Mitochondrial Dysfunction: A Cellular and Molecular Hub in Pathology of Metabolic Diseases and Infection
Abstract
1. Introduction
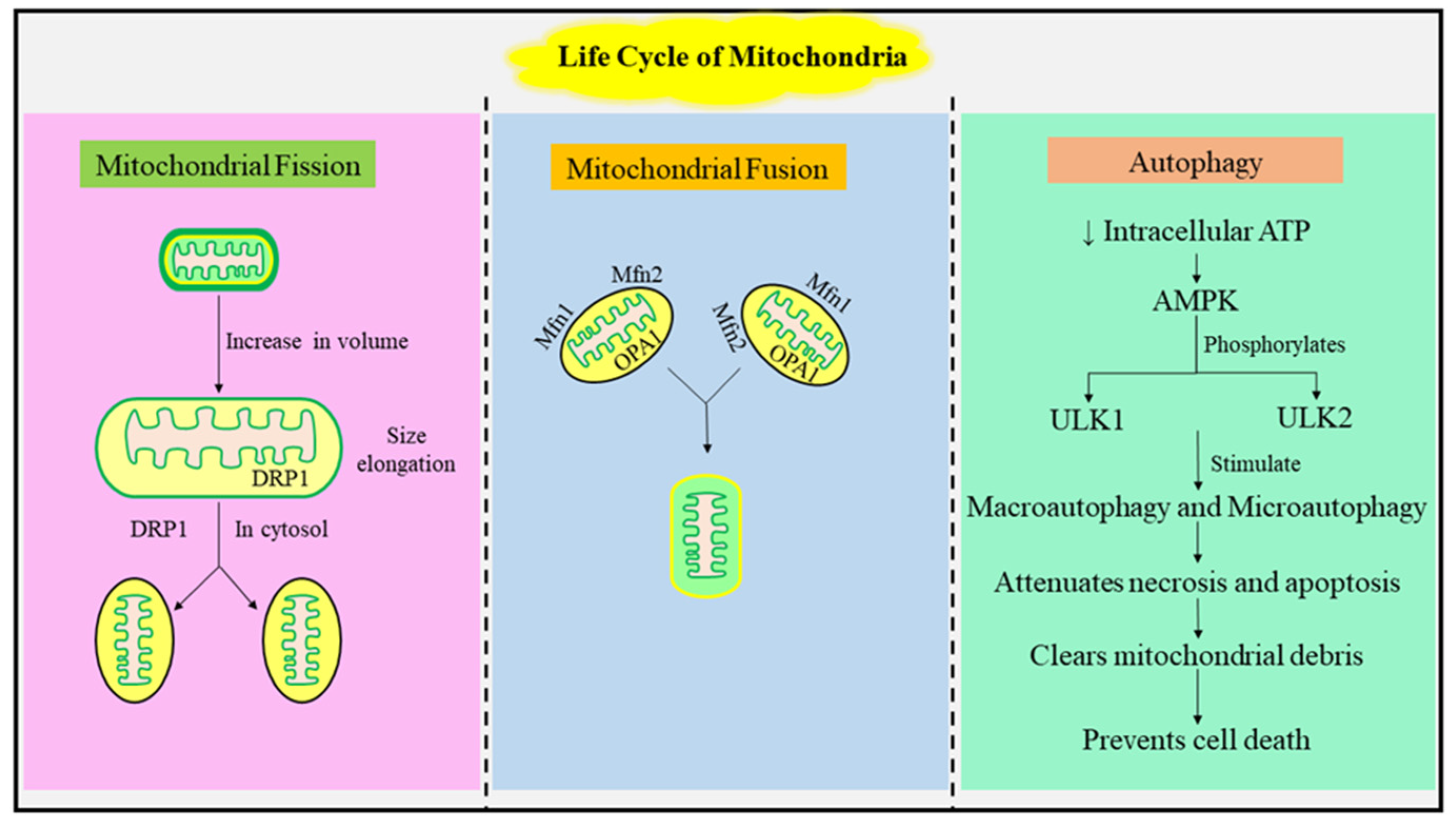
1.1. The Mitochondrial Life Cycle: Fusion, Fission, and Autophagy
1.2. Role of mtDNA in Maintenance of Metabolic Function
1.3. Nuclear DNA and Mitochondrial DNA Cooccurrence
1.4. Functional Validation of Mitochondria
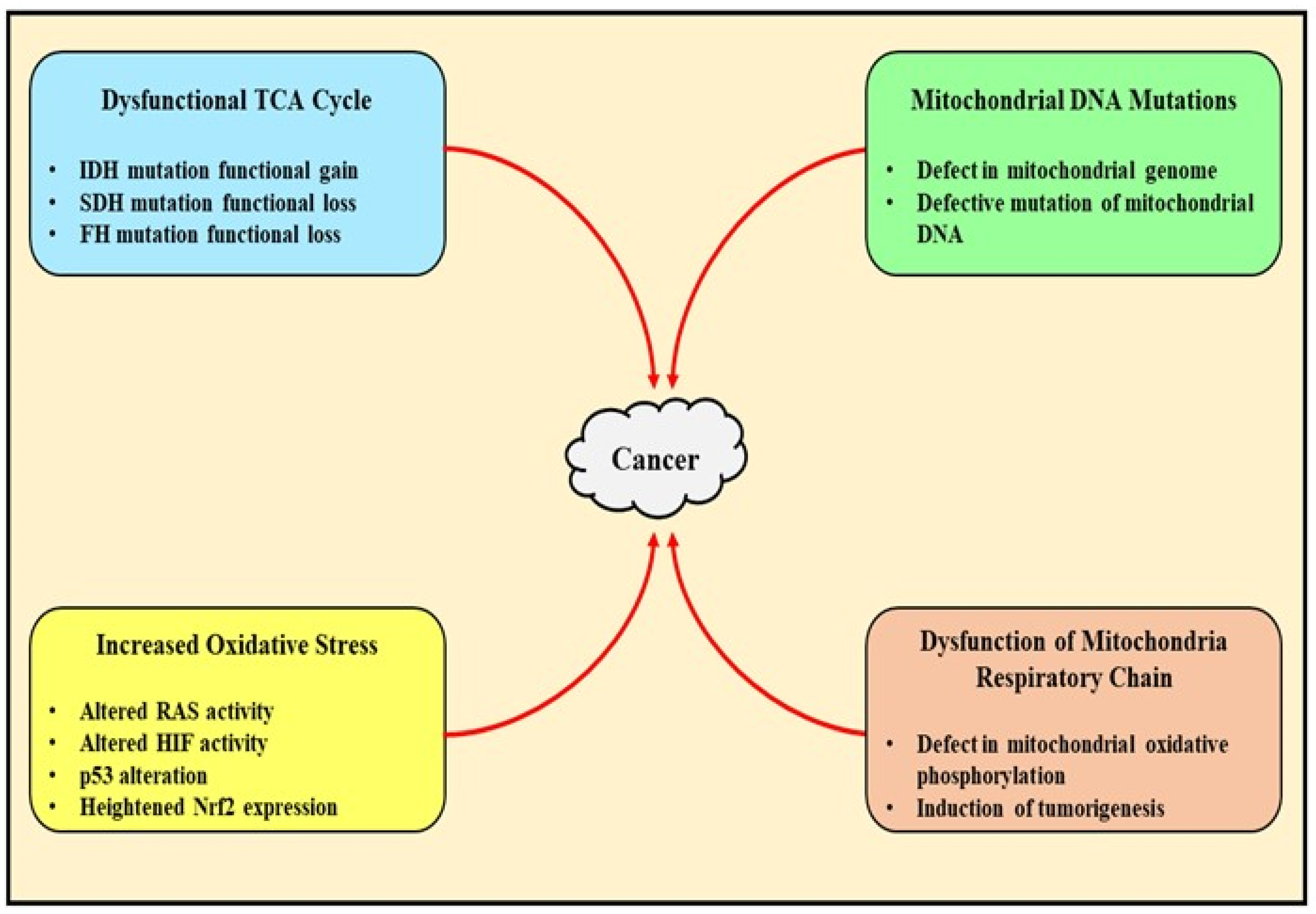
2. Pathophysiological Role of Mitochondrial Dysfunction in Cancer
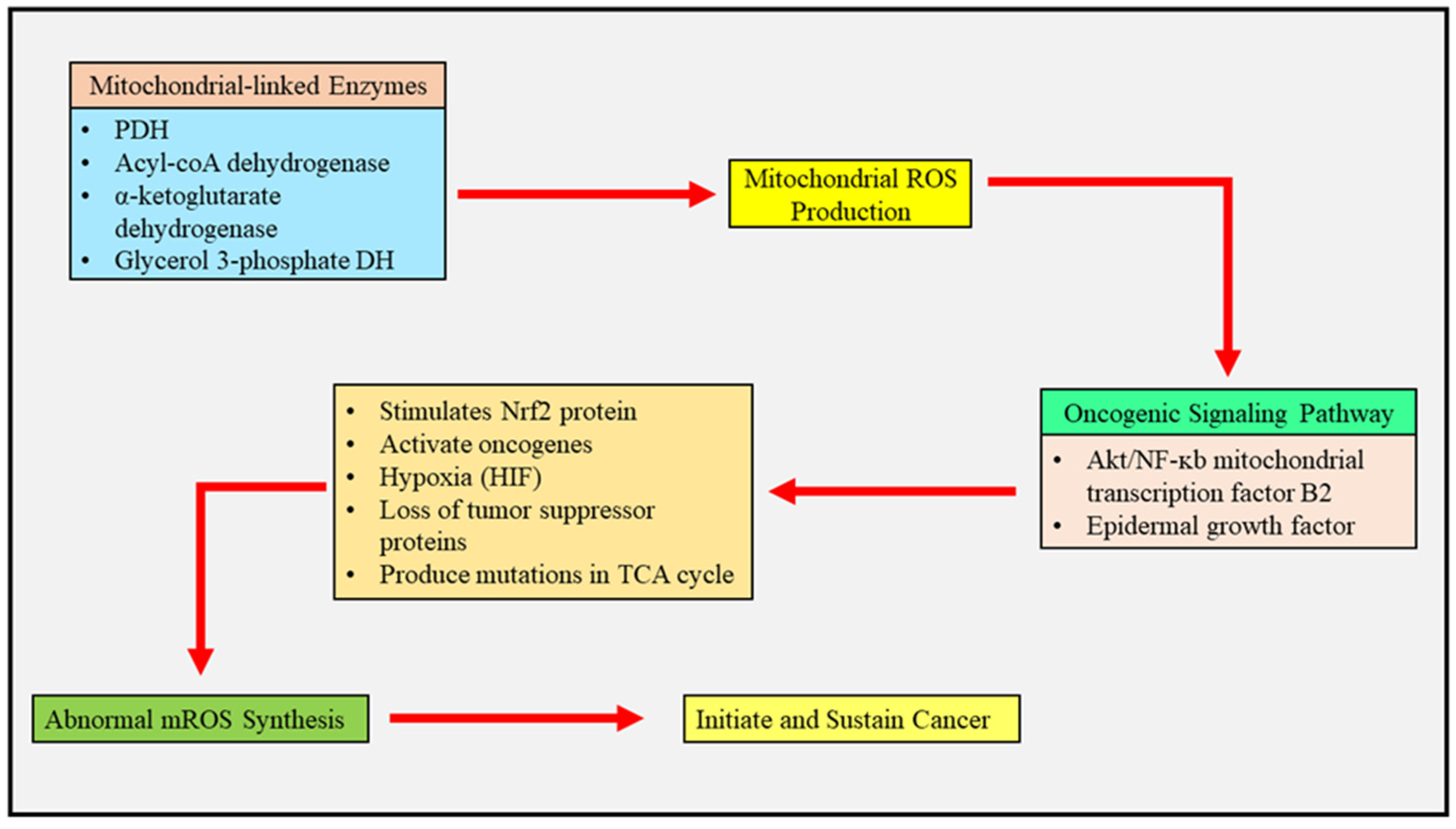
2.1. Mitochondrial Reactive Oxygen Species and Cancer Metabolism
2.2. Mitochondrial Metabolism Deregulation-Mediated Generation of Tumor-Related Proteins and Oncometabolites
2.3. Mitochondria as an Upcoming Target for Management of Cancer
3. Pathophysiological Role of Mitochondrial Dysfunction in Diabetes Mellitus
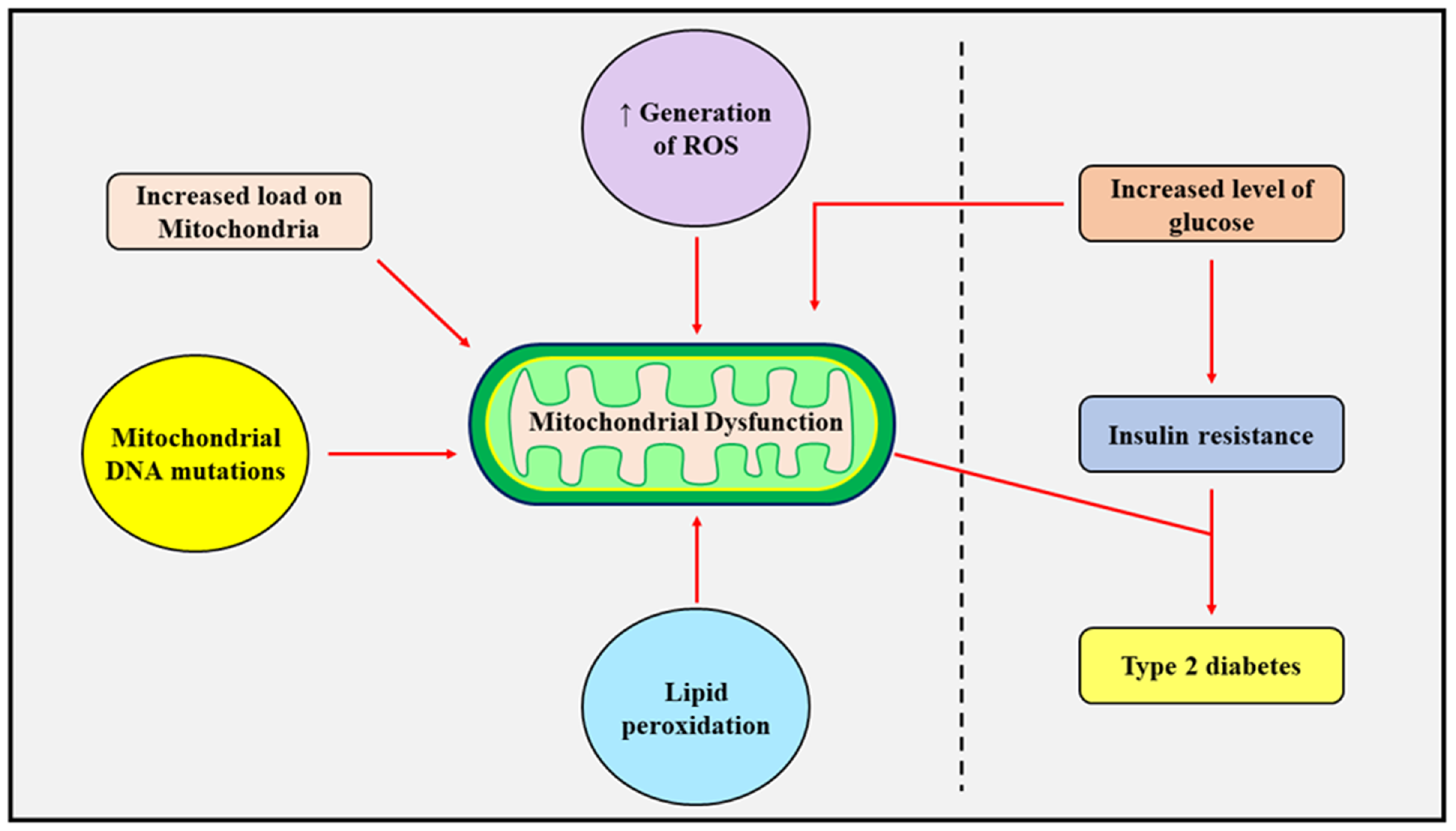
3.1. Insulin Resistance and Mitochondrial Dysfunction
3.2. Targeting of Mitochondrial Processes of Mitochondrial Fission and Mitochondrial Fusion to Prevent Mitochondrial Dysfunction
4. Pathophysiological Role of Mitochondrial Dysfunction in Obesity
4.1. Contribution of Mitochondrial Dysfunction in Consumption of Calories and ROS
4.2. Role of Mitochondria in Brown and White Adipose Tissues
4.3. Transcription Factors in Adipocytes and Mitochondria
5. Pathophysiological Role of Mitochondrial Dysfunction in Infectious Diseases
5.1. Infection Dynamics and Structure of Mitochondria
5.2. Metabolism of Mitochondria during Infection
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-DG | 2 deoxyglucose |
| ADP | adenosine diphosphate |
| ATP | adenosine triphosphate |
| BAT | black adipose tissues |
| DNA | deoxyribonucleic acid |
| DRP1 | dynamin-related protein 1 |
| ETC | electron transport chain |
| FADH2 | flavin adenine dinucleotide |
| FAO | fatty acid oxidation |
| FH | fumarate hydratase |
| FIS1 | mitochondrial fission 1 protein |
| HbA1c | hemoglobin A1c |
| HIF | Hypoxia-inducible factor |
| KEAP1 | kelch-like ECH associated protein 1 |
| LOF | loss of function |
| MAPK | mitogen activated protein kinase |
| Mdivi-1 | mitochondrial division inhibitor-1 |
| Mfn | mitofusins |
| MPT | mitochondrial permeability transition |
| mtDNA | mitochondrial deoxyribonucleic acid |
| Myf5 | myogenic factor 5 |
| NADH | nicotinamide adenine dinucleotide |
| NEFA | nonesterified fatty acids |
| NF-κB | nuclear factor kappa B |
| NLRP3 | NLR family pyrin domain containing 3 |
| Nox 4 | NADPH oxidase-4 |
| Nrf2 | nuclear factor erythroid 2-relaed factor 2 |
| PDH | pyruvate dehydrogenase |
| PGC | PPAR gamma coactivator |
| PHD | prolyl hydroxylase |
| PINK1 | PTEN induced kinase 1 |
| PPAR | peroxisome proliferator activated receptor |
| PRDM16 | PRD1-BF-1-R1Z1 homologous domain comprising protein 16 |
| PTEN | phosphatase and tensin homolog |
| ROS | reactive oxygen species |
| rRNA | ribosomal ribonucleic acid |
| SDH | succinate hydrogenase |
| TCA | tricarboxylic acid |
| TGF-β1 | transforming growth factor beta 1 |
| tRNA | transfer ribonucleic acid |
| UCP | uncoupling proteins |
| VES | vitamin E succinate |
| WAT | white adipose tissues |
| α-KG | alpha-ketoglutarate |
References
- Marchi, S.; Guilbaud, E.; Tait, S.W.G.; Yamazaki, T.; Galluzzi, L. Mitochondrial Control of Inflammation. Nat. Rev. Immunol. 2022, 23, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Reyes, I.; Diebold, L.P.; Kong, H.; Schieber, M.; Huang, H.; Hensley, C.T.; Mehta, M.M.; Wang, T.; Santos, J.H.; Woychik, R.; et al. TCA Cycle and Mitochondrial Membrane Potential Are Necessary for Diverse Biological Functions. Mol. Cell 2015, 61, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial Electron Transport Chain: Oxidative Phosphorylation, Oxidant Production, and Methods of Measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef] [PubMed]
- Vella, F. The cell. A Molecular Approach. Biochem. Educ. 1998, 26, 98–99. [Google Scholar] [CrossRef]
- Xia, M.; Zhang, Y.; Jin, K.; Lu, Z.; Zeng, Z.; Xiong, W. Communication between Mitochondria and Other Organelles: A Brand-New Perspective on Mitochondria in Cancer. Cell Biosci. 2019, 9, 27. [Google Scholar] [CrossRef]
- Chen, H.; Chan, D.C. Emerging Functions of Mammalian Mitochondrial Fusion and Fission. Hum. Mol. Genet. 2005, 14, R283–R289. [Google Scholar] [CrossRef]
- Jin, J.-Y.; Wei, X.-X.; Zhi, X.-L.; Wang, X.-H.; Meng, D. Drp1-Dependent Mitochondrial Fission in Cardiovascular Disease. Acta Pharmacol. Sin. 2020, 42, 655–664. [Google Scholar] [CrossRef]
- Olichon, A.; Landes, T.; Arnauné-Pelloquin, L.; Emorine, L.J.; Mils, V.; Guichet, A.; Delettre, C.; Hamel, C.; Amati-Bonneau, P.; Bonneau, D.; et al. Effects of OPA1 mutations on mitochondrial morphology and apoptosis: Relevance to ADOA pathogenesis. J. Cell. Physiol. 2006, 211, 423–430. [Google Scholar] [CrossRef]
- Quiles, J.M.; Gustafsson, B. The Role of Mitochondrial Fission in Cardiovascular Health and Disease. Nat. Rev. Cardiol. 2022, 19, 723–736. [Google Scholar] [CrossRef]
- Kulkarni, P.G.; Mohire, V.M.; Bhaisa, P.K.; Joshi, M.M.; Puranik, C.M.; Waghmare, P.P.; Banerjee, T. Mitofusin-2: Functional Switch between Mitochondrial Function and Neurodegeneration. Mitochondrion 2023, 69, 116–129. [Google Scholar] [CrossRef]
- Kim, S.-J.; Cheresh, P.; Jablonski, R.P.; Williams, D.B.; Kamp, D.W. The Role of Mitochondrial DNA in Mediating Alveolar Epithelial Cell Apoptosis and Pulmonary Fibrosis. Int. J. Mol. Sci. 2015, 16, 21486–21519. [Google Scholar] [CrossRef] [PubMed]
- Sabouny, R.; Shutt, T.E. Reciprocal Regulation of Mitochondrial Fission and Fusion. Trends Biochem. Sci. 2020, 45, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Kamenisch, Y.; Fousteri, M.; Knoch, J.; Von Thaler, A.-K.; Fehrenbacher, B.; Kato, H.; Becker, T.; Dollé, M.E.; Kuiper, R.; Majora, M.; et al. Proteins of Nucleotide and Base Excision Repair Pathways Interact in Mitochondria to Protect From Loss of Subcutaneous Fat, a Hallmark of Aging. J. Exp. Med. 2010, 207, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Valiente-Pallejà, A.; Tortajada, J.; Bulduk, B.K.; Vilella, E.; Garrabou, G.; Muntané, G.; Martorell, L. Comprehensive Summary of Mitochondrial DNA Alterations in the Postmortem Human Brain: A Systematic Review. Ebiomedicine 2022, 76, 103815. [Google Scholar] [CrossRef]
- Toews, D.P.L.; Brelsford, A. The Biogeography of Mitochondrial and Nuclear Discordance in Animals. Mol. Ecol. 2012, 21, 3907–3930. [Google Scholar] [CrossRef]
- Brischigliaro, M.; Fernandez-Vizarra, E.; Viscomi, C. Mitochondrial Neurodegeneration: Lessons from Drosophila melanogaster Models. Biomolecules 2023, 13, 378. [Google Scholar] [CrossRef]
- Shokolenko, I.; Alexeyev, M. Mitochondrial DNA: Consensuses and Controversies. DNA 2022, 2, 131–148. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Du, G.-H.; Zhang, J.-T. Assay of Mitochondrial Functions by Resazurin in Vitro. Acta Pharmacol. Sin. 2004, 25, 385–389. [Google Scholar]
- Kumar, A.; Raj, V.; Srivastava, A.; Ali, M.; Ghosh, A.K.; Rachamalla, M.; Kumar, D. Autophagy in Arsenic Exposed Population and Cancer Patients. In Autophagy and Metabolism; Academic Press: Cambridge, MA, USA, 2022; pp. 141–161. [Google Scholar] [CrossRef]
- Tuppen, H.A.; Blakely, E.L.; Turnbull, D.M.; Taylor, R.W. Mitochondrial DNA Mutations and Human Disease. Biochim. Biophys. Acta (BBA)-Bioenerg. 2010, 1797, 113–128. [Google Scholar] [CrossRef]
- Wei, W.; Tuna, S.; Keogh, M.J.; Smith, G.; Aitman, T.J.; Beales, P.L.; Bennett, D.L.; Gale, D.P.; Bitner-Glindzicz, M.A.K.; Black, G.C.; et al. Germline selection shapes human mitochondrial DNA diversity. Science 2019, 364, 6520. [Google Scholar] [CrossRef]
- Cannino, G.; Ciscato, F.; Masgras, I.; Martin, C.S.; Rasola, A. Metabolic Plasticity of Tumor Cell Mitochondria. Front. Oncol. 2018, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, R.J.; Singh, R.; Brewer, G.; Auger, C.; Lemire, J.; Appanna, V.D. α-Ketoglutarate Dehydrogenase and Glutamate Dehydrogenase Work in Tandem To Modulate the Antioxidant α-Ketoglutarate during Oxidative Stress in Pseudomonas fluorescens. J. Bacteriol. 2009, 191, 3804–3810. [Google Scholar] [CrossRef] [PubMed]
- Puttonen, K.A.; Lehtonen, Š.; Raasmaja, A.; Männistö, P.T. A Prolyl Oligopeptidase Inhibitor, Z-Pro-Prolinal, Inhibits Glyceraldehyde-3-Phosphate Dehydrogenase Translocation and Production of Reactive Oxygen Species in CV1-P Cells Exposed to 6-Hydroxydopamine. Toxicol. Vitr. 2006, 20, 1446–1454. [Google Scholar] [CrossRef]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in Cancer: Initiators, Amplifiers or an Achilles’ Heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Panieri, E.; Santoro, M.M. ROS Homeostasis and Metabolism: A Dangerous Liason in Cancer Cells. Cell Death Dis. 2016, 7, e2253. [Google Scholar] [CrossRef] [PubMed]
- Vatrinet, R.; Leone, G.; De Luise, M.; Girolimetti, G.; Vidone, M.; Gasparre, G.; Porcelli, A.M. The α-Ketoglutarate Dehydrogenase Complex in Cancer Metabolic Plasticity. Cancer Metab. 2017, 5, 3. [Google Scholar] [CrossRef]
- Pecinová, A.; Alán, L.; Brázdová, A.; Vrbacký, M.; Pecina, P.; Drahota, Z.; Houštěk, J.; Mráček, T. Role of Mitochondrial Glycerol-3-Phosphate Dehydrogenase in Metabolic Adaptations of Prostate Cancer. Cells 2020, 9, 1764. [Google Scholar] [CrossRef]
- De Santis, M.C.; Porporato, P.E.; Martini, M.; Morandi, A. Signaling Pathways Regulating Redox Balance in Cancer Metabolism. Front. Oncol. 2018, 8, 126. [Google Scholar] [CrossRef]
- Wang, S.-F.; Chen, S.; Tseng, L.-M.; Lee, H.-C. Role of the Mitochondrial Stress Response in Human Cancer Progression. Exp. Biol. Med. 2020, 245, 861–878. [Google Scholar] [CrossRef]
- Adam, J.; Hatipoglu, E.; O’Flaherty, L.; Ternette, N.; Sahgal, N.; Lockstone, H.; Baban, D.; Nye, E.; Stamp, G.W.; Wolhuter, K.; et al. Renal Cyst Formation in Fh1-Deficient Mice Is Independent of the Hif/Phd Pathway: Roles for Fumarate in KEAP1 Succination and Nrf2 Signaling. Cancer Cell 2011, 20, 524–537. [Google Scholar] [CrossRef]
- Sajnani, K.; Islam, F.; Smith, R.A.; Gopalan, V.; Lam, A.K.-Y. Genetic Alterations in Krebs Cycle and Its Impact on Cancer Pathogenesis. Biochimie 2017, 135, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Boland, M.L.; Chourasia, A.H.; MacLeod, K.F. Mitochondrial Dysfunction in Cancer. Front. Oncol. 2013, 3, 292. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.Y.; Tay, E.X.Y.; Ong, D.S.T.; Taneja, R. Mitochondrial Dysfunction at the Center of Cancer Therapy. Antioxid. Redox Signal. 2020, 32, 309–330. [Google Scholar] [CrossRef]
- Chen, R.; Lai, U.H.; Zhu, L.; Singh, A.; Ahmed, M.; Forsyth, N.R. Reactive Oxygen Species Formation in the Brain at Different Oxygen Levels: The Role of Hypoxia Inducible Factors. Front. Cell Dev. Biol. 2018, 6, 132. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, A.S.; Alswailem, M.; Alghamdi, B.; Al-Hindi, H. Fumarate Hydratase Is a Novel Gene for Familial Non-Medullary Thyroid Cancer. J. Clin. Endocrinol. Metab. 2022, 107, 2539–2544. [Google Scholar] [CrossRef]
- Murugan, A.K.; Alzahrani, A.S. Isocitrate Dehydrogenase IDH1 and IDH2 Mutations in Human Cancer: Prognostic Implications for Gliomas. Br. J. Biomed. Sci. 2022, 79, 10208. [Google Scholar] [CrossRef]
- Jo, H.; Park, Y.; Kim, T.; Kim, J.; Lee, J.S.; Kim, S.Y.; Chung, J.I.; Ko, H.Y.; Pyun, J.C.; Kim, K.S.; et al. Modulation of SIRT3 Expression through CDK4/6 Enhances the Anti-Cancer Effect of Sorafenib in Hepatocellular Carcinoma Cells. BMC Cancer 2020, 20, 332. [Google Scholar] [CrossRef]
- Missiroli, S.; Perrone, M.; Genovese, I.; Pinton, P.; Giorgi, C. Cancer Metabolism and Mitochondria: Finding Novel Mechanisms to Fight Tumours. Ebiomedicine 2020, 59, 102943. [Google Scholar] [CrossRef]
- Kim, Y.S.; Vallur, P.G.; Jones, V.M.; Worley, B.L.; Shimko, S.; Shin, D.-H.; Crawford, L.C.; Chen, C.-W.; Aird, K.M.; Abraham, T.; et al. Context-Dependent Activation of SIRT3 Is Necessary for Anchorage-Independent Survival and Metastasis of Ovarian Cancer Cells. Oncogene 2019, 39, 1619–1633. [Google Scholar] [CrossRef]
- Schmitt, S.; Zischka, H. Targeting Mitochondria for Cancer Therapy. Dtsch. Z. Fur Onkol. 2018, 50, 124–130. [Google Scholar] [CrossRef]
- Liu, X.; Romero, I.L.; Litchfield, L.M.; Lengyel, E.; Locasale, J.W. Metformin Targets Central Carbon Metabolism and Reveals Mitochondrial Requirements in Human Cancers. Cell Metab. 2016, 24, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Woodgate, R.; McManus, T.P.; Mead, S.; McCormick, J.J.; Maher, V.M. Evidence That in Xeroderma Pigmentosum Variant Cells, Which Lack DNA Polymerase η, DNA Polymerase ι Causes the Very High Frequency and Unique Spectrum of UV-Induced Mutations. Cancer Res. 2007, 67, 3018–3026. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Ju, R.; Gao, H.; Huang, Y.; Guo, L.; Zhang, D. Targeting Glutamine Utilization to Block Metabolic Adaptation of Tumor Cells under the Stress of Carboxyamidotriazole-Induced Nutrients Unavailability. Acta Pharm. Sin. B 2021, 12, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.H.; Park, K.S.; Lee, K.-U.; Lee, H.K. Mitochondrial Metabolism and Diabetes. J. Diabetes Investig. 2010, 1, 161–169. [Google Scholar] [CrossRef]
- Wang, C.-H.; Wei, Y.-H. Role of Mitochondrial Dysfunction and Dysregulation of Ca2+ Homeostasis in the Pathophysiology of Insulin Resistance and Type 2 Diabetes. J. Biomed. Sci. 2017, 24, 70. [Google Scholar] [CrossRef]
- Dong, L.-F.; Jameson, V.J.; Tilly, D.; Cerny, J.; Mahdavian, E.; Marín-Hernández, A.; Hernández-Esquivel, L.; Rodríguez-Enríquez, S.; Stursa, J.; Witting, P.K.; et al. Mitochondrial Targeting of Vitamin E Succinate Enhances Its Pro-apoptotic and Anti-cancer Activity via Mitochondrial Complex II. J. Biol. Chem. 2011, 286, 3717–3728. [Google Scholar] [CrossRef]
- Kim, J.; Wei, Y.; Sowers, J.R. Role of Mitochondrial Dysfunction in Insulin Resistance. Circ. Res. 2008, 102, 401–414. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Q.; Zhao, D.; Lian, F.; Li, X.; Qi, W. The Impact of Oxidative Stress-Induced Mitochondrial Dysfunction on Diabetic Microvascular Complications. Front. Endocrinol. 2023, 14, 1112363. [Google Scholar] [CrossRef]
- Pagel-Langenickel, I.; Bao, J.; Pang, L.; Sack, M.N. The Role of Mitochondria in the Pathophysiology of Skeletal Muscle Insulin Resistance. Endocr. Rev. 2009, 31, 25–51. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Orekhova, V.A.; Baig, M.S.; Bezsonov, E.E.; Starodubova, A.V.; Popkova, T.V.; Orekhov, A.N. The Role of Mitochondrial Mutations and Chronic Inflammation in Diabetes. Int. J. Mol. Sci. 2021, 22, 6733. [Google Scholar] [CrossRef]
- Takano, C.; Ogawa, E.; Hayakawa, S. Insulin Resistance in Mitochondrial Diabetes. Biomolecules 2023, 13, 126. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.F.; Hofmann, S.; Gerbitz, K.-D. Mitochondrial Dysfunction in Diabetes Mellitus. Adv. Cell Aging Gerontol. 2001, 7, 55–101. [Google Scholar] [CrossRef]
- Fletcher, J.A.; Deja, S.; Satapati, S.; Fu, X.; Burgess, S.C.; Browning, J.D. Impaired Ketogenesis and Increased Acetyl-CoA Oxidation Promote Hyperglycemia in Human Fatty Liver. J. Clin. Investig. 2019, 4, e127737. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zhang, S.; Li, J.; Liu, K.; Huang, F.; Liu, B. Metformin and Resveratrol Inhibit Drp1-Mediated Mitochondrial Fission and Prevent ER Stress-Associated NLRP3 Inflammasome Activation in the Adipose Tissue of Diabetic Mice. Mol. Cell. Endocrinol. 2016, 434, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Ritz, P.; Berrut, G. Mitochondrial Function, Energy Expenditure, Aging and Insulin Resistance. Diabetes Metab. 2005, 31, 5S67–5S73. [Google Scholar] [CrossRef]
- Diniz, M.S.; Tocantins, C.; Grilo, L.F.; Pereira, S.P. The Bitter Side of Sugar Consumption: A Mitochondrial Perspective on Diabetes Development. Diabetology 2022, 3, 583–595. [Google Scholar] [CrossRef]
- Kolac, U.K.; Eken, M.K.; Ünübol, M.; Yalcin, G.D.; Yalcin, A. The Effect of Gestational Diabetes on the Expression of Mitochondrial Fusion Proteins in Placental Tissue. Placenta 2021, 115, 106–114. [Google Scholar] [CrossRef]
- Pinti, M.V.; Fink, G.K.; Hathaway, Q.A.; Durr, A.J.; Kunovac, A.; Hollander, J.M. Mitochondrial Dysfunction in Type 2 Diabetes Mellitus: An Organ-Based Analysis. Am. J. Physiol. Metab. 2019, 316, E268–E285. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Sadykhov, N.K.; Kartuesov, A.G.; Borisov, E.E.; Sukhorukov, V.N.; Orekhov, A.N. The Role of Mitochondrial Abnormalities in Diabetic Cardiomyopathy. Int. J. Mol. Sci. 2022, 23, 7863. [Google Scholar] [CrossRef]
- Hesselink, M.K.C.; Schrauwen-Hinderling, V.; Schrauwen, P. Skeletal Muscle Mitochondria as a Target to Prevent or Treat Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2016, 12, 633–645. [Google Scholar] [CrossRef]
- Wada, J.; Nakatsuka, A. Mitochondrial Dynamics and Mitochondrial Dysfunction in Diabetes. Acta Med. Okayama 2016, 70, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Bournat, J.C.; Brown, C.W. Mitochondrial Dysfunction in Obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 446. [Google Scholar] [CrossRef] [PubMed]
- Preta, G.; Cronin, J.G.; Sheldon, I.M. Dynasore—Not Just a Dynamin Inhibitor. Cell Commun. Signal. 2015, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Baig, A.A.; Hussain, M.; Saeed, M.U.; Bilal, M.; Ahmed, N.; Chopra, H.; Hassan, M.; Rachamalla, M.; Putnala, S.K.; et al. Narrative on Hydrogen Therapy and Its Clinical Applications: Safety and Efficacy. Curr. Pharm. Des. 2022, 28, 2519–2537. [Google Scholar] [CrossRef]
- Singh, R.; Braga, M.; Reddy, S.T.; Lee, S.-J.; Parveen, M.; Grijalva, V.; Vergnes, L.; Pervin, S. Follistatin Targets Distinct Pathways To Promote Brown Adipocyte Characteristics in Brown and White Adipose Tissues. Endocrinology 2017, 158, 1217–1230. [Google Scholar] [CrossRef]
- Richard, A.J.; White, U.; Elks, C.M.; Stephens, J.M. Adipose Tissue: Physiology to Metabolic Dysfunction. In Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2020. [Google Scholar]
- Das, M.; Sauceda, C.; Webster, N.J.G. Mitochondrial Dysfunction in Obesity and Reproduction. Endocrinology 2020, 162, bqaa158. [Google Scholar] [CrossRef]
- Bhatraju, N.K.; Agrawal, A. Mitochondrial Dysfunction Linking Obesity and Asthma. Ann. Am. Thorac. Soc. 2017, 14, S368–S373. [Google Scholar] [CrossRef]
- Yin, X.; Lanza, I.R.; Swain, J.M.; Sarr, M.G.; Nair, K.S.; Jensen, M.D. Adipocyte Mitochondrial Function Is Reduced in Human Obesity Independent of Fat Cell Size. J. Clin. Endocrinol. Metab. 2014, 99, E209–E216. [Google Scholar] [CrossRef]
- Kusminski, C.M.; Scherer, P.E. Mitochondrial dysfunction in white adipose tissue. Trends Endocrinol. Metab. 2012, 23, 435–443. [Google Scholar] [CrossRef]
- Medina-Gómez, G. Mitochondria and Endocrine Function of Adipose Tissue. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 791–804. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; Huang, H.; Wei, Y. Mitochondrial Dysfunction Leads to Impairment of Insulin Sensitivity and Adiponectin Secretion in Adipocytes. FEBS J. 2012, 280, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Boudina, S.; Graham, T.E. Mitochondrial Function/Dysfunction in White Adipose Tissue. Exp. Physiol. 2014, 99, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Demine, S.; Renard, P.; Arnould, T. Mitochondrial Uncoupling: A Key Controller of Biological Processes in Physiology and Diseases. Cells 2019, 8, 795. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Yuan, L.; Yu, H.; Xi, Y.; Xiao, R. Mitochondrial Dysfunction and Oxidative Damage in the Brain of Diet-Induced Obese Rats But Not in Diet-Resistant Rats. Life Sci. 2014, 110, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Armeni, T.; Principato, G.; Quiles, J.L.; Pieri, C.; Bompadre, S.; Battino, M. Mitochondrial Dysfunctions during Aging: Vitamin E Deficiency or Caloric Restriction–Two Different Ways of Modulating Stress. J. Bioenerg. Biomembr. 2003, 35, 181–191. [Google Scholar] [CrossRef]
- Cedikova, M.; Kripnerová, M.; Dvorakova, J.; Pitule, P.; Grundmanova, M.; Babuska, V.; Mullerova, D.; Kuncova, J. Mitochondria in White, Brown, and Beige Adipocytes. Stem Cells Int. 2016, 2016, 6067349. [Google Scholar] [CrossRef]
- Saely, C.H.; Geiger, K.; Drexel, H. Brown versus White Adipose Tissue: A Mini-Review. Gerontology 2012, 58, 15–23. [Google Scholar] [CrossRef]
- Cinti, S. The Role of Brown Adipose Tissue in Human Obesity. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 569–574. [Google Scholar] [CrossRef]
- Masschelin, P.M.; Cox, A.R.; Chernis, N.; Hartig, S.M. The Impact of Oxidative Stress on Adipose Tissue Energy Balance. Front. Physiol. 2020, 10, 1638. [Google Scholar] [CrossRef]
- Sidossis, L.; Kajimura, S. Brown and Beige Fat in Humans: Thermogenic Adipocytes That Control Energy and Glucose Homeostasis. J. Clin. Investig. 2015, 125, 478–486. [Google Scholar] [CrossRef]
- Shinde, A.B.; Song, A.; Wang, Q.A. Brown Adipose Tissue Heterogeneity, Energy Metabolism, and Beyond. Front. Endocrinol. 2021, 12, 651763. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M.; Sanchez-Gurmaches, J.; Guertin, D.A. Brown Adipose Tissue Development and Metabolism. In Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2019; Volume 251. [Google Scholar]
- Becerril, S.; Gómez-Ambrosi, J.; Martín, M.; Moncada, R.; Sesma, P.; Burrell, M.A.; Frühbeck, G. Role of PRDM16 in the Activation of Brown fat Programming. Relevance to the Development of Obesity. Histol. Histopathol. 2013, 28, 1411–1425. [Google Scholar] [PubMed]
- Kawano, J.; Arora, R. The Role of Adiponectin in Obesity, Diabetes, and Cardiovascular Disease. J. CardioMetabolic Syndr. 2009, 4, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, A.; Oh, K.-J.; Lee, S.C.; Kim, W.K.; Bae, K.-H. The Role of Adipose Tissue Mitochondria: Regulation of Mitochondrial Function for the Treatment of Metabolic Diseases. Int. J. Mol. Sci. 2019, 20, 4924. [Google Scholar] [CrossRef]
- Sun, C.; Mao, S.; Chen, S.; Zhang, W.; Liu, C. PPARs-Orchestrated Metabolic Homeostasis in the Adipose Tissue. Int. J. Mol. Sci. 2021, 22, 8974. [Google Scholar] [CrossRef]
- Elesela, S.; Lukacs, N. Role of Mitochondria in Viral Infections. Life 2021, 11, 232. [Google Scholar] [CrossRef]
- Jiang, N.; Yang, M.; Han, Y.; Zhao, H.; Sun, L. PRDM16 Regulating Adipocyte Transformation and Thermogenesis: A Promising Therapeutic Target for Obesity and Diabetes. Front. Pharmacol. 2022, 13, 870250. [Google Scholar] [CrossRef]
- Khan, M.; Syed, G.H.; Kim, S.-J.; Siddiqui, A. Mitochondrial Dynamics and Viral Infections: A Close Nexus. Biochim. et Biophys. Acta (BBA)-Mol. Cell Res. 2015, 1853, 2822–2833. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, X.; Ding, T.; Zhong, Z.; Hu, H.; Xu, Z.; Deng, J. Mitochondrial Dynamics Imbalance: A Strategy for Promoting Viral Infection. Front. Microbiol. 2020, 11, 1992. [Google Scholar] [CrossRef]
- Lartigue, L.; Faustin, B. Mitochondria: Metabolic Regulators of Innate Immune Responses to Pathogens and Cell Stress. Int. J. Biochem. Cell Biol. 2013, 45, 2052–2056. [Google Scholar] [CrossRef]
- Reshi, L.; Wang, H.-V.; Hong, J.-R. Modulation of Mitochondria During Viral Infections. In Mitochondrial Diseases; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Ana, Y.; Marquez, J.R.; Fozzatti, L.; Baigorrí, R.; Marin, C.; Maletto, B.; Cerbán, F.; Radi, R.; Piacenza, L.; Stempin, C. An Exacerbated Metabolism and Mitochondrial Reactive Oxygen Species Contribute to Mitochondrial Alterations and Apoptosis in CD4 T Cells during the Acute Phase of Trypanosoma Cruzi Infection. Free Radic. Biol. Med. 2020, 163, 268–280, Corrigendum in Free Radic. Biol. Med. 2021, 166, 358–359. [Google Scholar] [CrossRef] [PubMed]
- Escoll, P.; Platon, L.; Buchrieser, C. Roles of Mitochondrial Respiratory Complexes during Infection. Immunometabolism 2019, 1, e190011. [Google Scholar] [CrossRef]
- Soto-Heredero, G.; de las Heras, M.M.G.; Gabandé-Rodríguez, E.; Oller, J.; Mittelbrunn, M. Glycolysis—A Key Player in the Inflammatory Response. FEBS J. 2020, 287, 3350–3369. [Google Scholar] [CrossRef] [PubMed]
- Spier, A.; Connor, M.G.; Steiner, T.; Carvalho, F.; Cossart, P.; Eisenreich, W.; Wai, T.; Stavru, F. Mitochondrial Respiration Restricts Listeria Monocytogenes infection by Slowing Down Host Cell Receptor Recycling. Cell Rep. 2021, 37, 109989. [Google Scholar] [CrossRef]
- Ledur, P.F.; Karmirian, K.; Pedrosa, C.D.S.G.; Souza, L.R.Q.; Assis-de-Lemos, G.; Martins, T.M.; Ferreira, J.D.C.C.G.; Reis, G.F.D.A.; Silva, E.S.; Silva, D.; et al. Zika Virus Infection Leads to Mitochondrial Failure, Oxidative Stress and DNA Damage in Human iPSC-Derived Astrocytes. Sci. Rep. 2020, 10, 1218. [Google Scholar] [CrossRef]
- Hardwick, J.M.; Soane, L. Multiple Functions of BCL-2 Family Proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a008722. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behl, T.; Makkar, R.; Anwer, M.K.; Hassani, R.; Khuwaja, G.; Khalid, A.; Mohan, S.; Alhazmi, H.A.; Sachdeva, M.; Rachamalla, M. Mitochondrial Dysfunction: A Cellular and Molecular Hub in Pathology of Metabolic Diseases and Infection. J. Clin. Med. 2023, 12, 2882. https://doi.org/10.3390/jcm12082882
Behl T, Makkar R, Anwer MK, Hassani R, Khuwaja G, Khalid A, Mohan S, Alhazmi HA, Sachdeva M, Rachamalla M. Mitochondrial Dysfunction: A Cellular and Molecular Hub in Pathology of Metabolic Diseases and Infection. Journal of Clinical Medicine. 2023; 12(8):2882. https://doi.org/10.3390/jcm12082882
Chicago/Turabian StyleBehl, Tapan, Rashita Makkar, Md. Khalid Anwer, Rym Hassani, Gulrana Khuwaja, Asaad Khalid, Syam Mohan, Hassan A. Alhazmi, Monika Sachdeva, and Mahesh Rachamalla. 2023. "Mitochondrial Dysfunction: A Cellular and Molecular Hub in Pathology of Metabolic Diseases and Infection" Journal of Clinical Medicine 12, no. 8: 2882. https://doi.org/10.3390/jcm12082882
APA StyleBehl, T., Makkar, R., Anwer, M. K., Hassani, R., Khuwaja, G., Khalid, A., Mohan, S., Alhazmi, H. A., Sachdeva, M., & Rachamalla, M. (2023). Mitochondrial Dysfunction: A Cellular and Molecular Hub in Pathology of Metabolic Diseases and Infection. Journal of Clinical Medicine, 12(8), 2882. https://doi.org/10.3390/jcm12082882









