Acute Pancreatitis Associated with Atypical Bacterial Pneumonia: Systematic Literature Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria—Case Selection
2.3. Data Extraction
2.4. Comprehensiveness of Reporting—Analysis
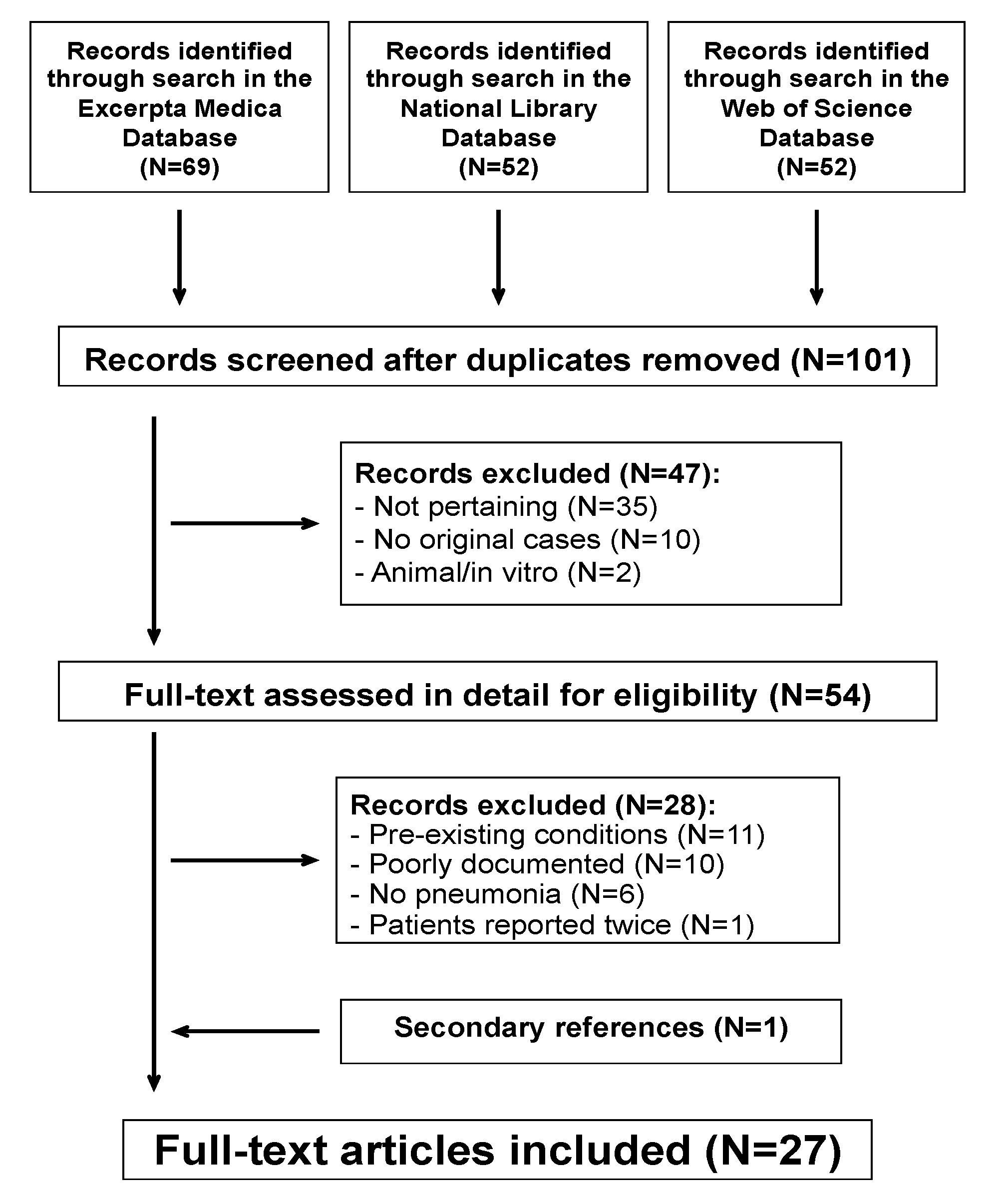
3. Results
3.1. Microbiological Diagnosis
3.2. Clinical and Laboratory Features
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basarab, M.; Macrae, M.B.; Curtis, C.M. Atypical pneumonia. Curr. Opin. Pulm. Med. 2014, 20, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Cunha, B.A.; Ortega, A.M. Atypical pneumonia. Extrapulmonary clues guide the way to diagnosis. Postgrad. Med. 1996, 99, 123–128, 131–132, Erratum in Postgrad. Med. 1996, 99, 64. [Google Scholar] [PubMed]
- Terraneo, L.; Lava, S.A.G.; Camozzi, P.; Zgraggen, L.; Simonetti, G.D.; Bianchetti, M.G.; Milani, G.P. Unusual eruptions associated with Mycoplasma pneumoniae respiratory infections: Review of the literature. Dermatology 2015, 231, 152–157. [Google Scholar] [CrossRef]
- Simoni, C.; Camozzi, P.; Faré, P.B.; Bianchetti, M.G.; Kottanattu, L.; Lava, S.A.G.; Milani, G.P. Myositis and acute kidney injury in bacterial atypical pneumonia: Systematic literature review. J. Infect. Public Health 2020, 13, 2020–2024. [Google Scholar] [CrossRef]
- Betti, C.; Camozzi, P.; Gennaro, V.; Bianchetti, M.G.; Scoglio, M.; Simonetti, G.D.; Milani, G.P.; Lava, S.A.G.; Ferrarini, A. Atypical bacterial pathogens and small-vessel leukocytoclastic vasculitis of the skin in children: Systematic literature review. Pathogens 2021, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Mårdh, P.A.; Ursing, B. Acute pancreatitis in mycoplasma pneumoniae infections. Br. Med. J. 1973, 267, 240–241. [Google Scholar] [CrossRef][Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Carroll, J.K.; Herrick, B.; Gipson, T.; Lee, S.P. Acute pancreatitis: Diagnosis, prognosis, and treatment. Am. Fam. Physician 2007, 75, 1513–1520. [Google Scholar]
- Badalov, N.; Baradarian, R.; Iswara, K.; Li, J.; Steinberg, W.; Tenner, S. Drug-induced acute pancreatitis: An evidence-based review. Clin. Gastroenterol. Hepatol. 2007, 5, 648–661.e3, quiz 644. [Google Scholar] [CrossRef]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis-2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Johnson, D.; Mayers, I. Multiple organ dysfunction syndrome: A narrative review. Can. J. Anaesth. 2001, 48, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Murad, M.H.; Sultan, S.; Haffar, S.; Bazerbachi, F. Methodological quality and synthesis of case series and case reports. BMJ Evid. Based Med. 2018, 23, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Mårdh, P.A.; Ursing, B. The occurrence of acute pancreatitis in Mycoplasma pneumoniae infection. Scand. J. Infect. Dis. 1974, 6, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Schmid, E.; Blaich, E. Akute Pankreatitis bei Infektion durch Mycoplasma pneumoniae (Acute pancreatitis in Mycoplasma pneumoniae infections). Z. Gastroenterol. 1976, 14, 536–537. [Google Scholar]
- Herbaut, C.; Tielemans, C.; Burette, A.; Dratwa, M. Mycoplasma pneumoniae infection and acute pancreatitis. Acta Clin. Belg. 1983, 38, 186–188. [Google Scholar] [CrossRef]
- Arriero Marín, J.M.; Gil Carbonell, J.; Mora Rufete, A.; Shum, C. Pancreatitis y hepatitis en el curso de neumonía por Mycoplasma pneumoniae (Pancreatitis and hepatitis in pneumonia caused by Mycoplasma pneumoniae). Rev. Clin. Esp. 1989, 185, 333. [Google Scholar]
- Van Bever, H.P.; Van Doorn, J.W.; Demey, H.E. Adult respiratory distress syndrome associated with Mycoplasma pneumoniae infection. Eur. J. Pediatr. 1992, 151, 227–228. [Google Scholar] [CrossRef] [PubMed]
- Theissen, O.; Kempf, J.; Loeb, J.P. Pancréatite aigue chez l’enfant, associée à un taux élevé d’anticorps anti-Mycoplasma pneumoniae (Acute pancreatitis in children, combined with high level of anti-Mycoplasma pneumoniae antibodies). Ann. Fr. Anesth. Reanim. 1994, 13, 143. [Google Scholar] [CrossRef]
- Vic, P.; Blondin, G.; Blayo, M.; Finel, E.; Daaboul, M.; Queinnec, C.; Broussine, L. Pancréatite aiguë et infection à Mycoplasma pneumoniae (Acute pancreatitis and Mycoplasma pneumoniae infection). Arch. Pédiatr. 2004, 11, 154. [Google Scholar] [CrossRef]
- Nakagawa, M.; Ogino, H.; Shimohira, M.; Hara, M.; Shibamoto, Y. Continuous regional arterial infusion therapy for acute necrotizing pancreatitis due to Mycoplasma pneumoniae infection in a child. Cardiovasc. Intervent. Radiol. 2009, 32, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Ficko, C.; Mellon, G.; Andriamanantena, D.; Merens, A.; Rapp, C. Pancréatite aiguë à Mycoplasma pneumoniae (Acute Mycoplasma pneumonia pancreatitis). Med. Mal. Infect. 2011, 41, 617–618. [Google Scholar] [CrossRef] [PubMed]
- Hopp, E.; Martínez, L.C.; Díaz, M.; Quintero, A.V.; Dominguez, M.; Di Girolamo, C.; Carreiro, M. Neumonía por Mycoplasma pneumoniae complicada con pancreatitis y hepatitis aguda: A propósito de un caso (Mycoplasma pneumoniae pneumonia complicated with acute pancreatitis and hepatitis: A case study). Rev. Soc. Venez Gestroenterol. 2013, 67, 106–110. [Google Scholar]
- Yang, A.; Kang, B.; Choi, S.Y.; Cho, J.B.; Kim, Y.J.; Jeon, T.Y.; Choe, Y.H. Acute necrotizing pancreatitis associated with Mycoplasma pneumoniae infection in a child. Pediatr. Gastroenterol. Hepatol. Nutr. 2015, 18, 209–215. [Google Scholar] [CrossRef]
- Benzaquen, M.; Lebowitz, D.; Belenotti, P.; Durand, J.M.; Serratrice, J. Acute pancreatitis and pneumonia due to Mycoplasma pneumoniae: A case report. BMC Res. Notes 2016, 9, 397. [Google Scholar] [CrossRef]
- Khan, H.R.A.; Singh, A.; Usman, O.; Rafiq, S.; Amin, A. Acute Pancreatitis: An Unusual Extrapulmonary Manifestation of Mycoplasma pneumoniae. Cureus 2022, 14, e25052. [Google Scholar] [CrossRef] [PubMed]
- Gordan, V.; Postic, B.; Zmyslinski, R.W.; Khan, A.H. Legionnaires’ disease complicated by acute pancreatitis: Case report. Mil. Med. 1980, 145, 345–347. [Google Scholar] [CrossRef]
- Jespersen, C.; Engbaek, K. Legionaersygdom med pancreaspåvirkning (Legionnaires’ disease with pancreatic involvement). Ugeskr. Laeger 1982, 144, 158. [Google Scholar]
- Michel, O.; Naeije, N.; Csoma, M.; Sergysels, R.; de Coster, A. Acute pancreatitis in Legionnaires’ disease. Eur. J. Respir. Dis. 1985, 66, 62–64. [Google Scholar] [PubMed]
- Bollaert, P.E.; Maurizi, M.; Laprevote-Heully, M.C.; Lambert, H.; Larcan, A. Pancréatite aiguë nécrotico-hémorrhagique au cours d’une maladie des légionnaires (Acute necrotic-hemorrhagic pancreatitis in Legionnaires’ disease). Presse Med. 1986, 15, 1732. [Google Scholar]
- Eitrem, R.; Forsgren, A.; Nilsson, C. Pneumonia and acute pancreatitis most probably caused by a Legionella longbeachae infection. Scand. J. Infect. Dis. 1987, 19, 381–382. [Google Scholar] [CrossRef] [PubMed]
- Craven, D.E.; Mark, E.J. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 37-1987. A 50-year-old man with bilateral pneumonia and respiratory failure. N. Engl. J. Med. 1987, 317, 694–702. [Google Scholar]
- Westblom, T.U.; Hamory, B.H. Acute pancreatitis caused by Legionella pneumophila. South Med. J. 1988, 81, 1200–1201. [Google Scholar] [CrossRef] [PubMed]
- Kesavan, C.R.; Pitchumoni, C.S.; Marino, W.D. Acute painless pancreatitis as a rare complication in Legionnaires disease. Am. J. Gastroenterol. 1993, 88, 468–469. [Google Scholar] [PubMed]
- Mégarbane, B.; Montambault, S.; Chary, I.; Guibert, M.; Axler, O.; Brivet, F.G. Acute pancreatitis caused by severe Legionella pneumophila infection. Infection 2000, 28, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Hadef, H.; Bilbault, P.; Arzouq, H.; Berna, C.; Phelipot, J.Y.; Jaeger, A. Violent abdominal pain: Severe Legionella pneumophila lung infection with acute pancreatitis. Am. J. Emerg. Med. 2006, 24, 371–372. [Google Scholar] [CrossRef]
- Puerto Alonso, J.L.; Díaz de Souza, P.; Miragaya García, D.; Sánchez Porto, A. Pancreatitis y colostasis disociada agudas: Manifestación inusual en la infección por Legionella pneumophila (Pancreatitis and dissociated cholestasis: An unusual manifestation in Legionella pneumophila induced-infections). Rev. Clin. Esp. 2011, 211, 379–380. [Google Scholar] [CrossRef]
- Franchini, S.; Marinosci, A.; Ferrante, L.; Sabbadini, M.G.; Tresoldi, M.; Dagna, L. Pancreatic involvement in Legionella pneumonia. Infection 2015, 43, 367–370. [Google Scholar] [CrossRef]
- Cancela Costa, A.; Chheang, C.; Thorens, O.; Lamy, O.; Prella, M.; Babaker, M.; Lamoth, F.; Greub, G. Pancreatitis, hypereosinophilia and bilateral pulmonary infiltrates as presentation of acute Q fever. New Microbes New Infect. 2021, 43, 100940. [Google Scholar] [CrossRef]
- Poddighe, D. Extra-pulmonary diseases related to Mycoplasma pneumoniae in children: Recent insights into the pathogenesis. Curr. Opin. Rheumatol. 2018, 30, 380–387. [Google Scholar] [CrossRef]
- Brewster, U.C. Acute renal failure associated with legionellosis. Ann. Intern. Med. 2004, 140, 406–407. [Google Scholar] [CrossRef] [PubMed]
- Sah, R.P.; Garg, P.; Saluja, A.K. Pathogenic mechanisms of acute pancreatitis. Curr. Opin. Gastroenterol. 2012, 28, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Campolo, M.; Casili, G.; Lanza, M.; Franco, D.; Filippone, A.; Peritore, A.F.; Cuzzocrea, S. Protective effects of xyloglucan in association with the polysaccharide gelose in an experimental model of gastroenteritis and urinary tract Infections. Int. J. Mol. Sci. 2018, 19, 1844. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Fusco, R.; D’Amico, R.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; Crupi, R.; Mandalari, G.; Cuzzocrea, S.; et al. Cashew (Anacardium occidentale L.) nuts modulate the Nrf2 and NLRP3 pathways in pancreas and lung after induction of acute pancreatitis by cerulein. Antioxidants 2020, 9, 992. [Google Scholar] [CrossRef] [PubMed]
- Leinikki, P.O.; Panzar, P.; Tykkä, H. Immunoglobulin M antibody response against Mycoplasma pneumoniae lipid antigen in patients with acute pancreatitis. J. Clin. Microbiol. 1978, 8, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Landry, M.L. Immunoglobulin M for acute infection: True or false? Clin. Vaccine Immunol. 2016, 23, 540–545. [Google Scholar] [CrossRef]
- Correia de Sá, T.; Soares, C.; Rocha, M. Acute pancreatitis and COVID-19: A literature review. World J. Gastrointest. Surg. 2021, 13, 574–584. [Google Scholar] [CrossRef]
- Parenti, D.M.; Steinberg, W.; Kang, P. Infectious causes of acute pancreatitis. Pancreas 1996, 13, 356–371. [Google Scholar] [CrossRef]
- Rawla, P.; Bandaru, S.S.; Vellipuram, A.R. Review of infectious etiology of acute pancreatitis. Gastroenterol. Res. 2017, 10, 153–158. [Google Scholar] [CrossRef]
| All Cases | Mycoplasma pneumoniae | Legionella Species | Coxiella burnetii | p-Values ⤬ | |
|---|---|---|---|---|---|
| n | 33 | 18 | 14 | 1 | |
| Females:Males, n | 14:19 | 11:7 | 2:12 | 1:0 | 0.0116 |
| Age | |||||
| years | 43 (21–56) | 25 (13–52) | 51 (45–57) | 30 | 0.0112 |
| <20 years, n (%) | 8 (24) | 8 (47) | 0 (0) | 0 (0) | 0.0044 |
| Temporal relationship to pneumonia | |||||
| Pre-infectious, n (%) | 1 (3.0) | 0 (0) | 0 (0) | 1 (0) | 0.9999 |
| Intra-infectious, n (%) | 29 (88) | 16 (89) | 13 (93) | 0 (0) | 0.9999 |
| Post-infectious, n (%) | 3 (9.1) | 2 (11) | 1 (7.1) | 0 (0) | 0.9999 |
| Abdominal features | |||||
| Abdominal pain, n (%) | 25 (76) | 16 (89) | 9 (64) | 0 (0) | 0.1948 |
| Nausea, vomiting, n (%) | 14 (44) | 10 (56) | 4 (29) | 0 (0) | 0.7249 |
| Ileus, n (%) | 7 (21) | 5 (28) | 2 (14) | 0 (0) | 0.4264 |
| Diarrhea, n (%) | 5 (15) | 0 (0) | 5 (36) | 0 (0) | 0.0099 |
| Jaundice, n (%) | 4 (12) | 0 (0) | 4 (29) | 0 (0) | 0.0278 |
| Elevated aminotransferases, n (%) | 13 (39) | 2 (11) | 11 (79) | 0 (0) | 0.0002 |
| Pancreatic imaging | |||||
| Interstitial edematous, n (%) | 14 (42) | 6 (33) | 8 (57) | 0 (0) | 0.1570 |
| Necrotizing, n (%) | 5 (15) | 3 (17) | 2 (14) | 0 (0) | 0.9999 |
| Further features | |||||
| Central nervous system dysfunction, n (%) | 15 (45) | 3 (17) | 12 (86) | 0 (0) | 0.0002 |
| Acute kidney injury, n (%) | 9 (27) | 1 (5.6) | 8 (57) | 0 (0) | 0.0037 |
| Multiple-organ dysfunction, n (%) | 7 (21) | 4 (22) | 3 (21) | 0 (0) | 0.9999 |
| Skin rashes, n (%) | 1 (3.0) | 1 (5.6) | 0 (0) | 0 (0) | 0.9999 |
| Length of hospitalization, days | 25 (19–36) | 21 (21–35) | 29 (20–41) | 14 | 0.4542 |
| Death, n (%) | 2 (6.1) | 1 (5.6) | 1 (7.1) | 0 (0) | 0.9999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graf, G.; Vassalli, G.A.M.; Kottanattu, L.; Bianchetti, M.G.; Agostoni, C.; Milani, G.P.; Lava, S.A.G.; Faré, P.B.; Janett, S. Acute Pancreatitis Associated with Atypical Bacterial Pneumonia: Systematic Literature Review. J. Clin. Med. 2022, 11, 7248. https://doi.org/10.3390/jcm11237248
Graf G, Vassalli GAM, Kottanattu L, Bianchetti MG, Agostoni C, Milani GP, Lava SAG, Faré PB, Janett S. Acute Pancreatitis Associated with Atypical Bacterial Pneumonia: Systematic Literature Review. Journal of Clinical Medicine. 2022; 11(23):7248. https://doi.org/10.3390/jcm11237248
Chicago/Turabian StyleGraf, Gwendolyn, Giulia A. M. Vassalli, Lisa Kottanattu, Mario G. Bianchetti, Carlo Agostoni, Gregorio P. Milani, Sebastiano A. G. Lava, Pietro B. Faré, and Simone Janett. 2022. "Acute Pancreatitis Associated with Atypical Bacterial Pneumonia: Systematic Literature Review" Journal of Clinical Medicine 11, no. 23: 7248. https://doi.org/10.3390/jcm11237248
APA StyleGraf, G., Vassalli, G. A. M., Kottanattu, L., Bianchetti, M. G., Agostoni, C., Milani, G. P., Lava, S. A. G., Faré, P. B., & Janett, S. (2022). Acute Pancreatitis Associated with Atypical Bacterial Pneumonia: Systematic Literature Review. Journal of Clinical Medicine, 11(23), 7248. https://doi.org/10.3390/jcm11237248








