossRef]
- Woolfenden, S.; Farrar, M.A.; Eapen, V.; Masi, A.; Wakefield, C.E.; Badawi, N.; Novak, I.; Nassar, N.; Lingam, R.; Dale, R.C. Delivering paediatric precision medicine: Genomic and environmental considerations along the causal pathway of childhood neurodevelopmental disorders. Dev. Med. Child Neurol. 2022, 64, 1077–1084. [Google Scholar] [CrossRef]
- Sewell, E.; Roberts, J.; Mukhopadhyay, S. Association of Infection in Neonates and Long-Term Neurodevelopmental Outcome. Clin. Perinatol. 2021, 48, 251–261. [Google Scholar] [CrossRef]
- Jain, V.G.; Kline, J.E.; He, L.; Kline-Fath, B.M.; Altaye, M.; Muglia, L.J.; DeFranco, E.A.; Ambalavanan, N.; Parikh, N.A.; Cincinnati Infant Neurodevelopment Early Prediction Study Investigators. Acute histologic chorioamnionitis independently and directly increases the risk for brain abnormalities seen on magnetic resonance imaging in very preterm infants. Am. J. Obstet. Gynecol. 2022, 227. [Google Scholar] [CrossRef]
- Estes, M.L.; McAllister, A.K. Maternal immune activation: Implications for neuropsychiatric disorders. Science 2016, 353, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Wienecke, L.M.; Cohen, S.; Bauersachs, J.; Mebazaa, A.; Chousterman, B.G. Immunity and inflammation: The neglected key players in congenital heart disease? Heart Fail. Rev. 2022, 27, 1957–1971. [Google Scholar] [CrossRef] [PubMed]
- Saand, A.R.; Yu, F.; Chen, J.; Chou, S.H. Systemic inflammation in hemorrhagic strokes—A novel neurological sign and therapeutic target? J. Cereb. Blood Flow Metab. 2019, 39, 959–988. [Google Scholar] [CrossRef] [PubMed]
- Stamatovic, S.M.; Phillips, C.M.; Martinez-Revollar, G.; Keep, R.F.; Andjelkovic, A.V. Involvement of Epigenetic Mechanisms and Non-coding RNAs in Blood-Brain Barrier and Neurovascular Unit Injury and Recovery After Stroke. Front. Neurosci. 2019, 13, 864. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMAScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
| Citation | CP Group/s and Comparator Details | Etiology of CP Group | Result Type Sample Type Method of Analysis: Inflammatory Markers | Reported Significant Findings Related to CP |
|---|---|---|---|---|
| Tao 2008 [19] | Children with CP: Type/topography/severity: not reported Mean age 5.2 years, range 0–10 (n = 31, 55% male) Neonatal controls with asphyxia or infection: Mean age 6.3 days, range 0–10 (n = 37, 54% male) Age-matched controls, no CP: Mean age 4.8 years, range 0–10 (n = 40, 58% male) | HIE and/or infection | Cytokine analysis Serum ELISA: TNF, IL-6 | ↑ TNF in CP vs. neonatal controls ↑ TNF in CP vs. age-matched controls ↑ IL-6 in CP vs. age-matched controls |
| Lin 2010 [5] | Children and adolescents with CP: Spastic/di, tri or quad/GMFCS II-V: Mean age (SD) 7.2 (±3.6) years (n = 32, 59% male) GA-matched controls, no CP: Mean age (SD) 6.2 (±2.2) years (n = 32, 44% male) | Preterm with PVL | Cytokine analysis Plasma ELISA: TNF, IL-6 | ↑ TNF in CP vs. GA-matched controls |
| Immune function Supernatant from PBMCs, +/− LPS ELISA: TNF, IL-6 Flow cytometry: TNF | ↑ TNF (ELISA) after LPS in CP vs. GA-matched controls ↑ TNF (flow cytometry) before vs. after LPS in CP group | |||
| Immune function RNA from PBMCs, +/− LPS RT-PCR: 84 TNF genes (TNF ligand/receptor signalling) | ↑ 10 genes (CAD, CASP2, CRADD, EDA2R, IKBKG, TAK1/TGF1a, JNK/JNK1, 4-1BB/CD137, TL1/TL1A, TRAF3) in CP vs. GA-matched controls | |||
| Koh 2015 [21] | Children with CP: Type/topography/severity: not reported Mean age not reported (n = 14, sex not reported) Adult controls, no CP Mean age not reported (n = 14, sex not reported) Cord blood from healthy neonates (n = 14, sex not reported) | Not reported | Immune function Mobilised PBMCs, +LPS/PMA and ionomycin Flow cytometry: TNF, IL-1β, IL-2, IL-3, IL-6, IL-8, IL-9, GM-CSF | ↓ IL-1β in CP vs. adult controls ↑ GM-CSF in CP vs. adult controls ↑ IL-8 in CP vs. adult controls |
| Wu 2015 [24] | Children with CP: Spastic/tri, quad, di, mono, hemi/GMFCS I-V Mean age (SD) 3.7 (±2.3) years (n = 54, 59% male) Age-matched controls, no CP: Mean age (SD) 4.6 (±3.1) years (n = 28, 54% male) | Not reported | Cytokine analysis Plasma ELISA: TNF | ↑ TNF in CP vs. age matched-control |
| Von Walden 2018 [26] | Children and adolescents with CP: Spastic/ topography not reported/GMFCS I-II, IV-V Mean age 15.5 years, range 9–18 (n = 18, another n = 2 with ABI, 85% male) Children and young adult controls, no CP: Mean age 15.1 years, range 7–21 (n = 10, 80% male) | Not reported | Cytokine analysis Skeletal muscle biopsy RT-PCR: IL-1β, IL-1R, IL-6, IL-6R, TNF, TWEAK, IL-8, IL-10 | ↑ IL-1β in CP vs. controls ↑ IL-6 in CP vs. controls ↑ TNF in CP vs. controls |
| Xia 2018 [25] | Infants and children with CP: Spastic and non-spastic/hemi, di, quad /GMFCS not reported Mean age for genotyping/plasma collection (SD) 16.2 (±12.7) months; 20.8 (±14.4) months (n = 282, 65% male) Infant and children controls, no CP: Mean (SD) age for genotyping/plasma collection 24.0 (±16.4) months; 21.6 (±13.8) months (n = 197, 77% male) | Mixed: HIE, PVL, other/not specified | Cytokine analysis Plasma 1 ELISA: IL-10 | ↑ IL-10 in CP vs. controls |
| Pingel 2019 [22] | Children and adolescents with CP: Type not reported/hemi, di, quad/GMFCS I-V: Mean age (SEM) 10.31 (±1.1) years (n = 14, 50% male) Adults with CP: 38.8 (±3.6) years (n = 10, 60% male) Adult controls, no CP: Mean age (SEM) 36.53 (±3.8) years (n = 10, sex not reported) | Not reported | Cytokine analysis Plasma ELISA: TGFβ1, CRP, IL-6 | ↑ TGFβ1 and CRP in children with CP vs. adult controls 2 |
| Ng 2021 [18] | Adults with CP: Type/topography/severity: not reported Mean age (SD) 25 (±5.39) years (n = 64, 55% male) Older adults’ with mild cognitive impairment, no CP (mean age (SD) 66.95 (±4.29) years) (n = 40, 30% males) Older adults’ controls, no MCI, no CP: (mean age (SD) 71.8 (±5.66) years) (n = 56, 20% males) | Not reported | Cytokine analysis Plasma ELISA: CRP | None |
| Taher 2021 [6] | Children and adolescents with CP: Type/topography/severity: not reported Mean age: “School-aged children”, age not reported (n = 10, 80% male) School aged children post NE, no CP (n = 10, 70% male) Mean age: not reported School aged children no NE, no CP (n = 23, 78% male) Mean age: not reported Neonates with NE (n = 30, 50% male) Mean age: not reported Neonates, no NE (n = 17, 53% male) Mean age: not reported | NE | Proportions of immune cell types Whole blood Flow cytometry: T cells (CD3+), B cells (CDCD3- CD19+), NK cells (CD3-/CD56+), MAIT cells (CD3+/Va7.2+/CD161+), iNKT cells (CD3+/Va24Ja18+) Vδ1 TCRs (CD3+/ Vδ1+), Vδ 2 TCRs (CD3+/ Vδ2+) | ↑ T-cells (absolute and % freq) in children with CP vs. school-aged children ↑ Vδ2 T cells (absolute and % freq) in children with CP vs. school-aged children ↑ iNKT cells (absolute and % freq) in children with CP vs. school-aged children ↑ CD4− CD8− T cell frequencies in children with CP vs. school aged children ↓ Vδ1 T cells (absolute and % freq) in children with CP vs. school aged children ↓ MAIT cell (% freq) in children with CP vs. school aged children ↓ B cell (% freq) in children with CP vs. school aged children |
| Cytokine analysis Serum ELISA: IFN-y, TNF, IL-2, IL-5, IL-6 IL-8, IL-9, IL-10, IL-15, IL-17A, IL-21, IL-22, IL-23 | ↓ IL-8 in children with CP vs. school aged children post NE | |||
| Zareen 2010 [9] | Children and adolescents with CP: Type not reported/topography not reported/GMFCS II, III, V Mean age (SD) 10.08 (±1.67) years, range 1–16 (n = 12, 67% male) Age-matched controls, no CP Mean age (SD) 9.08 (±1.08) years, range 6–14 (n = 12, 67% male) | Mixed: NE, congenital infection and stroke | Cytokine analysis Serum ELISA: IL-1α, IL-1β, IL-6, IL-8, IL-10 IL-18, IL-1Ra, TNF INF-γ, GM-CSF | None |
| Immune function Supernatant from whole blood + LPS ELISA IL-1α, IL-1β, IL-6, IL-8, IL-10, IL-18, IL-1Ra, TNF, IFN-γ, GM-CSF | ↑ IL-1α, IL-1β, IL-2, IL-6 after stim in CP vs. age-matched controls |
| Theme | Reported Significant Findings in Subgroups of Those with CP | Citation |
|---|---|---|
| Age | ↑ plasma TNF in CP vs. younger (1–3 years) and older (4–12 years) controls ↑ plasma TNF in younger subjects (1–3 years) with CP vs. older subjects (4–12 years) with CP ↑ TGFβ1 and CRP in children with CP vs. adults with CP | Wu 2015 [24] Pingel 2010 [22] |
| Type/Topography | ↑ plasma IL-10 in the CP group with spastic quadraplegia vs. controls Frequencies of allele and genotype those with spastic quadraplegia vs. controls of IL-10 polymorphisms: rs1554286, rs151811, rs3024490, rs1800871, and rs1800896 | Xia 2018 [25] |
| Severity | ↑ plasma TNF levels correlated with higher GMFCS in spastic diplegia or quadriplegia, and spastic diplegia | Wu 2015 [24] |
| ↑ whole blood NLR in children with severe motor impairment (GMFCS IV-V) vs. mild motor impairment (GMFCS II-III) living in a rehabilitation centre ↑ whole blood NLR positively correlated with higher GMFCS level | Riewruja 2020 [23] | |
| Aetiology | 4 unique discrete correlations in CSF related to inflammation, specific to preterm birth vs. term and extremely preterm: IL-10 and MIP-1β; IL-12p70 and TNF; IL-1Ra and IL-10; IL-1Ra and MIP-1β. 7 unique discrete correlations in CSF related to inflammation, specific to extreme preterm birth vs. term and preterm birth: IP10 and MIP-1β; IL-1α and IL-6; IL-1α and IL-10; IL-1α and RANTES; IL6 and IL10; IL-6 and RANTES; IL-10 and RANTES | Gorack-Postle 2021 [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paton, M.C.B.; Finch-Edmondson, M.; Dale, R.C.; Fahey, M.C.; Nold-Petry, C.A.; Nold, M.F.; Griffin, A.R.; Novak, I. Persistent Inflammation in Cerebral Palsy: Pathogenic Mediator or Comorbidity? A Scoping Review. J. Clin. Med. 2022, 11, 7368. https://doi.org/10.3390/jcm11247368
Paton MCB, Finch-Edmondson M, Dale RC, Fahey MC, Nold-Petry CA, Nold MF, Griffin AR, Novak I. Persistent Inflammation in Cerebral Palsy: Pathogenic Mediator or Comorbidity? A Scoping Review. Journal of Clinical Medicine. 2022; 11(24):7368. https://doi.org/10.3390/jcm11247368
Chicago/Turabian StylePaton, Madison C. B., Megan Finch-Edmondson, Russell C. Dale, Michael C. Fahey, Claudia A. Nold-Petry, Marcel F. Nold, Alexandra R. Griffin, and Iona Novak. 2022. "Persistent Inflammation in Cerebral Palsy: Pathogenic Mediator or Comorbidity? A Scoping Review" Journal of Clinical Medicine 11, no. 24: 7368. https://doi.org/10.3390/jcm11247368
APA StylePaton, M. C. B., Finch-Edmondson, M., Dale, R. C., Fahey, M. C., Nold-Petry, C. A., Nold, M. F., Griffin, A. R., & Novak, I. (2022). Persistent Inflammation in Cerebral Palsy: Pathogenic Mediator or Comorbidity? A Scoping Review. Journal of Clinical Medicine, 11(24), 7368. https://doi.org/10.3390/jcm11247368








