Peripheral Blood Mononuclear Cells Mitochondrial Respiration and Superoxide Anion after Heart Transplantation
Abstract
1. Introduction
2. Materials and Methods: Population and Parameters Determined
2.1. Population
2.2. Parameters Determined
2.3. Extraction of Circulating Peripheral Blood Mononuclear Cells (PBMCs)
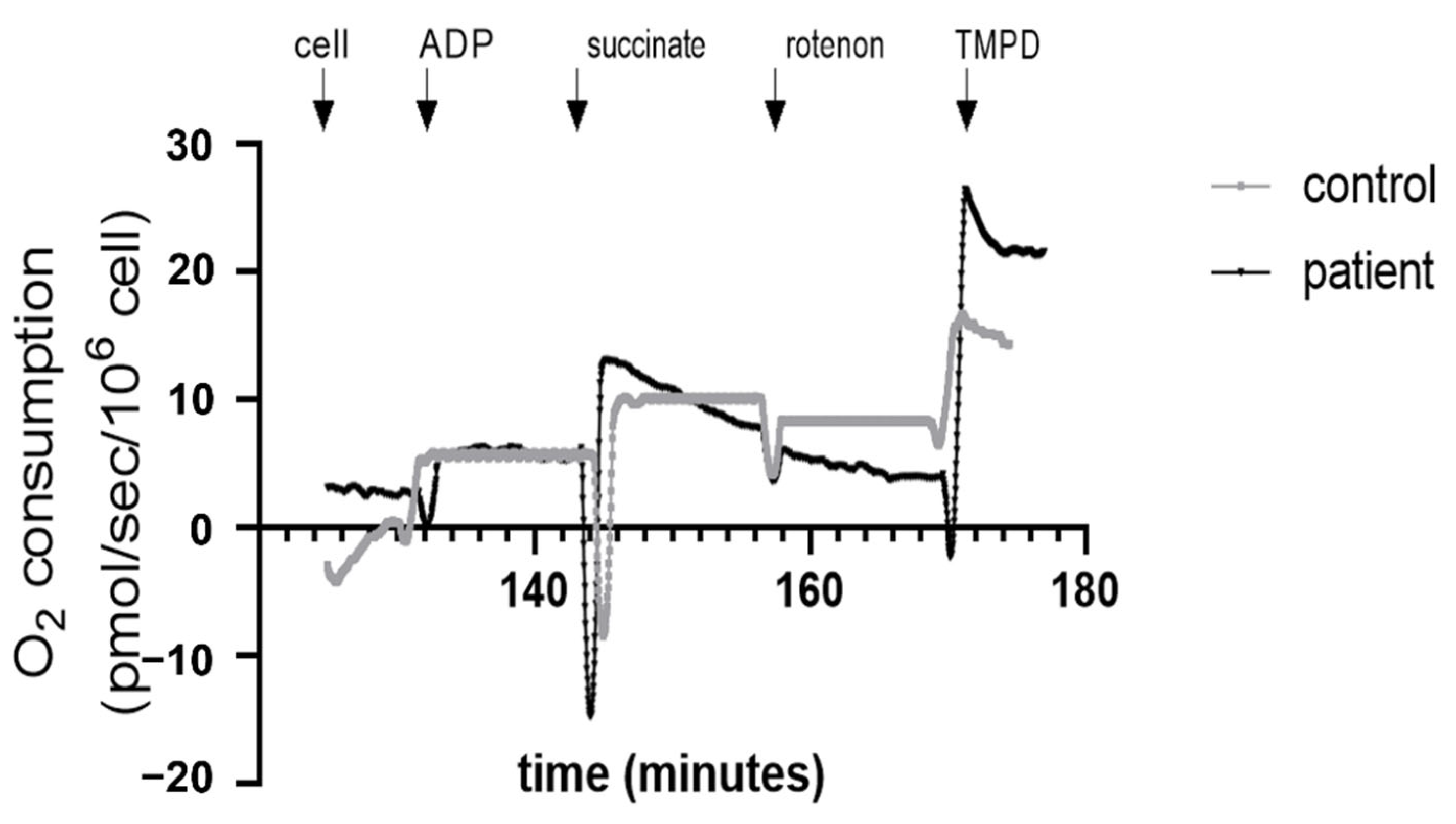
2.4. Mitochondrial Respiration of PBMCs
2.5. Measurement of Superoxide Anion
2.6. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Subject
3.2. Biological Characteristics of the Patients
3.3. Htx’s Cardiovascular Explorations
3.3.1. Echocardiography
3.3.2. Coronary Artery Angiography
3.3.3. Right Heart Biopsy
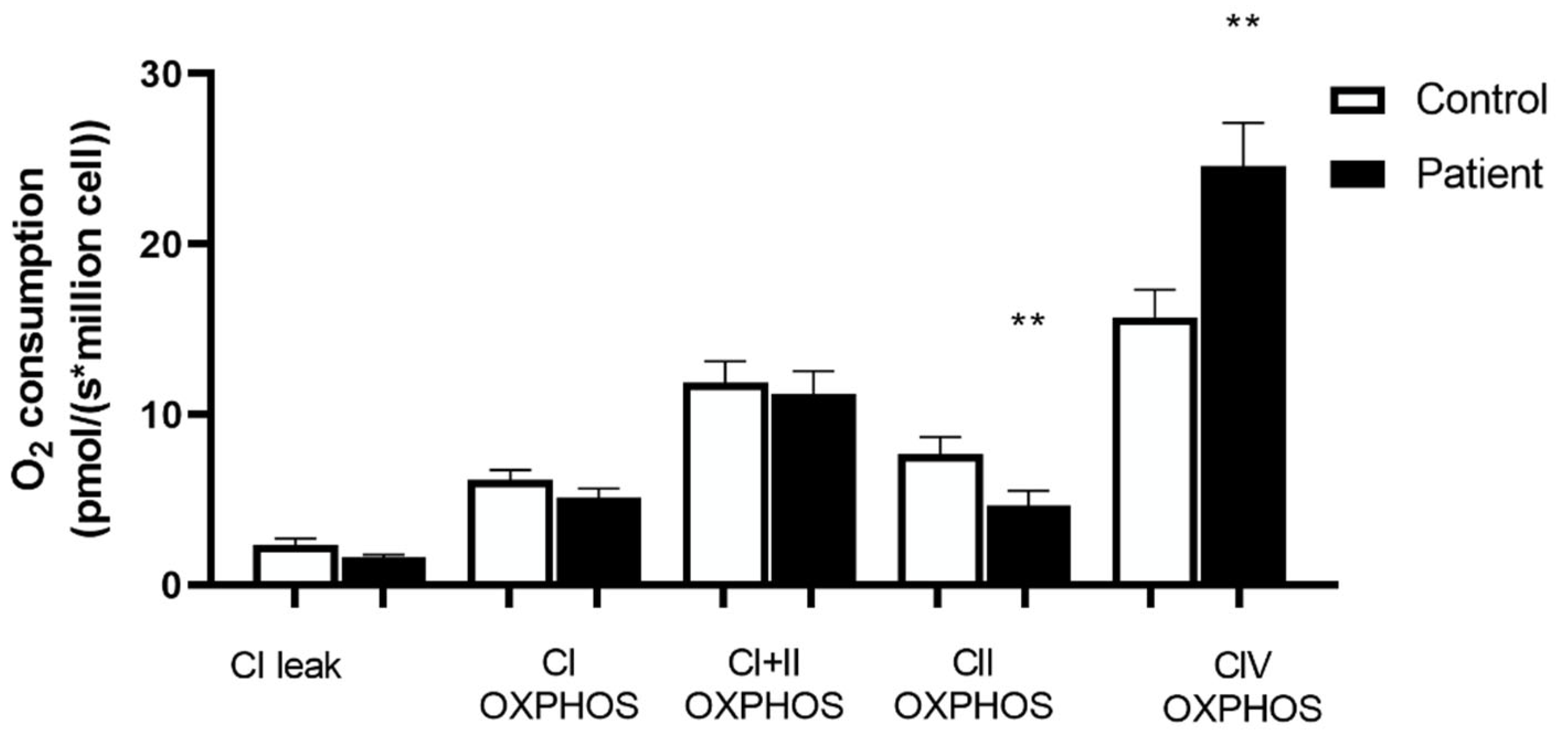
3.4. Decreased Complex II and Increased Complex IV Mitochondrial Respiration in Htx’s PBMCs
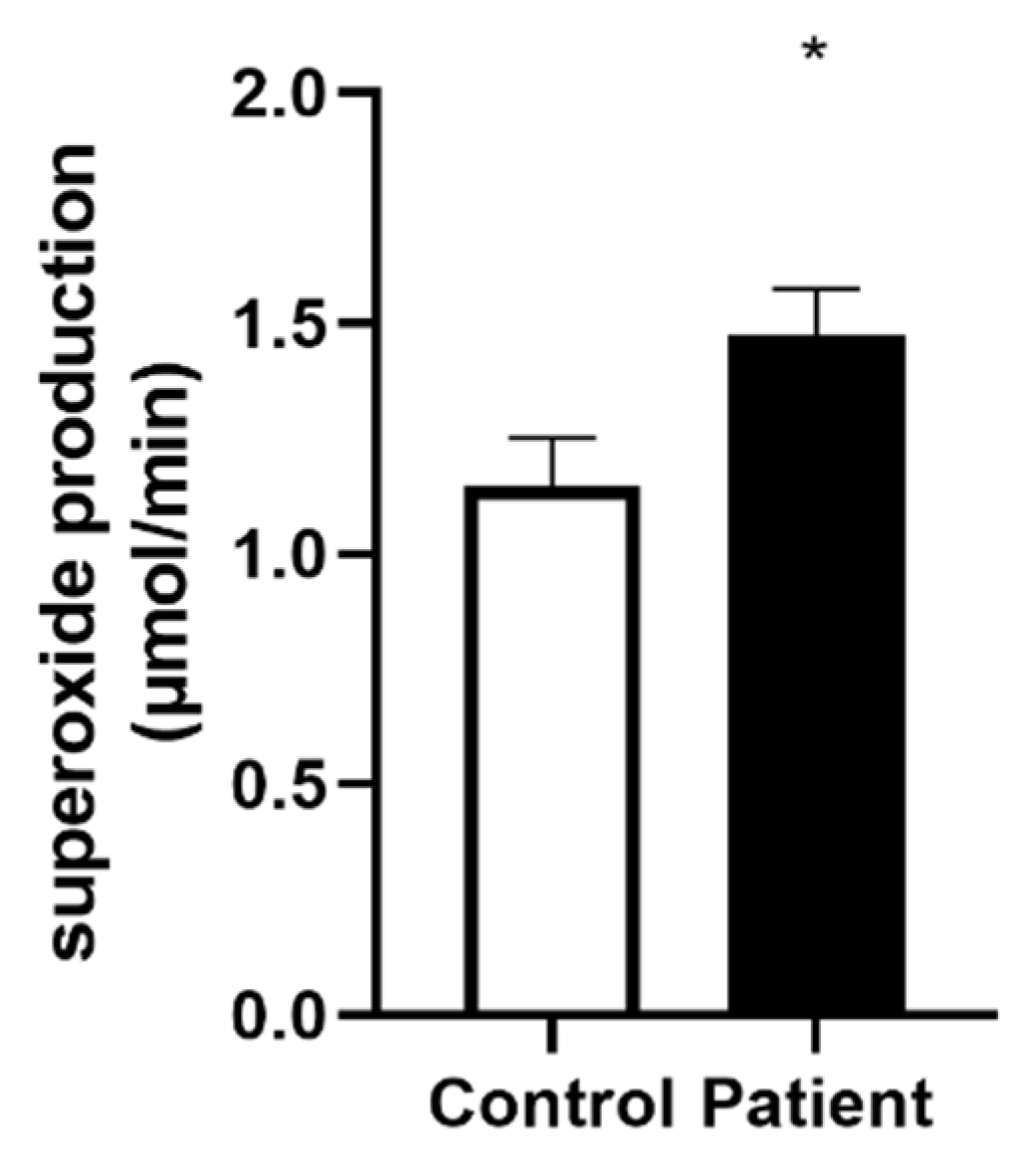
3.5. Increased Superoxide Anion Production after Heart Transplantation
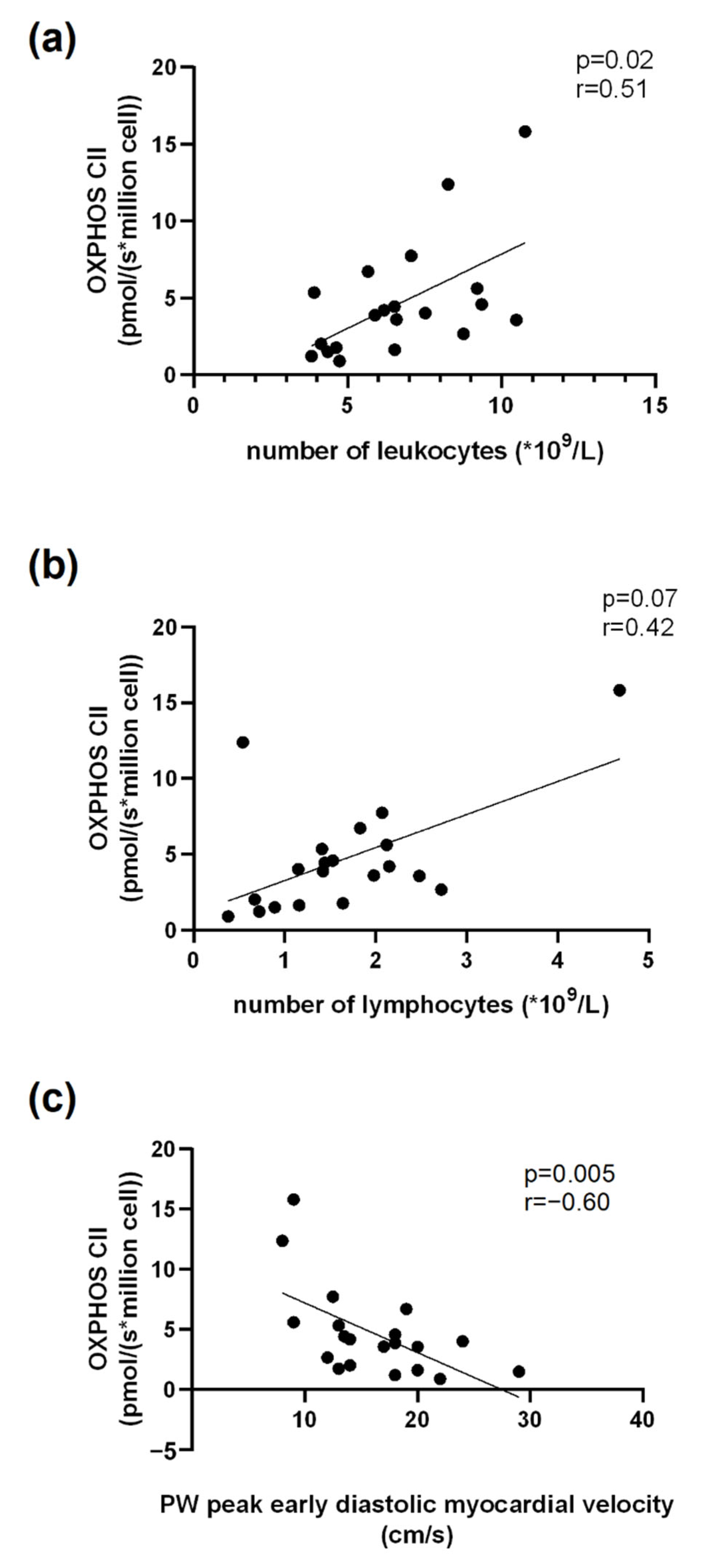
3.6. Correlations Related to Complex II Mitochondrial Respiration
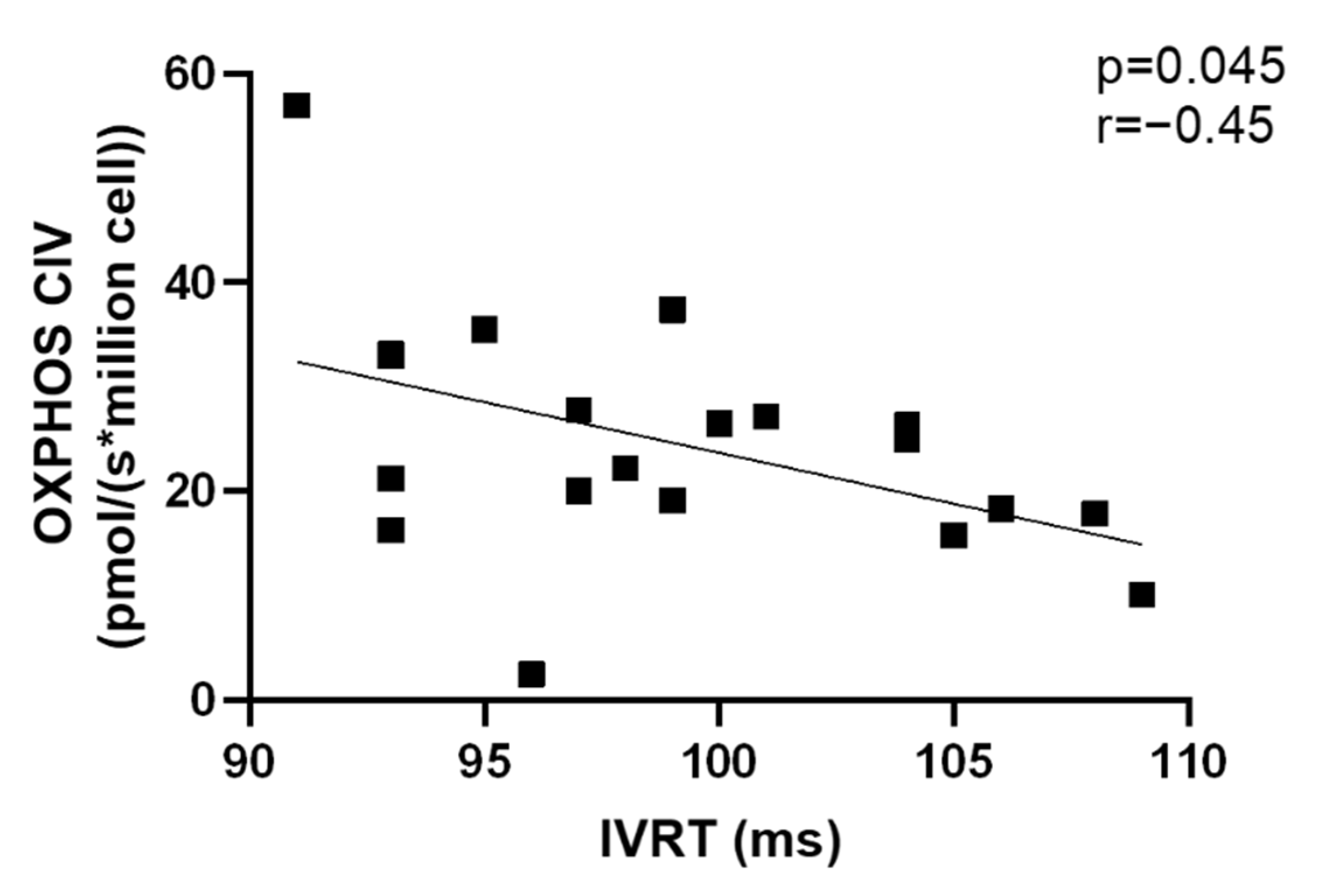
3.7. Correlations Related to Complex IV Mitochondrial Respiration
4. Discussion
4.1. Decreased PBMCs Mitochondrial Respiratory Chain Complex II Respiration after Heart Transplantation
4.2. Increased PBMCs Mitochondrial Respiratory Chain Complex IV Respiration after Heart Transplantation
4.3. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, D.A.; Perry, J.B.; Allen, M.E.; Sabbah, H.N.; Stauffer, B.L.; Shaikh, S.R.; Cleland, J.G.F.; Colucci, W.S.; Butler, J.; Voors, A.A.; et al. Mitochondrial Function as a Therapeutic Target in Heart Failure. Nat. Rev. Cardiol. 2017, 14, 238–250. [Google Scholar] [CrossRef]
- Badano, L.P.; Miglioranza, M.H.; Edvardsen, T.; Colafranceschi, A.S.; Muraru, D.; Bacal, F.; Nieman, K.; Zoppellaro, G.; Marcondes Braga, F.G.; Binder, T.; et al. European Association of Cardiovascular Imaging/Cardiovascular Imaging Department of the Brazilian Society of Cardiology Recommendations for the Use of Cardiac Imaging to Assess and Follow Patients after Heart Transplantation. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 919–948. [Google Scholar] [CrossRef] [PubMed]
- Lichscheidt, E.D.; Jespersen, N.R.; Nielsen, B.R.R.; Berg, K.; Seefeldt, J.; Nyengaard, J.R.; Bøtker, H.E.; Eiskjær, H. Abnormal Mitochondrial Function and Morphology in Heart Transplanted Patients with Cardiac Allograft Vasculopathy. J. Heart Lung Transplant. 2022, 41, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Stanley, W.C.; Recchia, F.A.; Lopaschuk, G.D. Myocardial Substrate Metabolism in the Normal and Failing Heart. Physiol. Rev. 2005, 85, 1093–1129. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-R.; Zweier, J.L. Cardiac Mitochondria and Reactive Oxygen Species Generation. Circ. Res. 2014, 114, 524–537. [Google Scholar] [CrossRef]
- Sauer, F.; Riou, M.; Charles, A.-L.; Meyer, A.; Andres, E.; Geny, B.; Talha, S. Pathophysiology of Heart Failure: A Role for Peripheral Blood Mononuclear Cells Mitochondrial Dysfunction? J. Clin. Med. 2022, 11, 741. [Google Scholar] [CrossRef]
- Li, P.; Wang, B.; Sun, F.; Li, Y.; Li, Q.; Lang, H.; Zhao, Z.; Gao, P.; Zhao, Y.; Shang, Q.; et al. Mitochondrial Respiratory Dysfunctions of Blood Mononuclear Cells Link with Cardiac Disturbance in Patients with Early-Stage Heart Failure. Sci. Rep. 2015, 5, 10229. [Google Scholar] [CrossRef]
- Zhou, B.; Tian, R. Mitochondrial Dysfunction in Pathophysiology of Heart Failure. J. Clin. Investig. 2018, 128, 3716–3726. [Google Scholar] [CrossRef]
- Shirakawa, R.; Yokota, T.; Nakajima, T.; Takada, S.; Yamane, M.; Furihata, T.; Maekawa, S.; Nambu, H.; Katayama, T.; Fukushima, A.; et al. Mitochondrial Reactive Oxygen Species Generation in Blood Cells Is Associated with Disease Severity and Exercise Intolerance in Heart Failure Patients. Sci. Rep. 2019, 9, 14709. [Google Scholar] [CrossRef]
- Kopecky, B.J.; Dun, H.; Amrute, J.M.; Lin, C.-Y.; Bredemeyer, A.L.; Terada, Y.; Bayguinov, P.O.; Koenig, A.L.; Frye, C.C.; Fitzpatrick, J.A.J.; et al. Donor Macrophages Modulate Rejection After Heart Transplantation. Circulation 2022, 146, 623–638. [Google Scholar] [CrossRef]
- Sumbalova, Z.; Garcia-Souza, L.; Calabria, E.; Volani, C.; Gnaiger, E. O2k-Protocols: Isolation of Peripheral Blood Mononuclear Cells and Platelets from Human Blood for HRR. Mitochondrial Physiol. Netw. 2020, 21, 1–15. [Google Scholar]
- Paradis, S.; Charles, A.-L.; Georg, I.; Goupilleau, F.; Meyer, A.; Kindo, M.; Laverny, G.; Metzger, D.; Geny, B. Aging Exacerbates Ischemia-Reperfusion-Induced Mitochondrial Respiration Impairment in Skeletal Muscle. Antioxidants 2019, 8, 168. [Google Scholar] [CrossRef] [PubMed]
- Seropian, I.M.; Romeo, F.J.; Pizarro, R.; Vulcano, N.O.; Posatini, R.A.; Marenchino, R.G.; Berrocal, D.H.; Belziti, C.A. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as Predictors of Survival after Heart Transplantation. ESC Heart Fail 2018, 5, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-R.; Chen, C.-L.; Pfeiffer, D.R.; Zweier, J.L. Mitochondrial Complex II in the Post-Ischemic Heart: Oxidative Injury and the Role of Protein S-Glutathionylation. J. Biol. Chem. 2007, 282, 32640–32654. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, P.; Wanagat, J.; Wang, Z.; Wang, Y.; Liem, D.A.; Ping, P.; Antoshechkin, I.A.; Margulies, K.B.; MacLellan, W.R. Divergent Mitochondrial Biogenesis Responses in Human Cardiomyopathy. Circulation 2013, 127, 1957–1967. [Google Scholar] [CrossRef]
- Garcia, A.M.; Sparagna, G.C.; Phillips, E.K.; Miyano, C.A.; Nunley, K.; Chatfield, K.C.; Stauffer, B.L.; Sucharov, C.; Miyamoto, S.D. Abstract 15615: Reactive Oxygen Species Accumulation and Mitochondrial Dysfunction in Peripheral Blood Mononuclear Cells Are Associated with Heart Failure in Patients with Single Ventricle Congenital Heart Disease. Circulation 2019, 140, A15615. [Google Scholar] [CrossRef]
- Zegelbone, P.M.; Baybayon-Grandgeorge, A.; Stauffer, B.; Sucharov, C.; Miyamoto, S.; Garcia, A.M. Abstract 8963: Mitochondrial Dysfunction in Peripheral Blood Mononuclear Cells Is Associated with Heart Failure in Patients with Single Ventricle Congenital Heart Disease. Circulation 2021, 144, A8963. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, D.D.-H.; Qiu, Y.; Airhart, S.; Liu, Y.; Stempien-Otero, A.; O’Brien, K.D.; Tian, R. Boosting NAD Level Suppresses Inflammatory Activation of PBMCs in Heart Failure. J. Clin. Invest. 2020, 130, 6054–6063. [Google Scholar] [CrossRef]
- Scheiber, D.; Zweck, E.; Albermann, S.; Jelenik, T.; Spieker, M.; Bönner, F.; Horn, P.; Schultheiss, H.-P.; Aleshcheva, G.; Escher, F.; et al. Human Myocardial Mitochondrial Oxidative Capacity Is Impaired in Mild Acute Heart Transplant Rejection. ESC Heart Fail 2021, 8, 4674–4684. [Google Scholar] [CrossRef]
- Escoll, P.; Platon, L.; Buchrieser, C. Roles of Mitochondrial Respiratory Complexes during Infection. Immunometabolism 2019, 1, e190011. [Google Scholar] [CrossRef]
- Rutter, J.; Winge, D.R.; Schiffman, J.D. Succinate Dehydrogenase—Assembly, Regulation and Role in Human Disease. Mitochondrion 2010, 10, 393–401. [Google Scholar] [CrossRef]
- Aldera, A.P.; Govender, D. Gene of the Month: SDH. J. Clin. Pathol. 2018, 71, 95–97. [Google Scholar] [CrossRef]
- Wojtovich, A.P.; Smith, C.O.; Haynes, C.M.; Nehrke, K.W.; Brookes, P.S. Physiological Consequences of Complex II Inhibition for Aging, Disease, and the MKATP Channel. Biochim. Biophys. Acta 2013, 1827, 598–611. [Google Scholar] [CrossRef]
- Dalla Pozza, E.; Dando, I.; Pacchiana, R.; Liboi, E.; Scupoli, M.T.; Donadelli, M.; Palmieri, M. Regulation of Succinate Dehydrogenase and Role of Succinate in Cancer. Semin. Cell Dev. Biol. 2020, 98, 4–14. [Google Scholar] [CrossRef]
- Bowman, A.; Birch-Machin, M.A. Age-Dependent Decrease of Mitochondrial Complex II Activity in Human Skin Fibroblasts. J. Investig. Derm. 2016, 136, 912–919. [Google Scholar] [CrossRef]
- Hokanson, J.F.; Mercier, J.G.; Brooks, G.A. Cyclosporine A Decreases Rat Skeletal Muscle Mitochondrial Respiration in Vitro. Am. J. Respir. Crit. Care Med. 1995, 151, 1848–1851. [Google Scholar] [CrossRef]
- Schultze, N.; Wanka, H.; Zwicker, P.; Lindequist, U.; Haertel, B. Mitochondrial Functions of THP-1 Monocytes Following the Exposure to Selected Natural Compounds. Toxicology 2017, 377, 57–63. [Google Scholar] [CrossRef]
- Nash, A.; Samoylova, M.; Leuthner, T.; Zhu, M.; Lin, L.; Meyer, J.N.; Brennan, T.V. Effects of Immunosuppressive Medications on Mitochondrial Function. J. Surg. Res. 2020, 249, 50–57. [Google Scholar] [CrossRef]
- Pottecher, J.; Guillot, M.; Belaidi, E.; Charles, A.-L.; Lejay, A.; Gharib, A.; Diemunsch, P.; Geny, B. Cyclosporine A Normalizes Mitochondrial Coupling, Reactive Oxygen Species Production, and Inflammation and Partially Restores Skeletal Muscle Maximal Oxidative Capacity in Experimental Aortic Cross-Clamping. J. Vasc. Surg. 2013, 57, 1100–1108.e2. [Google Scholar] [CrossRef]
- Infante, B.; Bellanti, F.; Correale, M.; Pontrelli, P.; Franzin, R.; Leo, S.; Calvaruso, M.; Mercuri, S.; Netti, G.S.; Ranieri, E.; et al. MTOR Inhibition Improves Mitochondria Function/Biogenesis and Delays Cardiovascular Aging in Kidney Transplant Recipients with Chronic Graft Dysfunction. Aging 2021, 13, 8026–8039. [Google Scholar] [CrossRef]
- Pérez, O.; Castro, P.; Jalil, J.; Zalaquett, R.; Morán, S.; Becker, P.; Corbalán, R.; Díaz-Araya, G.; Nettle, D.; Moraga, F.; et al. Persistencia del estrés oxidativo postrasplante cardíaco: Estudio comparativo entre pacientes con trasplante cardíaco y con insuficiencia cardíaca crónica estable. Rev. Española Cardiol. 2002, 55, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Núñez, J.; Miñana, G.; Bodí, V.; Núñez, E.; Sanchis, J.; Husser, O.; Llàcer, A. Low Lymphocyte Count and Cardiovascular Diseases. Curr. Med. Chem. 2011, 18, 3226–3233. [Google Scholar] [CrossRef] [PubMed]
- Chacko, B.K.; Kramer, P.A.; Ravi, S.; Johnson, M.S.; Hardy, R.W.; Ballinger, S.W.; Darley-Usmar, V.M. Methods for Defining Distinct Bioenergetic Profiles in Platelets, Lymphocytes, Monocytes, and Neutrophils, and the Oxidative Burst from Human Blood. Lab. Invest. 2013, 93, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Mansilla, N.; Racca, S.; Gras, D.E.; Gonzalez, D.H.; Welchen, E. The Complexity of Mitochondrial Complex IV: An Update of Cytochrome c Oxidase Biogenesis in Plants. Int. J. Mol. Sci. 2018, 19, 662. [Google Scholar] [CrossRef] [PubMed]
- Little, A.G.; Lau, G.; Mathers, K.E.; Leary, S.C.; Moyes, C.D. Comparative Biochemistry of Cytochrome c Oxidase in Animals. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2018, 224, 170–184. [Google Scholar] [CrossRef]
- Kadenbach, B. Complex IV—The Regulatory Center of Mitochondrial Oxidative Phosphorylation. Mitochondrion 2021, 58, 296–302. [Google Scholar] [CrossRef]
- Campbell, G.R.; Mahad, D.J. A Method to Detect Cytochrome c Oxidase Activity and Mitochondrial Proteins in Oligodendrocytes. In Oligodendrocytes; Lyons, D.A., Kegel, L., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 1936, pp. 333–342. ISBN 978-1-4939-9070-2. [Google Scholar]
- Kadenbach, B.; Hüttemann, M.; Arnold, S.; Lee, I.; Bender, E. Mitochondrial Energy Metabolism Is Regulated via Nuclear-Coded Subunits of Cytochrome c Oxidase11This Article Is Dedicated to the Memory of the Late Professor Lars Ernster. Free Radic. Biol. Med. 2000, 29, 211–221. [Google Scholar] [CrossRef]
- Bourens, M.; Fontanesi, F.; Soto, I.C.; Liu, J.; Barrientos, A. Redox and Reactive Oxygen Species Regulation of Mitochondrial Cytochrome c Oxidase Biogenesis. Antioxid. Redox Signal. 2013, 19, 1940–1952. [Google Scholar] [CrossRef]
- Durhuus, J.A.; Hansson, S.; Morville, T.; Kuhlman, A.B.; Dohlmann, T.L.; Larsen, S.; Helge, J.W.; Angleys, M.; Muniesa-Vargas, A.; Bundgaard, J.R.; et al. Simvastatin Improves Mitochondrial Respiration in Peripheral Blood Cells. Sci. Rep. 2020, 10, 17012. [Google Scholar] [CrossRef]
- Onwugbufor, M.; Levy, R.J.; Zurakowski, D.; Jonas, R.A.; Sinha, P. Myocardial Cytochrome Oxidase Activity Increases with Age and Hypoxemia in Patients with Congenital Heart Disease. Perfusion 2017, 32, 306–312. [Google Scholar] [CrossRef]
- Ederlé, C.; Charles, A.L.; Khayath, N.; Poirot, A.; Meyer, A.; Clere-Jehl, R.; Andres, E.; De Blay, F.; Geny, B. Mitochondrial Function in Peripheral Blood Mononuclear Cells (PBMC) Is Enhanced, Together with Increased Reactive Oxygen Species, in Severe Asthmatic Patients in Exacerbation. J. Clin. Med. 2019, 8, 1613. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.H.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Däbritz, J.H.M.; Gottlieb, E.; Latorre, I.; et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 2016, 167, 457–470.e13. [Google Scholar] [CrossRef] [PubMed]
- Alfatni, A.; Riou, M.; Charles, A.-L.; Meyer, A.; Barnig, C.; Andres, E.; Lejay, A.; Talha, S.; Geny, B. Peripheral Blood Mononuclear Cells and Platelets Mitochondrial Dysfunction, Oxidative Stress, and Circulating MtDNA in Cardiovascular Diseases. J. Clin. Med. 2020, 9, 311. [Google Scholar] [CrossRef] [PubMed]



| Healthy Controls | Heart Transplanted Patients | |
|---|---|---|
| Gender (M/F) | 17/3 | 18/2 |
| Age (years) | 59.6 ± 2.4 | 59.5± 2.5 |
| Body Mass Index (kg/m2) | 25.9 ± 0.98 | 24.4 ± 1.0 |
| Comorbidity (n, %) | ||
| Hypertension | 0 | 14, 70% |
| Diabetes | 0 | 6, 30% |
| Dyslipidaemia | 0 | 15, 75% |
| Initial cardiomyopathy (n, %) | ||
| Cardiac ischemia | 0 | 7, 35% |
| Dilated cardiomyopathy | 0 | 3, 15% |
| Non-obstructive cardiomyopathy | 0 | 3, 15% |
| Valvular | 0 | 2, 10% |
| Congenital | 0 | 2, 10% |
| Rhythmic | 0 | 1, 5% |
| Toxic | 0 | 1, 5% |
| Genetic | 0 | 1, 5% |
| Normal Values (Range) | Htx (Mean ± SEM) | |
|---|---|---|
| Glycemia (mmol/L) | 4.55–6.38 | 6.28 ± 0.44 |
| Creatinine (umol/L) ± SEM | 64–104 | 137 ± 16 |
| Cholesterol (g/L) ± SEM | 1.50–2.00 | 1.83 ± 0.1 n = 19 |
| LDLc (g/L) ± SEM | <1.60 | 0.95 ±0.1, n = 19 |
| Triglycerides (g/L) ± SEM | 0.35–1.50 | 1.97 ± 0.24, n = 19 |
| BNP (pg/mL) ± SEM | 10–150 | 221.42 ± 104.42, n = 19 |
| HB (hemoglobin level (g/dL) | 12–16 | 12.81 ± 0.39, n = 20 |
| Blood Cells Count (×109/L) | ||
| Leucocytes | 4–11 | 6.72± 0.47, n = 20 |
| Lymphocytes | 1–4 | 1.65 ± 0.21, n = 20 |
| Neutrophils | 1.40–7.70 | 4.29 ± 0.39 |
| Inflammation | ||
| CRP (mg/L) ± SEM | <5.0 | 6.31 ± 1.25, n = 20 |
| NLR (Neutrophil-to-lymphocyte ratio) | 1.53 ± 0.01, n = 20 | 3.56 ± 0.65, n = 20 |
| LLR (Leukocyte-to-lymphocyte ratio) | 3.09 ± 0.02, n = 20 | 5.16 ± 0.73, n = 20 |
| Normal Values | HTx | |
|---|---|---|
| Left ventricular ejection fraction (%) | >55 | 63 ± 1.6 |
| Right ventricular fractional shortening (%) | >32 | 47 ± 1 |
| Cardiac index (L/min/m2) | 2.5–3.5 | 3.16 ± 0.11 |
| Systolic pulmonary artery pressures (PAPs) (mmHg) | <35 | 32.7 ± 1.8 |
| E/A | 1–2 | 1.71 ± 0.09 |
| IVRT (Isovolumic Relaxation Time) (ms) | 60–100 | 99.05 ± 1.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfatni, A.; Charles, A.-L.; Sauer, F.; Riou, M.; Goupilleau, F.; Talha, S.; Meyer, A.; Andres, E.; Kindo, M.; Mazzucotelli, J.-P.; et al. Peripheral Blood Mononuclear Cells Mitochondrial Respiration and Superoxide Anion after Heart Transplantation. J. Clin. Med. 2022, 11, 7247. https://doi.org/10.3390/jcm11237247
Alfatni A, Charles A-L, Sauer F, Riou M, Goupilleau F, Talha S, Meyer A, Andres E, Kindo M, Mazzucotelli J-P, et al. Peripheral Blood Mononuclear Cells Mitochondrial Respiration and Superoxide Anion after Heart Transplantation. Journal of Clinical Medicine. 2022; 11(23):7247. https://doi.org/10.3390/jcm11237247
Chicago/Turabian StyleAlfatni, Abrar, Anne-Laure Charles, François Sauer, Marianne Riou, Fabienne Goupilleau, Samy Talha, Alain Meyer, Emmanuel Andres, Michel Kindo, Jean-Philippe Mazzucotelli, and et al. 2022. "Peripheral Blood Mononuclear Cells Mitochondrial Respiration and Superoxide Anion after Heart Transplantation" Journal of Clinical Medicine 11, no. 23: 7247. https://doi.org/10.3390/jcm11237247
APA StyleAlfatni, A., Charles, A.-L., Sauer, F., Riou, M., Goupilleau, F., Talha, S., Meyer, A., Andres, E., Kindo, M., Mazzucotelli, J.-P., Epailly, E., & Geny, B. (2022). Peripheral Blood Mononuclear Cells Mitochondrial Respiration and Superoxide Anion after Heart Transplantation. Journal of Clinical Medicine, 11(23), 7247. https://doi.org/10.3390/jcm11237247







