Vitamin D and Healthcare Service Utilization in Children: Insights from a Machine Learning Approach
Abstract
1. Introduction
2. Materials and Methods
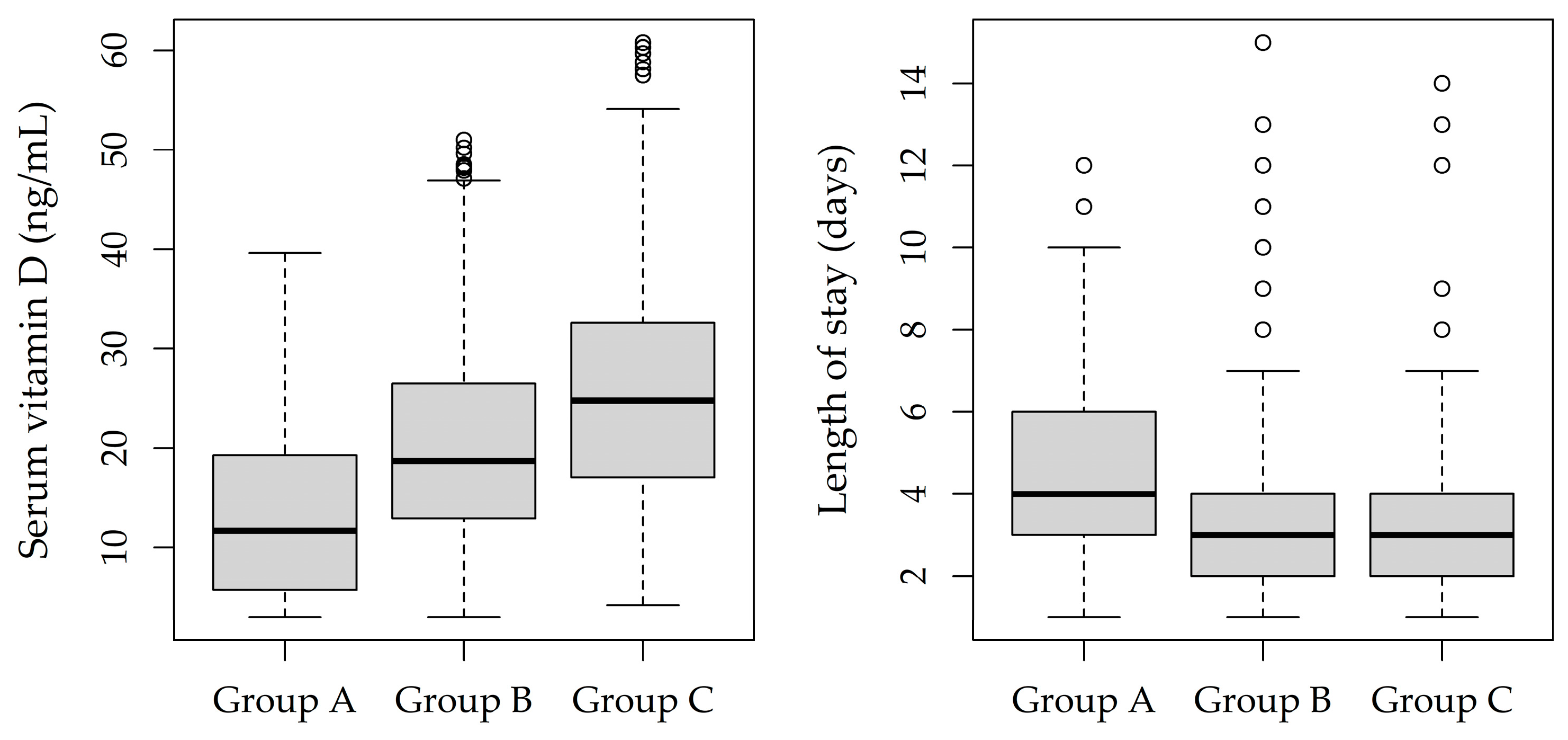
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thacher, T.D.; Clarke, B.L. Vitamin D insufficiency. Mayo Clin. Proc. 2011, 86, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Saggese, G.; Vierucci, F.; Prodam, F.; Cardinale, F.; Cetin, I.; Chiappini, E.; De’ Angelis, G.L.; Massari, M.; Miraglia Del Giudice, E.; Miraglia Del Giudice, M.; et al. Vitamin D in pediatric age: Consensus of the Italian Pediatric Society and the Italian Society of Preventive and Social Pediatrics, jointly with the Italian Federation of Pediatricians. Ital. J. Pediatr. 2018, 44, 51. [Google Scholar] [CrossRef]
- Huh, S.Y.; Gordon, C.M.; Kumar, J.; Muntner, P.; Kaskel, F.J.; Hailpern, S.M.; Melamed, M.L. Vitamin D deficiency in children and adolescents: Epidemiology, impact and treatment. Rev. Endocr. Metab. Disord. 2008, 9, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Muntner, P.; Kaskel, F.J.; Hailpern, S.M.; Melamed, M.L. Prevalence and Associations of 25-Hydroxyvitamin D Deficiency in US Children: NHANES 2001–2004. Pediatrics 2009, 124, e362–e370. [Google Scholar] [CrossRef] [PubMed]
- Mansbach, J.M.; Ginde, A.A.; Camargo, C.A., Jr. Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years: Do children need more vitamin D? Pediatrics 2009, 124, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Domellof, M.; Fewtrell, M.; Hojsak, I.; Mihatsch, W.; Molgaard, C.; Shamir, R.; et al. Vitamin D in the Healthy European Paediatric Population. J. Craniofacial Surg. 2013, 56, 692–701. [Google Scholar] [CrossRef]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Vierucci, F.; Del Pistoia, M.; Fanos, M.; Gori, M.; Carlone, G.; Erba, P.; Massimetti, G.; Federico, G.; Saggese, G. Vitamin D status and predictors of hypovitaminosis D in Italian children and adolescents: A cross-sectional study. Eur. J. Pediatr. 2013, 172, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Marrone, G.; Rosso, I.; Moretti, R.; Valent, F.; Romanello, C. Is vitamin D status known among children living in Northern Italy? Eur. J. Nutr. 2012, 51, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Nouvenne, A.; Ticinesi, A.; Bonelli, P.; Salvagno, G.L.; Cervellin, G.; Guidi, G.C. The burden of vitamin D deficiency in a mediterranean country without a policy of food fortification. Acta Bio-Med. 2015, 86, 59–62. [Google Scholar]
- Franchi, B.; Piazza, M.; Sandri, M.; Mazzei, F.; Maffeis, C.; Boner, A.L. Vitamin D at the onset of type 1 diabetes in Italian children. Eur. J. Pediatr. 2013, 173, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.H.; Cheetham, T.D. Diagnosis and management of vitamin D deficiency. BMJ 2010, 340, b5664. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.J. Clinical practice. Vitamin D Insufficiency. N. Engl. J. Med. 2011, 364, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Della Giustina, A.; Landi, M.; Bellini, F.; Bosoni, M.; Ferrante, G.; Onorari, M.; Travaglini, A.; Pingitore, G.; Passalacqua, G.; Tripodi, S. Vitamin D, allergies and asthma: Focus on pediatric patients. World Allergy Organ. J. 2014, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, A.; Gangemi, S.; La Grutta, S.; Malizia, V.; Riccobono, L.; Colombo, P.; Cibella, F.; Profita, M. 25-Hydroxyvitamin D, IL-31, and IL-33 in Children with Allergic Disease of the Airways. Mediat. Inflamm. 2014, 2014, 520241. [Google Scholar] [CrossRef] [PubMed]
- Zaffanello, M.; Ferrante, G.; Fasola, S.; Piazza, M.; Piacentini, G.; La Grutta, S. Personal and Environmental Risk Factors at Birth and Hospital Admission: Direct and Vitamin D-Mediated Effects on Bronchiolitis Hospitalization in Italian Children. Int. J. Environ. Res. Public Health 2021, 18, 747. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Mosaad, Y.M.; Mostafa, M.; Elwasify, M.; Youssef, H.M.; Omar, N.M. Vitamin D and Immune System. Vitam. Miner. 2017, 6, 1. [Google Scholar] [CrossRef]
- Kim, S.Y. The pleiomorphic actions of vitamin D and its importance for children. Ann. Pediatr. Endocrinol. Metab. 2013, 18, 45–54. [Google Scholar] [CrossRef]
- McNally, J.D.; Nama, N.; O’Hearn, K.; Sampson, M.; Amrein, K.; Iliriani, K.; McIntyre, L.; Fergusson, D.; Menon, K. Vitamin D deficiency in critically ill children: A systematic review and meta-analysis. Crit. Care 2017, 21, 287. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Singh, M.V.; Yadav, R.K.; Singh, D.K.; Siddiqui, S.A.; Christakos, S.; DeLuca, H.F. Vitamin D levels in paediatric intensive care unit patients and its relation to severity of illness: An Indian experience. Trop. Dr. 2021, 51, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Sankar, J.; Lotha, W.; Ismail, J.; Anubhuti, C.; Meena, R.S.; Sankar, M.J. Vitamin D deficiency and length of pediatric intensive care unit stay: A prospective observational study. Ann. Intensiv. Care 2016, 6, 3. [Google Scholar] [CrossRef]
- Masarweh, K.; Gur, M.; Leiba, R.; Bar-Yoseph, R.; Toukan, Y.; Nir, V.; Gut, G.; Ben-David, Y.; Hakim, F.; Bentur, L. Factors predicting length of stay in bronchiolitis. Respir. Med. 2020, 161, 105824. [Google Scholar] [CrossRef] [PubMed]
- Aurangzeb, B.; Whitten, K.E.; Harrison, B.; Mitchell, M.; Kepreotes, H.; Sidler, M.; Lemberg, D.A.; Day, A.S. Prevalence of malnutrition and risk of under-nutrition in hospitalized children. Clin. Nutr. 2012, 31, 35–40. [Google Scholar] [CrossRef]
- Raut, M.; Schein, J.; Mody, S.; Grant, R.; Benson, C.; Olson, W. Estimating the economic impact of a half-day reduction in length of hospital stay among patients with community-acquired pneumonia in the US. Curr. Med Res. Opin. 2009, 25, 2151–2157. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Whiting, S.J.; Schwalfenberg, G.K.; Genuis, S.J.; Kimball, S.M. Estimated economic benefit of increasing 25-hydroxyvitamin D concentrations of Canadians to or above 100 nmol/L. Dermato-Endocrinology 2016, 8, e1248324. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Cheng, L.; Han, P.; Zhu, Y.; Huang, W. Decision Tree-Based Data Stratification Method for the Minimization of the Masking Effect in Adverse Drug Reaction Signal Detection. Appl. Sci. 2021, 11, 11380. [Google Scholar] [CrossRef]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning: With Applications in R; Springer: New York, NY, USA, 2014. [Google Scholar]
- Therneau, T.; Atkinson, B.; Ripley, B.; Ripley, M.B. Package ‘Rpart’. 2015. Available online: http://cran.ma.ic.ac.uk/web/packages/rpart/rpart.pdf (accessed on 10 June 2022).
- Franchi, B.; Piazza, M.; Sandri, M.; Tenero, L.; Comberiati, P.; Boner, A.L.; Capristo, C. 25-hydroxyvitamin D serum level in children of different ethnicity living in Italy. Eur. J. Pediatr. 2015, 174, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; DeLuca, H.F. Minireview: Vitamin D: Is There a Role in Extraskeletal Health? Endocrinology 2011, 152, 2930–2936. [Google Scholar] [CrossRef] [PubMed]
- Powe, C.E.; Evans, M.K.; Wenger, J.; Zonderman, A.B.; Berg, A.H.; Nalls, M.; Tamez, H.; Zhang, D.; Bhan, I.; Karumanchi, S.A.; et al. Vitamin D–Binding Protein and Vitamin D Status of Black Americans and White Americans. N. Engl. J. Med. 2013, 369, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Earthman, C.P.; Beckman, L.M.; Masodkar, K.; Sibley, S.D. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: Considerations and implications. Int. J. Obes. 2012, 36, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Rusconi, R.E.; De Cosmi, V.; Gianluca, G.; Giavoli, C.; Agostoni, C. Vitamin D insufficiency in obese children and relation with lipid profile. Int. J. Food Sci. Nutr. 2015, 66, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Shulhai, A.-M.; Pavlyshyn, H.; Shulhai, O.; Furdela, V. The association between vitamin D deficiency and metabolic syndrome in Ukrainian adolescents with overweight and obesity. Ann. Pediatr. Endocrinol. Metab. 2021, 27, 113–120. [Google Scholar] [CrossRef]
- Harrison, C.M.; Gibson, A.T. Osteopenia in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2012, 98, F272–F275. [Google Scholar] [CrossRef]
- Walicka-Cupryś, K.; Zajkiewicz, K.; Drzał-Grabiec, J.; Perenc, L. Evaluation of vitamin D3 levels and morphotic parameters of blood in prematurely born children at six years of age. Sci. Rep. 2019, 9, 15089. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.; Long, K.; Agdere, L.; Kulpa, J.; Zarzoso-Fernandez, S.; Choudhary, D.; Sundarum, R. The association between vitamin D deficiency and hospitalization outcomes in pediatric patients with sickle cell disease. Blood Cells Mol. Dis. 2020, 82, 102415. [Google Scholar] [CrossRef] [PubMed]
- Haugen, J.; Basnet, S.; Hardang, I.M.; Sharma, A.; Mathisen, M.; Shrestha, P.; Valentiner-Branth, P.; Strand, T.A. Vitamin D status is associated with treatment failure and duration of illness in Nepalese children with severe pneumonia. Pediatr. Res. 2017, 82, 986–993. [Google Scholar] [CrossRef] [PubMed]
- McNally, J.D.; Menon, K.; Chakraborty, P.; Fisher, L.; Williams, K.A.; Al-Dirbashi, O.Y.; Doherty, D.R.; Choudhary, N.; Gupta, P.; Zhou, W.; et al. The Association of Vitamin D Status with Pediatric Critical Illness. Pediatrics 2012, 130, 429–436. [Google Scholar] [CrossRef]
- Michel, M.; Alberti, C.; Carel, J.-C.; Chevreul, K. Association of Pediatric Inpatient Socioeconomic Status with Hospital Efficiency and Financial Balance. JAMA Netw. Open 2019, 2, e1913656. [Google Scholar] [CrossRef] [PubMed]
- Dionne, A.; Bucholz, E.M.; Gauvreau, K.; Gould, P.; Son, M.B.F.; Baker, A.L.; de Ferranti, S.D.; Fulton, D.R.; Friedman, K.G.; Newburger, J.W. Impact of Socioeconomic Status on Outcomes of Patients with Kawasaki Disease. J. Pediatr. 2019, 212, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Dewan, P.; Shah, D.; Sharma, N.; Bedi, N.; Kaur, I.R.; Bansal, A.K.; Madhu, S.V. Vitamin D supplementation for treatment and prevention of pneumonia in under-five children: A randomized double-blind placebo controlled trial. Indian Pediatr. 2016, 53, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.; Gupta, P. Vitamin D supplementation for severe pneumonia—A randomized controlled trial. Indian Pediatr. 2012, 49, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Somnath, S.H.; Biswal, N.; Chandrasekaran, V.; Jagadisan, B.; Bobby, Z. Therapeutic effect of vitamin D in acute lower respiratory infection: A randomized controlled trial. Clin. Nutr. ESPEN 2017, 20, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Manaseki-Holland, S.; Qader, G.; Masher, M.I.; Bruce, J.; Mughal, M.Z.; Chandramohan, D.; Walraven, G. Effects of vitamin D supplementation to children diagnosed with pneumonia in Kabul: A randomised controlled trial. Trop. Med. Int. Health 2010, 15, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Rajshekhar, C.S.; Vanaki, R.; Badakali, A.V.; Pol, R.R.; Yelamali, B.C. Efficacy of vitamin D supplementation in the treatment of severe pneumonia in children aged less than five years. Int. J. Contemp. Pediatr. 2016, 3, 96–99. [Google Scholar] [CrossRef][Green Version]
- Das, R.R.; Singh, M.; Naik, S.S. Vitamin D as an adjunct to antibiotics for the treatment of acute childhood pneumonia. Cochrane Database Syst. Rev. 2018, 7, CD011597. [Google Scholar] [CrossRef]
- Chowdhury, F.; Bin Shahid, A.S.M.S.; Tabassum, M.; Parvin, I.; Ghosh, P.K.; Hossain, M.I.; Alam, N.H.; Faruque, A.S.G.; Huq, S.; Shahrin, L.; et al. Vitamin D supplementation among Bangladeshi children under-five years of age hospitalised for severe pneumonia: A randomised placebo controlled trial. PLoS ONE 2021, 16, e0246460. [Google Scholar] [CrossRef]
- Grant, W.B.; Cross, H.S.; Garland, C.F.; Gorham, E.D.; Moan, J.; Peterlik, M.; Porojnicu, A.C.; Reichrath, J.; Zittermann, A. Estimated benefit of increased vitamin D status in reducing the economic burden of disease in western Europe. Prog. Biophys. Mol. Biol. 2009, 99, 104–113. [Google Scholar] [CrossRef] [PubMed]


| Age (years), mean (SD) | 6.3 (4.3) |
| Gender, n (%) | |
| Male | 445 (54) |
| Female | 382 (46) |
| BMI z-score, mean (SD) | 0.6 (1.7) |
| Ethnicity, n (%) | |
| Caucasian | 691 (84) |
| African | 45 (5) |
| North African | 43 (5) |
| Indian | 27 (3) |
| Hispanic | 14 (2) |
| Asian | 7 (1) |
| Gestational age, n (%) | |
| Full-term born (≥37 weeks) | 749 (91) |
| Preterm born (<37 weeks) | 78 (9) |
| Birth weight (kg), mean (SD) | 3.2 (0.6) |
| Hospitalization season, n (%) | |
| Spring | 213 (26) |
| Summer | 230 (28) |
| Autumn | 201 (24) |
| Winter | 183 (22) |
| Serum vitamin D (ng/mL), mean (SD) | 20.7 (11.2) |
| Disease group (ICD-9 code), n (%) | |
| Respiratory diseases (460–519) | 230 (28) |
| Nutritional-metabolic diseases (240–279) | 195 (24) |
| Ill-defined conditions (780–799) | 88 (11) |
| Infections (001–139) | 60 (7) |
| Digestive diseases (520–579) | 53 (6) |
| Nervous diseases (320–389) | 43 (5) |
| Injuries and poisonings (800–999) | 43 (5) |
| Genitourinary diseases (580–629) | 41 (5) |
| Musculoskeletal diseases (710–739) | 25 (3) |
| Blood diseases (280–289) | 25 (3) |
| Skin diseases (680–709) | 24 (3) |
| Length of stay (days), mean (SD) | 3.7 (2.2) |
| Group A n = 129 (16%) | Group B n = 518 (62%) | Group C n = 180 (22%) | p-Value 1 | |
|---|---|---|---|---|
| Serum vitamin D (ng/mL), mean (SD) | 13.7 (9.4) | 20.5 (10.0) | 26.2 (12.6) | <0.001 |
| Age (years), mean (SD) | 5.7 (4.2) | 6.6 (4.4) | 5.9 (3.9) | 0.052 |
| Gender, n (%) | 0.753 | |||
| Male | 70 (54) | 274 (53) | 101 (56) | |
| Female | 59 (46) | 244 (47) | 79 (44) | |
| BMI z-score, mean (SD) | 0.2 (1.5) | 0.8 (1.8) | 0.4 (1.4) | <0.001 |
| Gestational age, n (%) | 0.041 | |||
| Full-term born (≥37 weeks) | 120 (93) | 459 (89) | 170 (94) | |
| Preterm born (<37 weeks) | 9 (7) | 59 (11) | 10 (6) | |
| Birth weight (kg) | 3.2 (0.6) | 3.2 (0.6) | 3.3 (0.5) | 0.074 |
| Hospitalization season, n (%) | 0.205 | |||
| Spring | 44 (34) | 119 (23) | 50 (28) | |
| Summer | 28 (22) | 149 (29) | 53 (29) | |
| Autumn | 30 (23) | 130 (25) | 41 (23) | |
| Winter | 27 (21) | 120 (23) | 36 (20) | |
| Length of stay (days), mean (SD) | 4.6 (2.5) | 3.6 (2.1) | 3.4 (2.2) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrante, G.; Fasola, S.; Piazza, M.; Tenero, L.; Zaffanello, M.; La Grutta, S.; Piacentini, G. Vitamin D and Healthcare Service Utilization in Children: Insights from a Machine Learning Approach. J. Clin. Med. 2022, 11, 7157. https://doi.org/10.3390/jcm11237157
Ferrante G, Fasola S, Piazza M, Tenero L, Zaffanello M, La Grutta S, Piacentini G. Vitamin D and Healthcare Service Utilization in Children: Insights from a Machine Learning Approach. Journal of Clinical Medicine. 2022; 11(23):7157. https://doi.org/10.3390/jcm11237157
Chicago/Turabian StyleFerrante, Giuliana, Salvatore Fasola, Michele Piazza, Laura Tenero, Marco Zaffanello, Stefania La Grutta, and Giorgio Piacentini. 2022. "Vitamin D and Healthcare Service Utilization in Children: Insights from a Machine Learning Approach" Journal of Clinical Medicine 11, no. 23: 7157. https://doi.org/10.3390/jcm11237157
APA StyleFerrante, G., Fasola, S., Piazza, M., Tenero, L., Zaffanello, M., La Grutta, S., & Piacentini, G. (2022). Vitamin D and Healthcare Service Utilization in Children: Insights from a Machine Learning Approach. Journal of Clinical Medicine, 11(23), 7157. https://doi.org/10.3390/jcm11237157








