Resistance on the Latest Oral and Intravenous P2Y12 ADP Receptor Blockers in Patients with Acute Coronary Syndromes: Fact or Myth?
Abstract
1. Introduction
2. Methods
3. Insufficient Response to ADPRB and the Risk of Future Events in Patients with ACS
4. Novel-Generation ADPRB
4.1. Prasugrel
4.2. Ticagrelor
4.3. Cangrelor
5. Prasugrel Resistance in Patients with ACS
6. Ticagrelor Resistance in Patients with ACS
7. Cangrelor Resistance in Patients with ACS
8. Risk Factors of Novel ADPRB Resistance
9. How to Manage Insufficient Response to Novel ADPRB?
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.-J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef] [PubMed]
- Schüpke, S.; Neumann, F.J.; Menichelli, M.; Mayer, K.; Bernlochner, I.; Wöhrle, J.; Richardt, G.; Liebetrau, C.; Witzenbichler, B.; Antoniucci, D.; et al. Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2019, 381, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Stone, G.W.; Mahaffey, K.W.; Gibson, C.M.; Steg, P.G.; Hamm, C.W.; Price, M.J.; Leonardi, S.; Gallup, D.; Bramucci, E.; et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N. Engl. J. Med. 2013, 368, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Samoš, M.; Fedor, M.; Kovář, F.; Duraj, L.; Stančiaková, L.; Galajda, P.; Staško, J.; Kubisz, P.; Mokáň, M. Ticagrelor: A safe and effective approach for overcoming clopidogrel resistance in patients with stent thrombosis? Blood Coagul. Fibrinolysis 2016, 27, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Horovitz, A.; Pons, A.-C.; Leroux, L.; Casassus, F. First report of a subacute stent thrombosis in a prasugrel resistant patient successfully managed with ticagrelor. Platelets 2013, 25, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Musallam, A.; Lev, E.I.; Roguin, A. Stent thrombosis in a patient with high on-treatment platelet reactivity despite ticagrelor treatment. Eur. Heart J. Acute Cardiovasc. Care 2014, 4, 85–87. [Google Scholar] [CrossRef]
- Thomas, M.R.; Storey, R.F. Clinical significance of residual platelet reactivity in patients treated with platelet P2Y12 inhibitors. Vasc. Pharmacol. 2016, 84, 25–27. [Google Scholar] [CrossRef]
- Małek, Ł.A.; Spiewak, M.; Filipiak, K.J.; Grabowski, M.; Szpotańska, M.; Rosiak, M.; Główczyńska, R.; Imiela, T.; Huczek, Z.; Opolski, G. Persistent platelet activation is related to very early cardiovascular events in patients with acute coronary syndromes. Kardiol. Pol. 2007, 65, 40–45. [Google Scholar]
- Wang, L.; Wang, X.; Chen, F. Clopidogrel resistance is associated with long-term thrombotic events in patients implanted with drug-eluting stents. Drugs R D 2010, 10, 219–224. [Google Scholar] [CrossRef]
- Liang, Z.-Y.; Han, Y.-L.; Zhang, X.-L.; Li, Y.; Yan, C.-H.; Kang, J. The impact of gene polymorphism and high on-treatment platelet reactivity on clinical follow-up: Outcomes in patients with acute coronary syndrome after drug-eluting stent implantation. EuroIntervention 2013, 9, 316–327. [Google Scholar] [CrossRef]
- Costache, I.I.; Rusu, C.; Ivanov, I.; Popescu, R.; Petriş, A. Impact of clopidogrel response on the clinical evolution in patients with acute coronary syndromes. Rev. Med. Chir. Soc. Med. Nat. IASI 2012, 116, 962–967. [Google Scholar] [PubMed]
- Li, M.; Wang, H.; Xuan, L.; Shi, X.; Zhou, T.; Zhang, N.; Huang, Y. Associations between P2RY12 gene polymorphisms and risks of clopidogrel resistance and adverse cardiovascular events after PCI in patients with acute coronary syndrome. Medicine 2017, 96, e6553. [Google Scholar] [CrossRef] [PubMed]
- Guha, S.; Mookerjee, S.; Guha, P.; Sardar, P.; Deb, S.; Das Roy, P.; Karmakar, R.; Mani, S.; Hema, M.B.; Pyne, S.; et al. Antiplatelet drug resistance in patients with recurrent acute coronary syndrome undergoing conservative management. Indian Heart J. 2010, 61, 348–352. [Google Scholar]
- Samoš, M.; Šimonová, R.; Kovář, F.; Duraj, L.; Fedorová, J.; Galajda, P.; Staško, J.; Fedor, M.; Kubisz, P.; Mokáň, M. Clopidogrel resistance in diabetic patient with acute myocardial infarction due to stent thrombosis. Am. J. Emerg. Med. 2014, 32, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Dunn, S.P.; Marshall, H.M.; Cohen, M.G.; Smyth, S.S. A case report of simultaneous thrombosis of two coronary artery stents in association with clopidogrel resistance. Clin. Cardiol. 2007, 30, 200–203. [Google Scholar] [CrossRef]
- Schäfer, A.; Bonz, A.W.; Eigenthaler, M.; Bauersachs, J. Late thrombosis of a drug-eluting stent during combined anti-platelet therapy in a clopidogrel nonresponsive diabetic patient: Shall we routinely test platelet function? Thromb Haemost 2007, 97, 862–865. [Google Scholar]
- Jung, H.-J.; Sir, J.-J. Recurrent myocardial infarction due to one subacute and two very late thrombotic events of drug-eluting stent associated with clopidogrel resistance. J. Invasive Cardiol. 2011, 23, E13–E18. [Google Scholar]
- Kim, Y.-S.; Lee, S.-R. Successful Prasugrel Therapy for Recurrent Left Main Stent Thrombosis in a Clopidogrel Hyporesponder. Tex. Heart Inst. J. 2015, 42, 483–486. [Google Scholar] [CrossRef]
- Redfors, B.; Ben-Yehuda, O.; Lin, S.-H.; Furer, A.; Kirtane, A.J.; Witzenbichler, B.; Weisz, G.; Stuckey, T.D.; Maehara, A.; Généreux, P.; et al. Quantifying Ischemic Risk After Percutaneous Coronary Intervention Attributable to High Platelet Reactivity on Clopidogrel (From the Assessment of Dual Antiplatelet Therapy with Drug-Eluting Stents Study). Am. J. Cardiol. 2017, 120, 917–923. [Google Scholar] [CrossRef]
- Olędzki, S.; Kornacewicz-Jach, Z.; Safranow, K.; Kiedrowicz, R.; Gawrońska-Szklarz, B.; Jastrzębska, M.; Gorący, J. Variability of platelet response to clopidogrel is not related to adverse cardiovascular events in patients with stable coronary artery disease undergoing percutaneous coronary intervention. Eur. J. Clin. Pharmacol. 2017, 73, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Bolliger, D.; Filipovic, M.; Matt, P.; Tanaka, K.A.; Gregor, M.; Zenklusen, U.; Seeberger, M.D.; Buse, G.L. Reduced aspirin responsiveness as assessed by impedance aggregometry is not associated with adverse outcome after cardiac surgery in a small low-risk cohort. Platelets 2015, 27, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.J.; Kirtane, A.J.; Zhang, Y.; Witzenbichler, B.; Weisz, G.; Stuckey, T.D.; Brodie, B.R.; Rinaldi, M.J.; Neumann, F.-J.; Metzger, D.C.; et al. Impact of high on-aspirin platelet reactivity on outcomes following successful percutaneous coronary intervention with drug-eluting stents. Am. Heart J. 2018, 205, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Redfors, B.; Chen, S.; Généreux, P.; Witzenbichler, B.; Weisz, G.; Stuckey, T.D.; Maehara, A.; McAndrew, T.; Mehran, R.; Ben-Yehuda, O.; et al. Relationship Between Stent Diameter, Platelet Reactivity, and Thrombotic Events After Percutaneous Coronary Artery Revascularization. Am. J. Cardiol. 2019, 124, 1363–1371. [Google Scholar] [CrossRef]
- Gori, T.; Polimeni, A.; Indolfi, C.; Räber, L.; Adriaenssens, T.; Münzel, T. Predictors of stent thrombosis and their implications for clinical practice. Nat. Rev. Cardiol. 2018, 16, 243–256. [Google Scholar] [CrossRef]
- Price, M.J.; Berger, P.B.; Teirstein, P.S.; Tanguay, J.F.; Angiolillo, D.J.; Spriggs, D.; Puri, S.; Robbins, M.; Garratt, K.N.; Bertrand, O.F.; et al. Standard- vs. high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: The GRAVITAS randomized trial. JAMA 2011, 305, 1097–1105. [Google Scholar] [CrossRef]
- Chandrasekhar, J.; Baber, U.; Mehran, R.; Aquino, M.; Sartori, S.; Yu, J.; Kini, A.; Sharma, S.; Skurk, C.; Shlofmitz, R.A.; et al. Impact of an integrated treatment algorithm based on platelet function testing and clinical risk assessment: Results of the TRIAGE Patients Undergoing Percutaneous Coronary Interventions to Improve Clinical Outcomes Through Optimal Platelet Inhibition study. J. Thromb. Thrombolysis 2016, 42, 186–196. [Google Scholar] [CrossRef]
- Xing, Z.; Tang, L.; Zhu, Z.; Huang, J.; Peng, X.; Hu, X. Platelet reactivity-adjusted antiplatelet therapy in patients with percutaneous coronary intervention: A meta-analysis of randomized controlled trials. Platelets 2017, 29, 589–595. [Google Scholar] [CrossRef]
- Robledo-Nolasco, R.; Godínez-Montes de Oca, A.; Zaballa-Contreras, J.F.; Suárez-Cuenca, J.A.; Mondragón-Terán, P.; Rubio-Guerra, A.F.; Meléndez-Alcántara, M.A. Efficacy of Change to New P2Y12 Receptor Antagonists in Patients High on Treatment Platelet Reactivity Undergoing Percutaneous Coronary Intervention. Clin. Appl. Thromb. Hemost. 2015, 21, 619–625. [Google Scholar] [CrossRef]
- Tantry, U.S.; Bonello, L.; Aradi, D.; Price, M.J.; Jeong, Y.-H.; Angiolillo, D.J.; Stone, G.W.; Curzen, N.; Geisler, T.; ten Berg, J.; et al. Consensus and Update on the Definition of On-Treatment Platelet Reactivity to Adenosine Diphosphate Associated with Ischemia and Bleeding. J. Am. Coll. Cardiol. 2013, 62, 2261–2273. [Google Scholar] [CrossRef]
- Fedor, M.; Samoš, M.; Šimonová, R.; Fedorová, J.; Škorňová, I.; Duraj, L.; Staško, J.; Kovář, F.; Mokáň, M.; Kubisz, P. Monitoring the efficacy of ADP inhibitor treatment in patients with acute STEMI post-PCI by VASP-P flow cytometry assay. Clin. Appl. Thromb. Hemost. 2015, 21, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Alvitigala, B.Y.; Gooneratne, L.V.; Constantine, G.R.; Wijesinghe, R.A.N.K.; Arawwawala, L.D.A.M. Pharmacokinetic, pharmacodynamic, and pharmacogenetic assays to monitor clopidogrel therapy. Pharmacol. Res. Perspect. 2020, 8, e00686. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, J.A.; Tobin, W.O.; Cox, D.; Coughlan, T.; Collins, R.; O’Neill, D.; Murphy, R.P.; McCabe, D.J. Prevalence of Ex Vivo High On-treatment Platelet Reactivity on Antiplatelet Therapy after Transient Ischemic Attack or Ischemic Stroke on the PFA-100® and VerifyNow®. J. Stroke Cerebrovasc. Dis. 2013, 22, e84–e92. [Google Scholar] [CrossRef] [PubMed]
- Crescente, M.; Mezzasoma, A.M.; Del Pinto, M.; Palmerini, F.; Di Castelnuovo, A.; Cerletti, C.; De Gaetano, G.; Gresele, P. Incomplete inhibition of platelet function as assessed by the platelet function analyzer (PFA-100) identifies a subset of cardiovascular patients with high residual platelet response while on aspirin. Platelets 2011, 22, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Koessler, J.; Kobsar, A.L.; Rajkovic, M.S.; Schafer, A.; Flierl, U.; Pfoertsch, S.; Bauersachs, J.; Steigerwald, U.; Rechner, A.R.; Walter, U. The new INNOVANCE® PFA P2Y cartridge is sensitive to the detection of the P2Y12 receptor inhibition. Platelets 2011, 22, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Paniccia, R.; Antonucci, E.; Gori, A.M.; Marcucci, R.; Poli, S.; Romano, E.; Valente, S.; Giglioli, C.; Fedi, S.; Gensini, G.F.; et al. Comparison of Different Methods to Evaluate the Effect of Aspirin on Platelet Function in High-Risk Patients with Ischemic Heart Disease Receiving Dual Antiplatelet Treatment. Am. J. Clin. Pathol. 2007, 128, 143–149. [Google Scholar] [CrossRef]
- Kweon, O.J.; Lim, Y.K.; Kim, B.; Lee, M.-K.; Kim, A.H.R. Effectiveness of Platelet Function Analyzer-100 for Laboratory Detection of Anti-Platelet Drug-Induced Platelet Dysfunction. Ann. Lab. Med. 2019, 39, 23–30. [Google Scholar] [CrossRef]
- Guha, S.; Mookerjee, S.; Lahiri, P.; Mani, S.; Saha, J.; Guha, S.; Majumdar, D.; Mandal, M.; Bhattacharya, R. A study of platelet aggregation in patients with acute myocardial infarction at presentation and after 48 hrs of initiating standard anti platelet therapy. Indian Heart J. 2013, 63, 409–413. [Google Scholar]
- Dobesh, P.P.; Varnado, S.; Doyle, M. Antiplatelet Agents in Cardiology: A Report on Aspirin, Clopidogrel, Prasugrel, and Ticagrelor. Curr. Pharm. Des. 2016, 22, 1918–1932. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Bhatt, D.L.; Cohen, M.; Steg, P.G.; Storey, R.F.; Jensen, E.C.; Magnani, G.; Bansilal, S.; Fish, M.P.; Im, K.; et al. Long-Term Use of Ticagrelor in Patients with Prior Myocardial Infarction. N. Engl. J. Med. 2015, 372, 1791–1800. [Google Scholar] [CrossRef]
- Wang, W.; Wang, B.; Chen, Y.; Wei, S. Late Stent Thrombosis After Drug-Coated Balloon Coronary Angioplasty for In-Stent Restenosis. Int. Heart J. 2021, 62, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Cho, J.Y.; Yun, K.H.; Oh, S.K. Successful Therapy with Ticagrelor in Three-Vessel Stent Thrombosis Related to Clopidogrel Resistance. Can. J. Cardiol. 2021, 37, 1278–1280. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M. Cangrelor: A Review in Percutaneous Coronary Intervention. Drugs 2015, 75, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bernardo, N.L.; Waksman, R. Cangrelor for the Rescue of Intra-Procedural Stent Thrombosis in Percutaneous Coronary Intervention. Cardiovasc. Revascularization Med. 2019, 20, 624–625. [Google Scholar] [CrossRef]
- Bonello, L.; Pansieri, M.; Mancini, J.; Bonello, R.; Maillard, L.; Barnay, P.; Rossi, P.; Ait-Mokhtar, O.; Jouve, B.; Collet, F.; et al. High On-Treatment Platelet Reactivity after Prasugrel Loading Dose and Cardiovascular Events after Percutaneous Coronary Intervention in Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2011, 58, 467–473. [Google Scholar] [CrossRef]
- Skorňová, I.; Samoš, M.; Šimonová, R.; Žolková, J.; Stančiaková, L.; Vádelová, L.; Bolek, T.; Urban, L.; Kovář, F.; Staško, J.; et al. On-treatment platelet reactivity in the era of new ADP receptor blockers: Data from a real-world clinical practice. Acta Med. Martiniana 2018, 18, 34–39. [Google Scholar] [CrossRef]
- Kuliczkowski, W.; Atar, D.; Serebruany, V.L.; Aradi, D. Inter-patient variability and impact of proton pump inhibitors on platelet reactivity after prasugrel. Thromb. Haemost. 2012, 107, 338–345. [Google Scholar] [CrossRef]
- Cayla, G.; Cuisset, T.; Silvain, J.; O’Connor, S.A.; Kerneis, M.; Castelli, C.; Quilici, J.; Bonnet, J.-L.; Alessi, M.-C.; Morange, P.-E.; et al. Prasugrel Monitoring and Bleeding in Real World Patients. Am. J. Cardiol. 2013, 111, 38–44. [Google Scholar] [CrossRef]
- Laine, M.; Gaubert, M.; Frère, C.; Peyrol, M.; Thuny, F.; Yvorra, S.; Chelini, V.; Bultez, B.; Luigi, S.; Mokrani, Z.; et al. Comparison of Platelet reactivity following prAsugrel and ticagrelor loading dose in ST-Segment elevation myocardial infarction patients: The COMPASSION study. Platelets 2015, 26, 570–572. [Google Scholar] [CrossRef]
- Lemesle, G.; Schurtz, G.; Bauters, C.; Hamon, M. High on-treatment platelet reactivity with ticagrelor versus prasugrel: A systematic review and meta-analysis. J. Thromb. Haemost. 2015, 13, 931–942. [Google Scholar] [CrossRef]
- Siller-Matula, J.M.; Akca, B.; Neunteufl, T.; Maurer, G.; Lang, I.M.; Kreiner, G.; Berger, R.; Delle-Karth, G. Inter-patient variability of platelet reactivity in patients treated with prasugrel and ticagrelor. Platelets 2015, 27, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Selhorst, G.; Schmidtler, F.; Ott, A.; Hitzke, E.; Tomelden, J.; Antoni, D.; Hoffmann, E.; Rieber, J. Platelet reactivity in patients with acute coronary syndrome treated with prasugrel or ticagrelor in comparison to clopidogrel: A retrospective pharmacodynamic analysis. Platelets 2018, 30, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Verdoia, M.; Pergolini, P.; Nardin, M.; Rolla, R.; Barbieri, L.; Marino, P.; Carriero, A.; Suryapranata, H.; De Luca, G. Prevalence and predictors of high-on treatment platelet reactivity during prasugrel treatment in patients with acute coronary syndrome undergoing stent implantation. J. Cardiol. 2018, 73, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Verdoia, M.; Pergolini, P.; Nardin, M.; Rolla, R.; Suryapranata, H.; Kedhi, E.; De Luca, G. Ticagrelor and prasugrel in acute coronary syndrome: A single-arm crossover platelet reactivity study. J. Cardiovasc. Med. 2021, 22, 686–692. [Google Scholar] [CrossRef]
- Bonello, L.; Mancini, J.; Pansieri, M.; Maillard, L.; Rossi, P.; Collet, F.; Jouve, B.; Wittenberg, O.; Laine, M.; Michelet, P.; et al. Relationship between post-treatment platelet reactivity and ischemic and bleeding events at 1-year follow-up in patients receiving prasugrel. J. Thromb. Haemost. 2012, 10, 1999–2005. [Google Scholar] [CrossRef]
- Aradi, D.; Gross, L.; Trenk, D.; Geisler, T.; Merkely, B.; Kiss, R.G.; Komócsi, A.; Dézsi, C.A.; Ruzsa, Z.; Ungi, I.; et al. Platelet reactivity and clinical outcomes in acute coronary syndrome patients treated with prasugrel and clopidogrel: A pre-specified exploratory analysis from the TROPICAL-ACS trial. Eur. Heart J. 2019, 40, 1942–1951. [Google Scholar] [CrossRef]
- Silvano, M.; Zambon, C.-F.; De Rosa, G.; Plebani, M.; Pengo, V.; Napodano, M.; Padrini, R. A case of resistance to clopidogrel and prasugrel after percutaneous coronary angioplasty. J. Thromb. Thrombolysis 2010, 31, 233–234. [Google Scholar] [CrossRef]
- Ohno, Y.; Okada, S.; Kitahara, H.; Nishi, T.; Nakayama, T.; Fujimoto, Y.; Kobayashi, Y. Repetitive stent thrombosis in a patient who had resistance to both clopidogrel and prasugrel. J. Cardiol. Cases 2016, 13, 139–142. [Google Scholar] [CrossRef]
- Warlo, E.M.K.; Arnesen, H.; Seljeflot, I. A brief review on resistance to P2Y12 receptor antagonism in coronary artery disease. Thromb. J. 2019, 17, 1–9. [Google Scholar] [CrossRef]
- Alexopoulos, D.; Galati, A.; Xanthopoulou, I.; Mavronasiou, E.; Kassimis, G.; Theodoropoulos, K.C.; Makris, G.; Damelou, A.; Tsigkas, G.; Hahalis, G.; et al. Ticagrelor versus prasugrel in acute coronary syndrome patients with high on-clopidogrel platelet reactivity following percutaneous coronary intervention: A pharmacodynamic study. J. Am. Coll. Cardiol. 2012, 60, 193–199. [Google Scholar] [CrossRef]
- Alexopoulos, D.; Xanthopoulou, I.; Mavronasiou, E.; Stavrou, K.; Siapika, A.; Tsoni, E.; Davlouros, P. Randomized Assessment of Ticagrelor Versus Prasugrel Antiplatelet Effects in Patients with Diabetes. Diabetes Care 2013, 36, 2211–2216. [Google Scholar] [CrossRef] [PubMed]
- Laine, M.; Toesca, R.; Berbis, J.; Frere, C.; Barnay, P.; Pansieri, M.; Peyre, J.-P.; Michelet, P.; Bessereau, J.; Camilleri, E.; et al. Platelet reactivity evaluated with the VASP assay following ticagrelor loading dose in acute coronary syndrome patients undergoing percutaneous coronary intervention. Thromb. Res. 2013, 132, e15–e18. [Google Scholar] [CrossRef] [PubMed]
- Laine, M.; Frere, C.; Toesca, R.; Berbis, J.; Barnay, P.; Pansieri, M.; Michelet, P.; Bessereau, J.; Camilleri, E.; Ronsin, O.; et al. Ticagrelor versus prasugrel in diabetic patients with an acute coronary syndrome. Thromb. Haemost. 2014, 111, 273–278. [Google Scholar] [CrossRef]
- Verdoia, M.; Sartori, C.; Pergolini, P.; Nardin, M.; Rolla, R.; Barbieri, L.; Schaffer, A.; Marino, P.; Bellomo, G.; Suryapranata, H.; et al. Immature platelet fraction and high-on treatment platelet reactivity with ticagrelor in patients with acute coronary syndromes. J. Thromb. Thrombolysis 2015, 41, 663–670. [Google Scholar] [CrossRef]
- Barbieri, L.; Pergolini, P.; Verdoia, M.; Rolla, R.; Nardin, M.; Marino, P.; Bellomo, G.; Suryapranata, H.; De Luca, G. Platelet reactivity in patients with impaired renal function receiving dual antiplatelet therapy with clopidogrel or ticagrelor. Vasc. Pharmacol. 2016, 79, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-D.; Wang, X.-Y.; Xi, S.-Z.; Liu, J.; Qin, L.-A.; Jing, J.; Yin, T.; Chen, Y.-D. Relationship between ADP-induced platelet-fibrin clot strength and anti-platelet responsiveness in ticagrelor treated ACS patients. J. Geriatr. Cardiol. 2016, 13, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Laine, M.; Panagides, V.; Frère, C.; Cuisset, T.; Gouarne, C.; Jouve, B.; Thuny, F.; Paganelli, F.; Alessi, M.; Mancini, J.; et al. Platelet reactivity inhibition following ticagrelor loading dose in patients undergoing percutaneous coronary intervention for acute coronary syndrome. J. Thromb. Haemost. 2019, 17, 2188–2195. [Google Scholar] [CrossRef]
- Verdoia, M.; Sartori, C.; Pergolini, P.; Nardin, M.; Rolla, R.; Barbieri, L.; Schaffer, A.; Marino, P.; Bellomo, G.; Suryapranata, H.; et al. Prevalence and predictors of high-on treatment platelet reactivity with ticagrelor in ACS patients undergoing stent implantation. Vasc. Pharmacol. 2015, 77, 48–53. [Google Scholar] [CrossRef]
- Verdoia, M.; Rolla, R.; Negro, F.; Tonon, F.; Pergolini, P.; Nardin, M.; Marcolongo, M.; De Luca, G. Homocysteine levels and platelet reactivity in coronary artery disease patients treated with ticagrelor. Nutr. Metab. Cardiovasc. Dis. 2019, 30, 292–299. [Google Scholar] [CrossRef]
- Stavrou, K.; Koniari, I.; Gkizas, V.; Perperis, A.; Kontoprias, K.; Vogiatzi, C.; Bampouri, T.; Xanthopoulou, I.; Alexopoulos, D. Ticagrelor vs. prasugrel one-month maintenance therapy: Impact on platelet reactivity and bleeding events. Thromb. Haemost. 2014, 112, 551–557. [Google Scholar] [CrossRef]
- Alexopoulos, D.; Xanthopoulou, I.; Storey, R.F.; Bliden, K.P.; Tantry, U.S.; Angiolillo, D.J.; Gurbel, P.A. Platelet reactivity during ticagrelor maintenance therapy: A patient-level data meta-analysis. Am. Heart J. 2014, 168, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Gaglia, M.A., Jr.; Lipinski, M.J.; Lhermusier, T.; Steinvil, A.; Kiramijyan, S.; Pokharel, S.; Torguson, R.; Angiolillo, D.J.; Wallentin, L.; Storey, R.F.; et al. Comparison of Platelet Reactivity in Black Versus White Patients with Acute Coronary Syndromes After Treatment with Ticagrelor. Am. J. Cardiol. 2017, 119, 1135–1140. [Google Scholar] [CrossRef]
- Sweeny, J.M.; Angiolillo, D.J.; Franchi, F.; Rollini, F.; Waksman, R.; Raveendran, G.; Dangas, G.; Khan, N.D.; Carlson, G.F.; Zhao, Y.; et al. Impact of Diabetes Mellitus on the Pharmacodynamic Effects of Ticagrelor Versus Clopidogrel in Troponin-Negative Acute Coronary Syndrome Patients Undergoing Ad Hoc Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2017, 6, e005650. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Wei, Y.J.; Ding, P.; Zhang, J.; Li, T.C.; Wang, B.; Wang, M.S.; Li, Y.T.; Zhang, J.J.; Ren, Y.H.; et al. Antiplatelet Effect of Different Loading Doses of Ticagrelor in Patients with Non-ST-Elevation Acute Coronary Syndrome Undergoing. Can. J. Cardiol. 2017, 33, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Li, Y.; Qu, X.; Zhu, Y.; Tian, L.; Shen, Z.; Yang, X.; Shi, X. Comparison of platelet reactivity between prasugrel and ticagrelor in patients with acute coronary syndrome: A meta-analysis. BMC Cardiovasc. Disord. 2020, 20, 430. [Google Scholar] [CrossRef]
- Dai, L.; Xu, J.; Jiang, Y.; Chen, K. Impact of Prasugrel and Ticagrelor on Platelet Reactivity in Patients with Acute Coronary Syndrome: A Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 905607. [Google Scholar] [CrossRef]
- Malik, J. A Case of Ticagrelor Resistance. Eur. J. Case Rep. Intern. Med. 2021, 8, 002719. [Google Scholar] [CrossRef]
- Jariwala, P.; Bhatia, H.; Kumar, E.A.P. Sub-acute stent thrombosis secondary to ticagrelor resistance-Myth or reality! Indian Heart J. 2017, 69, 804–806. [Google Scholar] [CrossRef]
- Rollini, F.; Franchi, F.; Tello-Montoliu, A.; Patel, R.; Darlington, A.; Ferreiro, J.L.; Cho, J.R.; Muñiz-Lozano, A.; Desai, B.; Zenni, M.M.; et al. Pharmacodynamic effects of cangrelor on platelet P2Y12 receptor-mediated signaling in prasugrel-treated patients. JACC Cardiovasc. Interv. 2014, 7, 426–434. [Google Scholar] [CrossRef]
- Valenti, R.; Muraca, I.; Marcucci, R.; Ciatti, F.; Berteotti, M.; Gori, A.M.; Carrabba, N.; Migliorini, A.; Marchionni, N.; Valgimigli, M. “Tailored” antiplatelet bridging therapy with cangrelor: Moving toward personalized medicine. Platelets 2021, 33, 687–691. [Google Scholar] [CrossRef]
- Franchi, F.; Rollini, F.; Rivas, A.; Wali, M.; Briceno, M.; Agarwal, M.; Shaikh, Z.; Nawaz, A.; Silva, G.; Been, L.; et al. Platelet Inhibition with Cangrelor and Crushed Ticagrelor in Patients with ST-Segment–Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Circulation 2019, 139, 1661–1670. [Google Scholar] [CrossRef]
- Cuisset, T.; Gaborit, B.; Dubois, N.; Quilici, J.; Loosveld, M.; Beguin, S.; Loundou, A.D.; Moro, P.J.; Morange, P.E.; Alessi, M.-C.; et al. Platelet reactivity in diabetic patients undergoing coronary stenting for acute coronary syndrome treated with clopidogrel loading dose followed by prasugrel maintenance therapy. Int. J. Cardiol. 2013, 168, 523–528. [Google Scholar] [CrossRef]
- Pankert, M.; Quilici, J.; Loundou, A.D.; Verdier, V.; Lambert, M.; Deharo, P.; Bonnet, G.; Gaborit, B.; Morange, P.E.; Valéro, R.; et al. Impact of Obesity and the Metabolic Syndrome on Response to Clopidogrel or Prasugrel and Bleeding Risk in Patients Treated After Coronary Stenting. Am. J. Cardiol. 2014, 113, 54–59. [Google Scholar] [CrossRef]
- Silvain, J.; Cayla, G.; Hulot, J.-S.; Finzi, J.; Kerneis, M.; O’Connor, S.A.; Bellemain-Appaix, A.; Barthélémy, O.; Beygui, F.; Collet, J.-P.; et al. High on-thienopyridine platelet reactivity in elderly coronary patients: The SENIOR-PLATELET study. Eur. Heart J. 2011, 33, 1241–1249. [Google Scholar] [CrossRef]
- Cuisset, T.; Loosveld, M.; Morange, P.E.; Quilici, J.; Moro, P.J.; Saut, N.; Gaborit, B.; Castelli, C.; Beguin, S.; Grosdidier, C.; et al. CYP2C19*2 and *17 Alleles Have a Significant Impact on Platelet Response and Bleeding Risk in Patients Treated with Prasugrel After Acute Coronary Syndrome. JACC Cardiovasc. Interv. 2012, 5, 1280–1287. [Google Scholar] [CrossRef]
- Grosdidier, C.; Quilici, J.; Loosveld, M.; Camoin, L.; Moro, P.J.; Saut, N.; Gaborit, B.; Pankert, M.; Cohen, W.; Lambert, M.; et al. Effect of CYP2C19*2 and *17 Genetic Variants on Platelet Response to Clopidogrel and Prasugrel Maintenance Dose and Relation to Bleeding Complications. Am. J. Cardiol. 2013, 111, 985–990. [Google Scholar] [CrossRef]
- Alexopoulos, D.; Xanthopoulou, I.; Perperis, A.; Siapika, A.; Stavrou, K.; Tsoni, E.; Davlouros, P.; Hahalis, G. Factors Affecting Residual Platelet Aggregation in Prasugrel Treated Patients. Curr. Pharm. Des. 2013, 19, 5121–5126. [Google Scholar] [CrossRef]
- Samoš, M.; Fedor, M.; Kovář, F.; Galajda, P.; Bolek, T.; Stančiaková, L.; Fedorová, J.; Staško, J.; Kubisz, P.; Mokáň, M. The Impact of Type 2 Diabetes on the Efficacy of ADP Receptor Blockers in Patients with Acute ST Elevation Myocardial Infarction: A Pilot Prospective Study. J. Diabetes Res. 2016, 2016, 2909436. [Google Scholar] [CrossRef]
- Adamski, P.; Buszko, K.; Sikora, J.; Niezgoda, P.; Fabiszak, T.; Ostrowska, M.; Barańska, M.; Karczmarska-Wódzka, A.; Navarese, E.P.; Kubica, J. Determinants of high platelet reactivity in patients with acute coronary syndromes treated with ticagrelor. Sci. Rep. 2019, 9, 3924. [Google Scholar] [CrossRef]
- Ilardi, F.; Gargiulo, G.; Paolillo, R.; Ferrone, M.; Cimino, S.; Giugliano, G.; Schiattarella, G.G.; Verde, N.; Stabile, E.; Perrino, C.; et al. Impact of chronic kidney disease on platelet aggregation in patients with acute coronary syndrome. J. Cardiovasc. Med. 2020, 21, 660–666. [Google Scholar] [CrossRef]
- Bolek, T.; Samoš, M.; Šimonová, R.; Kovář, F.; Fedor, M.; Galajda, P.; Staško, J.; Kubisz, P.; Mokáň, M. Does Pantoprazole Affect the On-Treatment Platelet Reactivity in Patients With Acute STEMI Treated With ADP Receptor Blockers?—A Pilot Prospective Study. Am. J. Ther. 2017, 24, e162–e166. [Google Scholar] [CrossRef] [PubMed]
- Ferreiro, J.L.; Ueno, M.; Tello-Montoliu, A.; Tomasello, S.D.; Seecheran, N.; Desai, B.; Rollini, F.; Guzman, L.A.; Bass, T.A.; Angiolillo, D.J. Impact of Prasugrel Reload Dosing Regimens on High On-Treatment Platelet Reactivity Rates in Patients on Maintenance Prasugrel Therapy. JACC Cardiovasc. Interv. 2013, 6, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Christ, G.; Hafner, T.; Siller-Matula, J.M.; Francesconi, M.; Grohs, K.; Wilhelm, E.; Podczeck-Schweighofer, A. Platelet Inhibition by Abciximab Bolus-Only Administration and Oral ADP Receptor Antagonist Loading in Acute Coronary Syndrome Patients: The Blocking and Bridging Strategy. Thromb. Res. 2013, 132, e36–e41. [Google Scholar] [CrossRef] [PubMed]
- Niazi, A.K.; Dinicolantonio, J.J.; Lavie, C.J.; O’Keefe, J.H.; Meier, P.; Bangalore, S. Triple versus dual antiplatelet therapy in acute coronary syndromes: Adding cilostazol to aspirin and clopidogrel? Cardiology 2013, 126, 233–243. [Google Scholar] [CrossRef]
- Bassez, C.; Deharo, P.; Pankert, M.; Bonnet, G.; Quilici, J.; Lambert, M.; Verdier, V.; Morange, P.; Alessi, M.-C.; Bonnet, J.-L.; et al. Effectiveness of switching ‘low responders’ to prasugrel to ticagrelor after acute coronary syndrome. Int. J. Cardiol. 2014, 176, 1184–1185. [Google Scholar] [CrossRef]
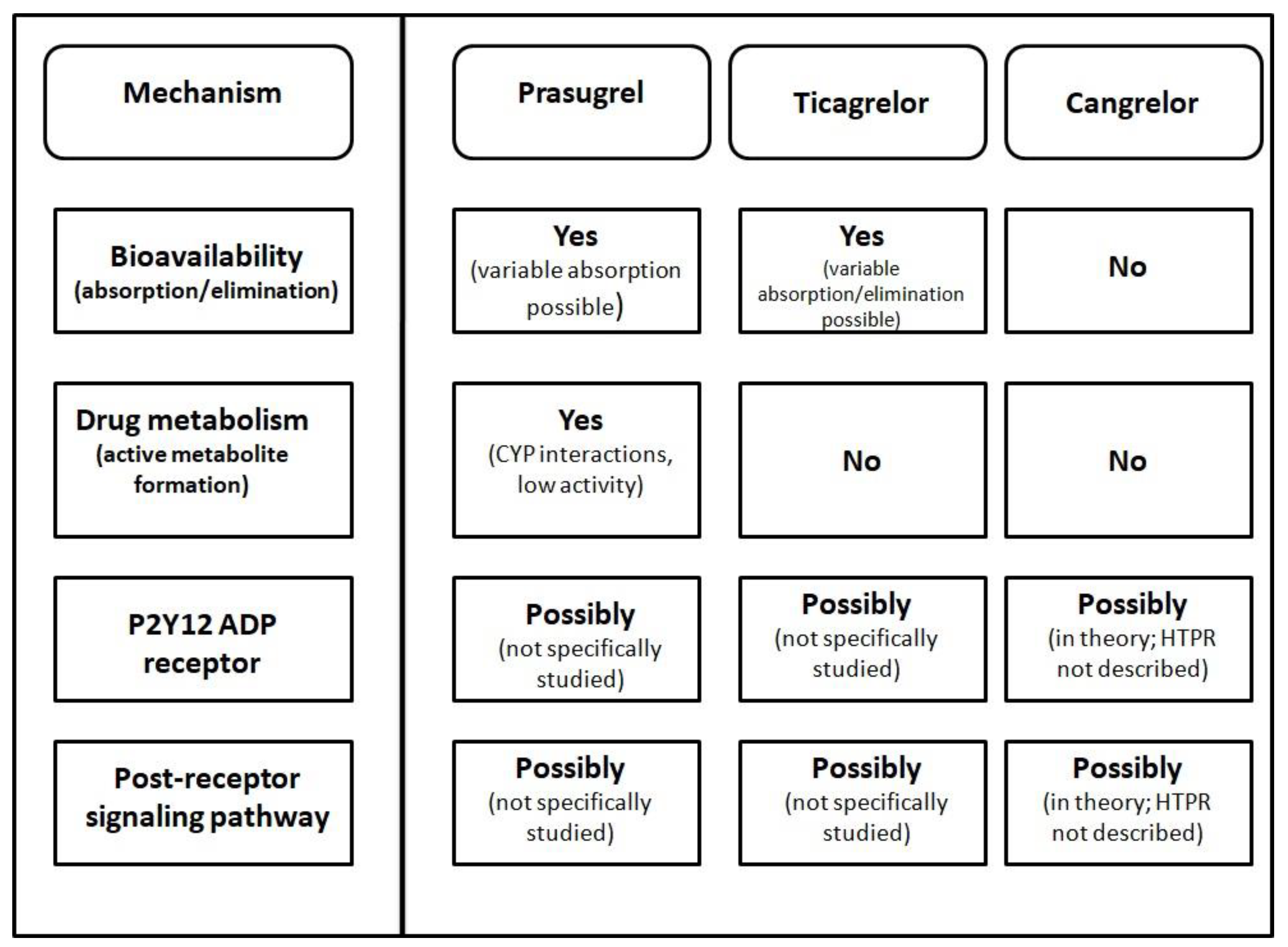
| Drug | Route of Administration Dosing | Bioavailability | Receptor Inhibition | Time to Peak Platelet Inhibition | Clinical Application | HTPR (Prevalence) |
|---|---|---|---|---|---|---|
| Prasugrel | Oral Loading dose of 60 mg followed by 10 mg once daily (5 mg in elderly and low body weight) | Prodrug | Irreversible | 0.5–2 h | ACS with PCI | Described (1.6–25%; higher if time from drug administration to blood sampling is too short) |
| Ticagrelor | Oral Loading dose of 180 mg followed by 90 mg twice daily (60 mg twice daily in CAD) | Direct-acting | Reversible | 1.5–2 h | ACS High ischemic risk CAD | Described (8.6–13.7% if tested 30 to 90 days post drug loading; 0.0–1.9% on long-term therapy) |
| Cangrelor | Intravenous Bolus injection of 30 ug/kg followed by continuous intravenous infusion of 4 ug/kg/min. | Direct-acting | Reversible | 2 min | PCI (if not pretreated with oral agent) | Not described |
| Study | Type of Study | Studied Population | Number of Patients | Test for HTPR | Cut Off | Main Results |
|---|---|---|---|---|---|---|
| Bonello et al. [45] | Prospective multicenter (non-randomized) | PCI for ACS | 301 | VASP-P | VASP-P: PRI > 50% | HTPR in 25.2% of patients; significantly higher PRI in those with thrombotic events |
| Škorňová et al. [46] | Prospective single-center (non-randomized) | STEMI with primary PCI | 44 | LTA with ADP induction, VASP-P | LTA: >50%, VASP-P: PRI > 50% | HTPR in 8.7% of patients |
| Aradi et al. [47] | Prospective multicenter (non-randomized) | high risk ACS | 104 | LTA, Multiplate® | LTA: >46%, Multiplate®: >47 AU | inter-patient variability after prasugrel loading dosing; no effect of PPI on prasugrel activity |
| Cayla et al. [48] | Prospective two high-volume centers (non-randomized) | ACS with PCI | 444 | VASP-P, VerifyNow®, LTA | VASP-P: PRI ≥ 50%, VerifyNow®: ≥235 PRU, LTA: ≥46.2% | HTPR in 3.2–6.8% of patients according to method used for detection |
| Laine et al. [49] | Prospective single-center (randomized) | STEMI with primary PCI | 44 | VASP-P | VASP-P: PRI ≥ 50% | HTPR in 9.1% of patients |
| Lemesle et al. [50] | Meta-analysis (14 studies included) | CAD | 1822 | VASP-P, VerifyNow® | VASP-P: PRI ≥ 50%, different cut off for VerifyNow in included studies (208–235 PRU) | HTPR in 9.8% of patients |
| Siller-Matula et al. [51] | Prospective single-center (non-randomized) | ACS | 200 | Multiplate® | Multiplate®: >46 AU | HTPR in 3% of patients |
| Selhorst et al. [52] | Retrospective single-center | ACS with primary PCI | 809 | Multiplate® | Multiplate®: >468 AUC | HTPR in 1.6% of patients |
| Verdoia et al. [53] | Prospective single-center (non-randomized) | ACS with PCI | 190 | Multiplate® | Multiplate®: >417 AUC | HTPR in 10% of patients |
| Verdoia et al. [54] | Prospective single-center (non-randomized) | ACS with PCI | 105 | Multiplate® | Multiplate®: >417 AUC | HTPR in 12.3% of patients |
| Study | Type of Study | Studied Population | Number of Patients | Test for HTPR | Cut Off | Main Results |
|---|---|---|---|---|---|---|
| Alexopoulos et al. [60] | Prospective single-center (randomized) | ACS with PCI and HTPR on clopidogrel | 44 | VerifyNow® | VerifyNow®: ≥235 PRU | HTPR in 0% of patients |
| Alexopoulos et al. [61] | Prospective single-center (randomized) | ACS with PCI and T2D | 30 | VerifyNow® | VerifyNow®: ≥230 PRU | HTPR in 0% of patients |
| Laine et al. [62] | Prospective multicenter (non-randomized) | ACS with PCI | 115 | VASP-P | VASP-P: PRI ≥ 50% | HTPR in 3.5% of patients |
| Škorňová et al. [46] | Prospective single-center (non-randomized) | STEMI with primary PCI | 44 | LTA with ADP induction, VASP-P | LTA: >50%, VASP-P: PRI > 50% | HTPR in 14.3% of patients |
| Lemesle et al. [50] | Meta-analysis (14 studies included) | CAD | 1822 | VASP-P, VerifyNow® | VASP-P: PRI ≥ 50%, different cut off for VerifyNow in included studies (208–235 PRU) | HTPR in 1.5% of patients |
| Siller-Matula et al. [51] | Prospective single-center (non-randomized) | ACS | 200 | Multiplate® | Multiplate®: >46 AU | HTPR in 2% of patients |
| Laine et al. [63] | Prospective single-center (randomized) | ACS with PCI and T2D | 100 | VASP-P | VASP-P: PRI ≥ 50% | HTPR in 6% of patients |
| Verdoia et al. [64] | Prospective single-center (non-randomized) | ACS | 190 | Multiplate® | Multiplate®: >417 AUC | HTPR in 11% of patients |
| Barbieri et al. [65] | Prospective single-center (non-randomized) | PCI | 537 | Multiplate® | Multiplate®: >417 AUC | HTPR in 12.7% of patients |
| Li et al. [66] | Prospective single-center (non-randomized) | ACS | 176 | TEG® | TEG®: MA > 47 mm | HTPR in 3.98% of patients |
| Laine et al. [67] | Prospective multicenter (non-randomized) | ACS with PCI | 530 | VASP-P | VASP-P: PRI ≥ 50% | HTPR in 5.3% of patients |
| Verdoia et al. [54] | Prospective single-center (non-randomized) | ACS with PCI | 105 | Multiplate® | Multiplate®: >417 AUC | HTPR in 8.6% of patients |
| Verdoia et al. [68] | Prospective single-center (non-randomized) | ACS with PCI | 195 | Multiplate® | Multiplate®: >417 AUC | HTPR in 13.3% of patients |
| Verdoia et al. [69] | Prospective single-center (non-randomized) | ACS with PCI | 432 | Multiplate® | Multiplate®: >417 AUC | HTPR in 11.4% of patients |
| Laine et al. [49] | Prospective single-center (randomized) | STEMI with primary PCI | 44 | VASP-P | VASP-P: PRI ≥ 50% | HTPR in 0% of patients |
| Selhorst et al. [52] | Retrospective single-center | ACS with primary PCI | 809 | Multiplate® | Multiplate®: >468 AUC | HTPR in 1.9% of patients |
| Alexopoulos et al. [70] | Prospective single-center (non-randomized) | ACS with PCI | 512 | VerifyNow® | VerifyNow®: >208 PRU | HTPR in 0% of patients |
| Alexopoulos et al. [71] | Meta-analysis (8 studies included) | CAD or ACS (with or without PCI) | 445 | VerifyNow® | VerifyNow®: >230 PRU | HTPR in 0% of patients |
| Gaglia et al. [72] | Prospective single-center (non-randomized) | ACS and black rase | 29 | LTA, VASP-P, VerifyNow® | LTA: >60%, VASP-P: PRI > 50%, VerifyNow®: >208 PRU | HTPR in 0% of patients |
| Sweeny et al. [73] | post-hoc analysis of prospective multicenter study (randomized) | ACS (troponin negative) with PCI | 100 | VerifyNow® | VerifyNow®: >208 PRU | HTPR in 5.9% of patients |
| Liu et al. [74] | Prospective multicenter (randomized) | NSTE ACS | 278 | VASP-P | VASP-P: PRI ≥ 50% | HTPR in 3.1–5.3% of patients (according to ticagrelor loading dose) |
| Wen et al. [75] | Meta-analysis (14 studies included) | ACS | 2629 | VASP-P, VerifyNow® | VASP-P: PRI ≥ 50%, VerifyNow®: ≥208 PRU or ≥230 PRU | HTPR in 0.66–2.67% of patients (according to method used for detection) |
| Dai et al. [76] | Meta-analysis (25 studies included) | ACS | 5098 | VASP-P, VerifyNow®, Multiplate® | Not reported | low incidence of HTPR on ticagrelor maintenance dosing (exact rate was not reported) |
| Musallam et al. [7] | Case report | stent thrombosis on ticagleror therapy | 1 | VerifyNow® | - | HTPR (339 PRU) at the time of stent thrombosis |
| Malik [77] | Case report | stent thrombosis on ticagleror therapy | 1 | TEG® | TEG®: MA > 47 mm | HTPR (MA of 66 mm) at the time of stent thrombosis |
| Jariwala et al. [78] | Case report | stent thrombosis on ticagleror therapy | 1 | Not tested | - | stent thrombosis on ticagrelor—stent-related complication excluded by intravascular coronary imaging |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blaško, P.; Samoš, M.; Bolek, T.; Stančiaková, L.; Škorňová, I.; Péč, M.J.; Jurica, J.; Staško, J.; Mokáň, M. Resistance on the Latest Oral and Intravenous P2Y12 ADP Receptor Blockers in Patients with Acute Coronary Syndromes: Fact or Myth? J. Clin. Med. 2022, 11, 7211. https://doi.org/10.3390/jcm11237211
Blaško P, Samoš M, Bolek T, Stančiaková L, Škorňová I, Péč MJ, Jurica J, Staško J, Mokáň M. Resistance on the Latest Oral and Intravenous P2Y12 ADP Receptor Blockers in Patients with Acute Coronary Syndromes: Fact or Myth? Journal of Clinical Medicine. 2022; 11(23):7211. https://doi.org/10.3390/jcm11237211
Chicago/Turabian StyleBlaško, Peter, Matej Samoš, Tomáš Bolek, Lucia Stančiaková, Ingrid Škorňová, Martin Jozef Péč, Jakub Jurica, Ján Staško, and Marián Mokáň. 2022. "Resistance on the Latest Oral and Intravenous P2Y12 ADP Receptor Blockers in Patients with Acute Coronary Syndromes: Fact or Myth?" Journal of Clinical Medicine 11, no. 23: 7211. https://doi.org/10.3390/jcm11237211
APA StyleBlaško, P., Samoš, M., Bolek, T., Stančiaková, L., Škorňová, I., Péč, M. J., Jurica, J., Staško, J., & Mokáň, M. (2022). Resistance on the Latest Oral and Intravenous P2Y12 ADP Receptor Blockers in Patients with Acute Coronary Syndromes: Fact or Myth? Journal of Clinical Medicine, 11(23), 7211. https://doi.org/10.3390/jcm11237211






