Psychiatric Symptoms in Patients with Cerebral Endometriosis: A Case Report and Literature Review
Abstract
1. Introduction
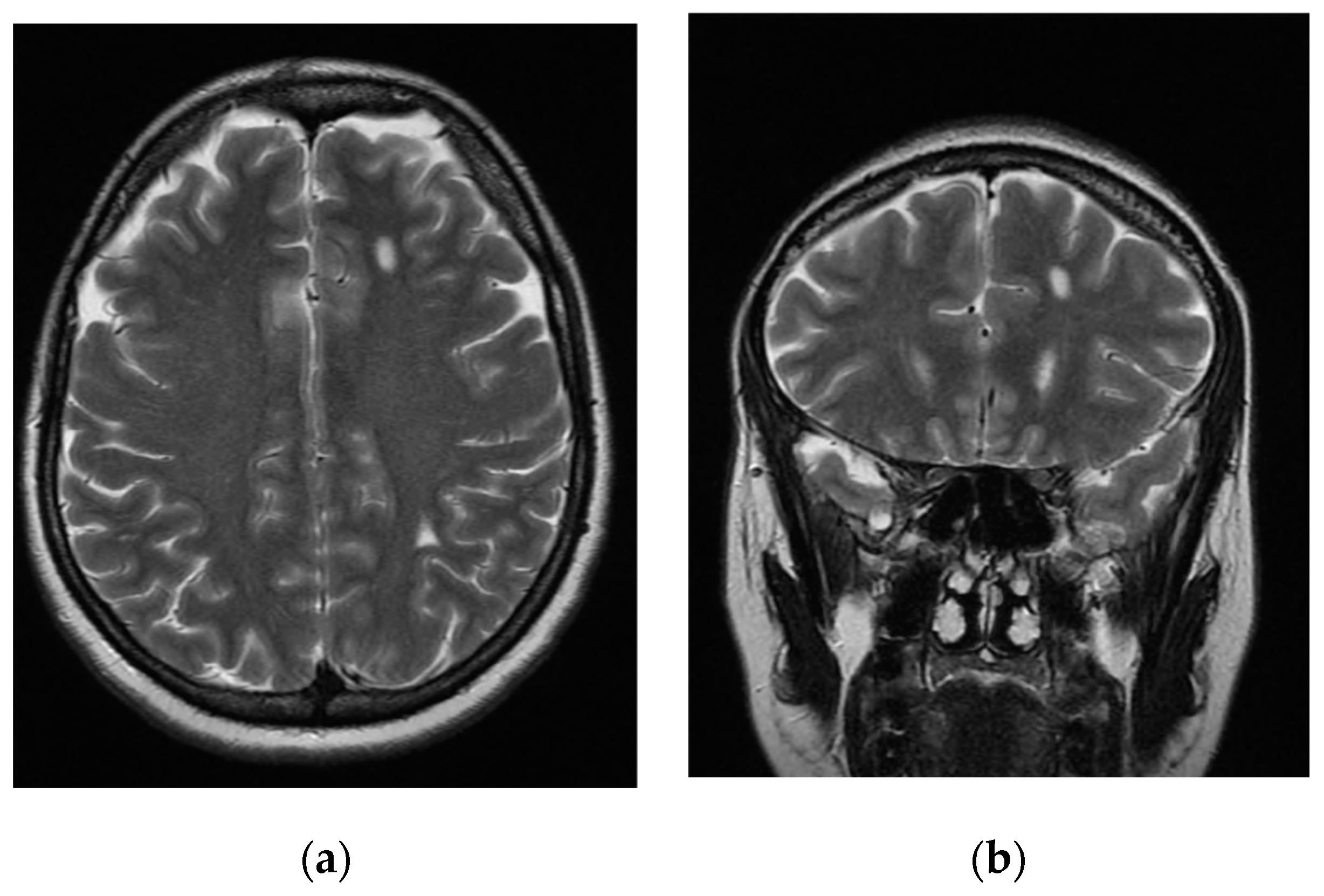
2. Case Report
3. Literature Review
3.1. Materials and Methods
3.1.1. Search
3.1.2. Eligibility Criteria
3.1.3. Abstract Screening and Study Selection
3.2. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koninckx, P.R.; Fernandes, R.; Ussia, A.; Schindler, L.; Wattiez, A.; Al-Suwaidi, S.; Amro, B.; Al-Maamari, B.; Hakim, Z.; Tahlak, M. Pathogenesis Based Diagnosis and Treatment of Endometriosis. Front. Endocrinol. 2021, 12, 745548. [Google Scholar] [CrossRef] [PubMed]
- Dmowski, W.P.; Lesniewicz, R.; Rana, N.; Pepping, P.; Noursalehi, M. Changing trends in the diagnosis of endometriosis: A comparative study of women with pelvic endometriosis presenting with chronic pelvic pain or infertility. Fertil. Steril. 1997, 67, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Dun, E.C.; Kho, K.A.; Morozov, V.V.; Kearney, S.; Zurawin, J.L.; Nezhat, C.H. Endometriosis in adolescents. JSLS J. Soc. Laparoendosc. Surg. 2015, 19, e2015.00019. [Google Scholar] [CrossRef]
- Counseller, V.S.; Crenshaw, J.L.J. A clinical and surgical review of endometriosis. Am. J. Obstet. Gynecol. 1951, 62, 930–942. [Google Scholar] [CrossRef]
- Ozawa, Y.; Murakami, T.; Terada, Y.; Yaegashi, N.; Okamura, K.; Kuriyama, S.; Tsuji, I. Management of the pain associated with endometriosis: An update of the painful problems. Tohoku J. Exp. Med. 2006, 210, 175–288. [Google Scholar] [CrossRef]
- Acién, P.; Velasco, I. Endometriosis: A disease that remains enigmatic. ISRN Obstet. Gynecol. 2013, 2013, 242149. [Google Scholar] [CrossRef] [PubMed]
- Sonavane, S.K.; Kantawala, K.P.; Menias, C.O. Beyond the boundaries-endometriosis: Typical and atypical locations. Curr. Probl. Diagn. Radiol. 2011, 40, 219–232. [Google Scholar] [CrossRef]
- Jenkins, S.; Olive, D.L.; Haney, A.F. Endometriosis: Pathogenetic implications of the anatomic distribution. Obstet Gynecol. 1986, 67, 335–338. [Google Scholar] [PubMed]
- Nezhat, C.; Falik, R.; McKinney, S.; King, L.P. Pathophysiology and management of urinary tract endometriosis. Nat. Rev. Urol. 2017, 14, 359–372. [Google Scholar] [CrossRef]
- Machairiotis, N.; Stylianaki, A.; Dryllis, G.; Zarogoulidis, P.; Kouroutou, P.; Tsiamis, N.; Katsikogiannis, N.; Sarika, E.; Courcoutsakis, N.; Tsiouda, T.; et al. Extrapelvic endometriosis: A rare entity or an under diagnosed condition? Diagn. Pathol. 2013, 8, 194. [Google Scholar] [CrossRef]
- De Sousa, A.C.S.; Capek, S.; Amrami, K.K.; Spinner, R.J. Neural involvement in endometriosis: Review of anatomic distribution and mechanisms. Clin. Anat. 2015, 28, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Lukac, S.; Schmid, M.; Pfister, K.; Janni, W.; Schäffler, H.; Dayan, D. Extragenital Endometriosis in the Differential Diagnosis of Non-Gynecological Diseases. Dtsch. Arztebl. Int. 2022, 119, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Ek, M.; Roth, B.; Ekström, P.; Valentin, L.; Bengtsson, M.; Ohlsson, B. Gastrointestinal symptoms among endometriosis patients-A case-cohort study. BMC Womens Health 2015, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Macek, C. Neurological deficits, back pain tied to endometriosis. JAMA 1983, 249, 686. [Google Scholar] [CrossRef] [PubMed]
- Rafique, S.; Decherney, A.H. Medical Management of Endometriosis. Clin. Obstet. Gynecol. 2017, 60, 485–496. [Google Scholar] [CrossRef]
- Martone, S.; Troìa, L.; Marcolongo, P.; Luisi, S. Role of medical treatment of endometriosis. Minerva Obstet. Gynecol. 2021, 73, 304–316. [Google Scholar] [CrossRef]
- Guo, S.-W. Recurrence of endometriosis and its control. Hum. Reprod. Update 2009, 15, 441–461. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.M.; Gattrell, W.T.; Gude, K.; Singh, S.S. Reevaluating response and failure of medical treatment of endometriosis: A systematic review. Fertil. Steril. 2017, 108, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Mettler, L.; Ruprai, R.; Alkatout, I. Impact of medical and surgical treatment of endometriosis on the cure of endometriosis and pain. BioMed Res. Int. 2014, 2014, 264653. [Google Scholar] [CrossRef]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- As-Sanie, S.; Kim, J.; Schmidt-Wilcke, T.; Sundgren, P.C.; Clauw, D.J.; Napadow, V.; Harris, R.E. Functional Connectivity is Associated with Altered Brain Chemistry in Women With Endometriosis-Associated Chronic Pelvic Pain. J. Pain 2016, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- As-Sanie, S.; Harris, R.E.; Napadow, V.; Kim, J.; Neshewat, G.; Kairys, A.; Williams, D.; Clauw, D.J.; Schmidt-Wilcke, T. Changes in regional gray matter volume in women with chronic pelvic pain: A voxel-based morphometry study. Pain 2012, 153, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Maulitz, L.; Stickeler, E.; Stickel, S.; Habel, U.; Tchaikovski, S.N.; Chechko, N. Endometriosis, psychiatric comorbidities and neuroimaging: Estimating the odds of an endometriosis brain. Front. Neuroendocrinol. 2022, 65, 100988. [Google Scholar] [CrossRef]
- Gambadauro, P.; Carli, V.; Hadlaczky, G. Depressive symptoms among women with endometriosis: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2019, 220, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.G.; Campo, G.; Papaleo, E.; Marazziti, D.; Maremmani, I. The Importance of a Multi-Disciplinary Approach to the Endometriotic Patients: The Relationship between Endometriosis and Psychic Vulnerability. J. Clin. Med. 2021, 10, 1616. [Google Scholar] [CrossRef] [PubMed]
- Sanders, S.A.; Graham, C.A.; Bass, J.L.; Bancroft, J. A prospective study of the effects of oral contraceptives on sexuality and well-being and their relationship to discontinuation. Contraception 2001, 64, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Patten, S.B.; Barbui, C. Drug-induced depression: A systematic review to inform clinical practice. Psychother. Psychosom. 2004, 73, 207–215. [Google Scholar] [CrossRef]
- Cavaggioni, G.; Lia, C.; Resta, S.; Antonielli, T.; Panici, P.B.; Megiorni, F.; Porpora, M.G. Are Mood and Anxiety Disorders and Alexithymia Associated with Endometriosis? A Prelim. Study 2014, 2014, 786830. [Google Scholar] [CrossRef]
- Chen, S.F.; Yang, Y.C.; Hsu, C.Y.; Shen, Y.C. Risk of bipolar disorder in patients with endometriosis: A nationwide population-based cohort study. J. Affect. Disord. 2020, 270, 36–41. [Google Scholar] [CrossRef]
- Gao, M.; Koupil, I.; Sjöqvist, H.; Karlsson, H.; Lalitkumar, S.; Dalman, C.; Kosidou, K. Psychiatric comorbidity among women with endometriosis: Nationwide cohort study in Sweden. Am. J. Obstet. Gynecol. 2020, 223, 415.e1–415.e16. [Google Scholar] [CrossRef]
- Kumar, V.; Khan, M.; Vilos, G.A.; Sharma, V. Revisiting the Association Between Endometriosis and Bipolar Disorder. J. Obstet. Gynaecol. Canada 2011, 33, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.O.; Comite, F.; Mallouh, C.; Zadunaisky, L.; Hutchinson-Williams, K.; Cherksey, B.D.; Yeager, C. Bipolar mood disorder and endometriosis: Preliminary findings. Am. J. Psychiatry 1987, 144, 1588–1591. [Google Scholar] [CrossRef]
- Saha, R.; Jakhar, K. Oligodendroglioma presenting as chronic mania. Shanghai Arch. Psychiatry 2015, 27, 183–27185. [Google Scholar] [CrossRef]
- Madhusoodanan, S.; Ting, M.B.; Farah, T.; Ugur, U. Psychiatric aspects of brain tumors: A review. World J. Psychiatry 2015, 5, 273–285. [Google Scholar] [CrossRef]
- Wechsler, D. WAIS-IV: Wechsler Adult Intelligence Scale, 4th ed.; Pearson: London, UK, 2008. [Google Scholar]
- Orsini, A.; Pezzuti, L.; Hulbert, S. The Unitary Ability of IQ and Indexes in WAIS-IV. Eur. J. Psychol. Assess 2017, 33, 453–459. [Google Scholar] [CrossRef][Green Version]
- Pezzuti, L. The GAI and CPI in the Italian standardization of the WAIS-IV and their clinical implications. Appl. Psychol. Bull. 2016, 276, 19–38. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Duke, R.; Fawcett, P.; Booth, J. Recurrent subarachnoid hemorrhage due to endometriosis. Neurology 1995, 45, 1000–1002. [Google Scholar] [CrossRef]
- Thibodeau, L.L.; Prioleau, G.R.; Manuelidis, E.E.; Merino, M.J.; Heafner, M.D. Cerebral endometriosis. Case report. J. Neurosurg. 1987, 66, 609–610. [Google Scholar] [CrossRef]
- Ichida, M.; Gomi, A.; Hiranouchi, N.; Fujimoto, K.; Suzuki, K.; Yoshida, M.; Nokubi, M.; Masuzawa, T. A case of cerebral endometriosis causing catamenial epilepsy. Neurology 1993, 43, 2708–2709. [Google Scholar] [CrossRef]
- Sarma, D.; Iyengar, P.; Marotta, T.R.; Terbrugge, K.G.; Gentili, F.; Halliday, W. Cerebellar endometriosis. AJR Am. J. Roentgenol. 2004, 182, 1543–1546. [Google Scholar] [CrossRef] [PubMed]
- Vilos, G.A.; Hollett-Caines, J.; Abu-Rafea, B.; Ahmad, R.; Mazurek, M.F. Resolution of catamenial epilepsy after goserelin therapy and oophorectomy: Case report of presumed cerebral endometriosis. J. Minim. Invasive Gynecol. 2011, 18, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Maniglio, P.; Ricciardi, E.; Meli, F.; Tomao, F.; Peiretti, M.; Caserta, D. Complete remission of cerebral endometriosis with dienogest: A case report. Gynecol. Endocrinol. 2018, 34, 837–839. [Google Scholar] [CrossRef]
- Meggyesy, M.; Friese, M.; Gottschalk, J.; Kehler, U. Case Report of Cerebellar Endometriosis. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2020, 81, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Antonio, M.; Walter, A.; Giorgia, R.; Anna, C.; Luigi, A.; Domenico, I. A surgical solution for a catamenial epilepsy medical therapy-resistant: A presumed case of cerebral endometriosis? J. Endometr. Pelvic. Pain Disord. 2021, 13, 66–68. [Google Scholar] [CrossRef]
- Satzer, D.; Bond, D.J. Mania secondary to focal brain lesions: Implications for understanding the functional neuroanatomy of bipolar disorder. Bipolar. Disord. 2016, 18, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L. Frontal-Subcortical Circuits and Human Behavior. Arch. Neurol. 1993, 50, 873–880. [Google Scholar] [CrossRef]
- Barahona-Corrêa, J.B.; Cotovio, G.; Costa, R.M.; Ribeiro, R.; Velosa, A.; Silva, V.C.E.; Sperber, C.; Karnath, H.O.; Senova, S.; Oliveira-Maia, A.J. Right-sided brain lesions predominate among patients with lesional mania: Evidence from a systematic review and pooled lesion analysis. Transl. Psychiatry 2020, 10, 139. [Google Scholar] [CrossRef]
- Murai, T.; Fujimoto, S. Rapid cycling bipolar disorder after left temporal polar damage. Brain Inj. 2003, 17, 355–358. [Google Scholar] [CrossRef]
- Jampala, V.C.; Abrams, R. Mania secondary to left and right hemisphere damage. Am. J. Psychiatry 1983, 140, 1197–1199. [Google Scholar] [CrossRef]
- Elefante, C.; Brancati, G.E.; Torrigiani, S.; Amadori, S.; Ricciardulli, S.; Pistolesi, G.; Lattanzi, L.; Perugi, G. Bipolar Disorder and Manic-like Symptoms in Alzheimer’s, Vascular and Frontotemporal Dementia: A Systematic Review. Curr. Neuropharmacol. 2022; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
| Age | Gynecological History | Neurological Signs or Symptoms | Instrumental Examinations | Lesion Site(s) | Findings of the Lesion(s) | Histological Diagnosis | Catamenial Symptoms | Treatment | Remission | |
|---|---|---|---|---|---|---|---|---|---|---|
| Thibodeau et al., 1987 [40] | 20 | Normal menstrual history; 2 therapeutic abortions | Generalized seizure; 3-year history of 4–5 min occipital headaches | CT, EEG, cerebral angiography | One lesion in the posteroinferior parietal right lobe | Hypodense ring enhancing well circumscribed lesion on CT; moderate generalized slowing, especially over the right temporal area on EEG | Yes | No | Brain surgery and danazol 800 mg/day for 6 months | Yes |
| Ichida et al., 1993 [41] | 31 | Regular menses; 2 pregnancies | Partial seizures once every 30 min for around half of the first day of the cycle | CT, MRI, EEG, cerebral angiography | 2 lesions in precentral gyrus, 1 lesion in postcentral gyrus | 3 ring-enhancing lesions on CT; T2-weighted MRI images revealed central areas of decreased attenuation | Yes | Yes | Brain surgery and danazol 600 mg/day for 6 months | Yes |
| Sarma et al., 2004 [42] | 40 | No history of pelvic pain or infertility; 1 caesarean birth | Gait disturbance, headaches | CT, MRI | 1 multiloculated cystic mass arising from cerebellar vermis | Mild rim enhancement, shading sign, and fluid–fluid levels on MRI | Yes | No | Brain surgery | Yes |
| Vilos et al., 2011 [43] | 41 | Chronic pelvic pain, menorrhagia, dysmenorrhea, and myoma; 3 caesarean births; tubal ligation | Sensory motory abnormalities, pains on the face, paroxysmal headaches, focal seizures | CT, MRI, EEG | Circumscribed abnormality in the centrum semiovale | 1 brain lesion without significant gadolinium enhancement on MRI; no abnormalities on EEG | No | Yes | Endometrial balloon ablation, GnRH-a, antiepileptics, salpingo-oophorectomy | Yes |
| Maniglio et al., 2018 [44] | 39 | Pelvic endometriosis with dysmenorrhea, dyschezia, and chronic pain | Catamenial epilepsy with hallucinations | MRI | Cerebral hemosiderosis deposits in globus pallidus | Hemosiderin deposits | No | Yes | Progestin therapy, GnRH-a, dienogest | Yes |
| Meggyesy et al., 2020 [45] | 39 | Menorrhagia and amenorrhea | Chronic hydrocephalus, gait disturbances, epilepsy, progressive neurological impairment causing death | MRI | 1 single cyst in the fourth ventricle, then multiple infratentorial cysts | No fluid suggestive of intracystal bleeds on MRI | Yes | No | Brain surgery, hormonal treatment | No (cyst recurrence) |
| Antonio et al., 2021 [46] | 44 | Ovarian endometrioma, dysmenorrhea, pelvic pain, and dyschezia; 1 caesarean birth | Catamential epilepsy, ocular disorder, limb paraesthesia/hypoaesthesia | CT, MRI, EEG, liquor examination | Not found | Not found | No | Yes | Hormonal treatment, GnRH-a salpingo-oophorectomy, antiepileptics | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elefante, C.; Brancati, G.E.; Oragvelidze, E.; Lattanzi, L.; Maremmani, I.; Perugi, G. Psychiatric Symptoms in Patients with Cerebral Endometriosis: A Case Report and Literature Review. J. Clin. Med. 2022, 11, 7212. https://doi.org/10.3390/jcm11237212
Elefante C, Brancati GE, Oragvelidze E, Lattanzi L, Maremmani I, Perugi G. Psychiatric Symptoms in Patients with Cerebral Endometriosis: A Case Report and Literature Review. Journal of Clinical Medicine. 2022; 11(23):7212. https://doi.org/10.3390/jcm11237212
Chicago/Turabian StyleElefante, Camilla, Giulio Emilio Brancati, Elene Oragvelidze, Lorenzo Lattanzi, Icro Maremmani, and Giulio Perugi. 2022. "Psychiatric Symptoms in Patients with Cerebral Endometriosis: A Case Report and Literature Review" Journal of Clinical Medicine 11, no. 23: 7212. https://doi.org/10.3390/jcm11237212
APA StyleElefante, C., Brancati, G. E., Oragvelidze, E., Lattanzi, L., Maremmani, I., & Perugi, G. (2022). Psychiatric Symptoms in Patients with Cerebral Endometriosis: A Case Report and Literature Review. Journal of Clinical Medicine, 11(23), 7212. https://doi.org/10.3390/jcm11237212









