1. Introduction
The incidence of urethral stricture in the United States is as high as 0.6% with an annual healthcare cost of 191 million, which is expected to increase as the population ages [
1]. Urethral stricture refers to the pathological narrowing of the urethral lumen secondary to scar formation in the subepithelial connective tissue and can occur anywhere along the anterior urethra, the portion distal to the genitourinary diaphragm that is enveloped in corpus spongiosum. Within the anterior urethra, the bulbar urethra is the most common site for stricture development, comprising almost 50% of all urethral strictures treated, with the majority in the United States being of either iatrogenic or idiopathic in origin [
2,
3]. Urethroplasty remains the gold standard in management of this complex disease and has been an area of active innovation since the first urethroplasty in the late 19th century. This review will cover some of the new urethroplasty techniques and how they incorporate into existing treatment pathways. We will focus on non-transecting techniques that preserve blood supply, tissue, and minimize morbidity.
2. Diagnosis and Evaluation
Initial evaluation starts with a focused genitourinary history, patient reported outcome index, uroflowmetry, post-void residual, and urinalysis. If the patient has been managed with intermittent self-catheterization or indwelling urethral catheter, a SPC should be placed to allow for urethral rest. Radiologic imaging—retrograde urethrogram (RUG) and/or voiding cystourethrogram (VCUG)—is critical to defining stricture length, location, lumen, as is cystourethroscopy to assessing tissue quality and ruling out malignancy [
4].
The surgeon should stage the stricture by length, lumen, and location prior to a surgical intervention. These factors, in addition to patient history, stricture etiology, and patient goals are key in the medical decision making
3. Management Options and Treatment Algorithm for Bulbar Urethral Strictures
While initial endoscopic management for appropriately selected patients can achieve short-term success, repetitive treatment is usually unsuccessful [
5,
6]. Long-term outcomes are poor, with approximately 50% of patients failing at 1 year, and durable cure achieved in less than 30% [
7,
8]. Current guidelines favor urethroplasty in managing urethral strictures and only indicate dilation or direct vision internal urethrotomy (DVIU) in strictures less than 2 cm in length, or in situations where the increased anesthesia requirement, cost, and higher morbidity of urethroplasty outweigh its higher success rate [
9]
Although a more invasive and technically challenging, urethroplasty has been shown to have superior outcomes when compared to endoscopic procedures, with a long-term success rate of 80–95% [
2,
10,
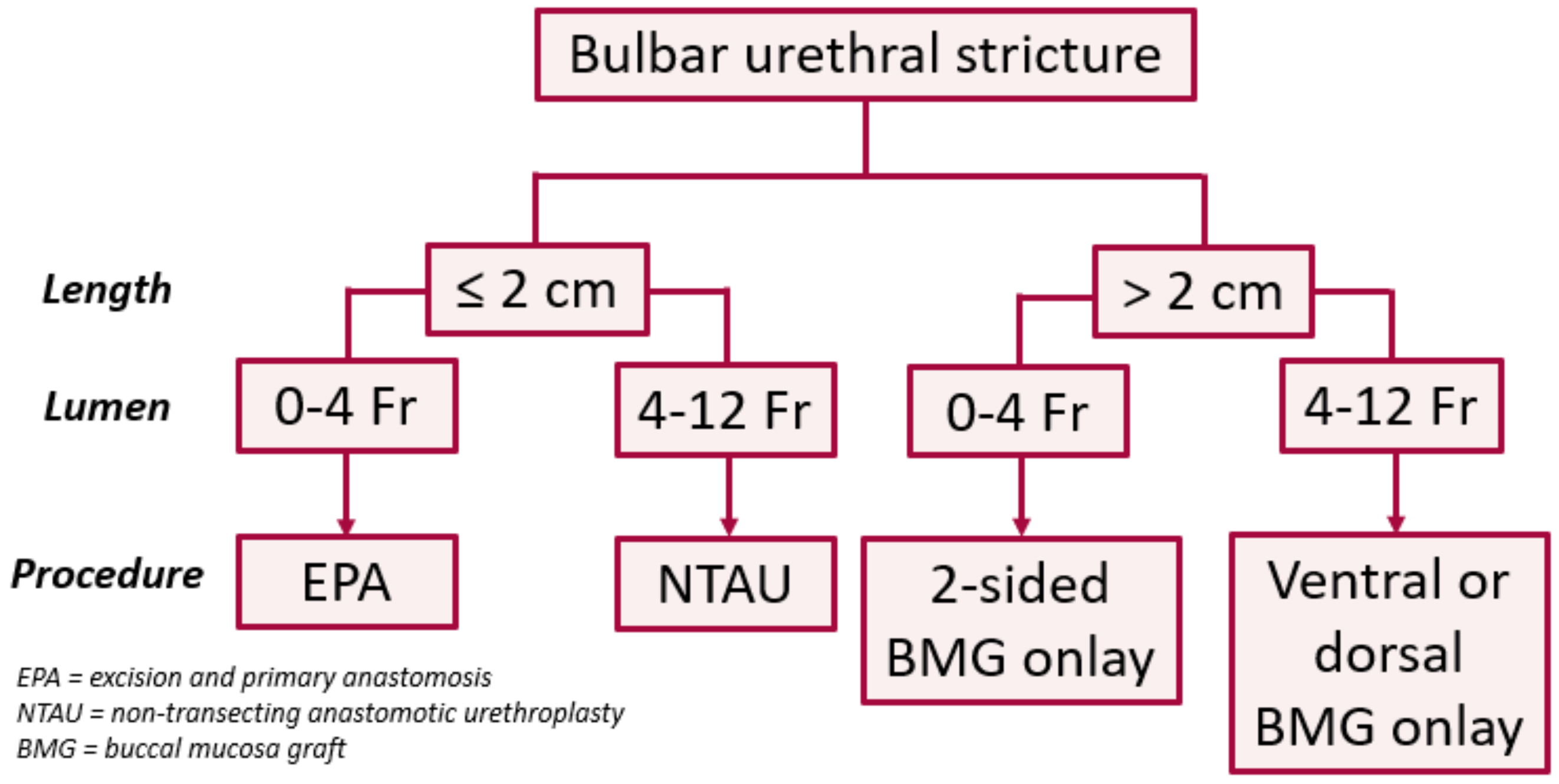
11]. In the bulbar urethra, the authors use a treatment algorithm based on length and lumen caliber (
Figure 1) [
12]. In patients with a short (≤2 cm) obliterative (0–4 Fr) strictures, excision and primary anastomosis (EPA) is our treatment of choice, especially for traumatic strictures with severe spongiofibrosis. If the stricture is short but non-obliterative (>4 Fr), we prefer a non-transecting anastomotic urethroplasty (NTAU). In patients with longer (>2 cm) strictures, we prefer non-transecting techniques using buccal mucosa graft (BMG) onlay, ac 1-sided dorsal graft if the stricture lumen is >4 Fr and a two-sided (ventral and dorsal) graft if <4 Fr. While flexible, we prefer the dorsal approach in most circumstances. The 4 Fr cut-off employed here is based on our clinical experience with passage of a standard open-ended catheter into the bladder at the beginning of the case.
4. Bulbar Urethroplasty: Transecting vs. Non-Transecting Techniques
For bulbar urethral strictures, an EPA urethroplasty has been the gold standard, with a well-established, durable success rate of 90–95% in properly selected patients [
13,
14]. Traditional EPAs require full transection of urethra and bulbospongiosus. The scar and surrounding fibrosis are completely excised, and both ends of the urethra are spatulated and reconnected in a tension free manner. The antegrade flow from the bulbourethral arteries is often interrupted. The distal aspect of the urethra then relies partially on retrograde flow from the dorsal penile artery via connections within the glans.
While the stricture patency rates are excellent, concerns have been raised regarding post-operative sexual dysfunction related to both neural and vascular disruptions associated with mobilization and transection of the bulbar urethra [
15]. This is not always well captured on existing patient reported outcomes, such as the International Index of Erectile Function (IIEF-5). A recent randomized trial addressed this gap by developing a novel outcome measure specific to urethroplasty with which they were able to detect significant differences in penile complications, including shortening and reduced glans filling, despite similar IIEF-5 scores between transecting and non-transecting groups (
Table 1) [
16].
Another concern around traditional EPAs relates to the evolving etiology of stricture disease. Iatrogenic strictures related to treatment of prostate hypertrophy and cancer are on the rise [
21]. Radiation in particular can be challenging to reconstruction, and roughly 13% of patients will have a stricture recurrence after an EPA in this setting [
22]. Limiting dissection in the radiated field is important in order to preserve blood flow for any subsequent procedure for continence, recurrence, or metachronous stricture [
23].
In contrast, non-transecting techniques encompass a variety of maneuvers for managing bulbar urethral strictures without full transection of the corpus spongiosum. Grafting techniques, in which the lumen is opened and augmented with buccal mucosa can be thought of as a non-transecting technique, although they were not specifically developed as such [
24]. Over the past 15 years, tissue-sparing approaches have been explicitly developed to address the perceived short-comings with EPA. In 2007, Jordan et al., introduced a vessel sparing (VS-EPA) technique designed to preserve proximal blood flow from the bulbourethral arteries in post-prostatectomy patients in order to prevent erosion of a future artificial urinary sphincter or male sling [
25]. This was further refined by Mundy in 2012 and has become an invaluable tool in reconstructive armamentarium [
23]. An international collaboration demonstrated the long-term patency of this technique; 95% of the 68 patients who underwent a VS-EPA remained patent at 17 month follow-up [
26]. It has been shown that patients undergoing VS-EPA had a lesser negative impact on their erectile function compared to those undergoing EPA [
18].
5. Surgical Technique and Intra-Operative Decision Making
When performing a non-transecting urethroplasty (NTU), surgical dissection is kept to a minimum. Ventrolateral dissection is minimized in order to protect the bulbar arteries. Attempts to preserve the urethral artery within the corpus spongiosum are made by incising the urethra dorsally, though in cases of deep spongiofibrosis, the urethral artery might be compromised. While pre-operative planning with RUG to determine the stricture characteristics guides the surgical technique in most instances, intraoperative findings may alter the surgical needs. For this reason, the authors start with 1-sided dissection to access the dorsal urethra in most cases in efforts to minimize trauma to the local tissue and maintain surgical options.
The patient is placed in a low lithotomy position (no more than 90 degrees of hip flexion). A flexible cystoscope is used to bypass the stricture with a sensor wire, followed by a 5-Fr open ended catheter. A midline perineal incision is made and carried down to the bulbospongiosus muscle. The bulbar urethra is then un-roofed from its surrounding ventral attachments and mobilized on one side from its lateral and dorsal attachment to the tunica albuginea of the corpora cavernosa. Typically, the dissection borders are proximally to the perineal membrane and distally towards the penile suspensory ligament. This is done unilaterally to reduce tissue trauma. A dorsal longitudinal stricturotomy is performed until a 20-French Bougie can be easily passed proximally and distally within healthy appearing urethral mucosa. Stay stitches are used to facilitate visualization and rotate the urethra into the surgical field. At this time, the full length of stricture and degree of spongiofibrosis is determined, which will dictate the surgical technique. Tissue mobility, additional urethral length for spatulation if transection is being considered, as well as the extent of clinical significant fibrosis to be addressed during the procedure are all important to consider.
If the stricture is short (<2 cm), decision making is driven by lumen, degree of spongiofibrosis, and location within the bulbar urethra. Short strictures with minimal underlying fibrosis located within the proximal bulbar urethra can be managed with a stricturoplasty using the Heineke–Mikulicz (HM) principle by closing the longitudinal stricturotomy in a transverse manner [
27]. Good urethral mobility and elasticity are critical for the success of this technique. For short strictures with moderate underlying fibrosis, a non-transecting excision of the stricture and mucosal anastomosis, as described by Mundy et al., can be performed [
23,
28]. This involves excising the abnormal mucosa and underlying spongiofibrosis with preservation of healthy ventral sponge, thus minimizing disruption in longitudinal blood flow. The mucosal ends are then anastomosed together and the dorsal stricturotomy is closed in a transverse manner. If there is an obliterative stricture with significant circumferential spongiofibrosis, as is often the case for traumatic bulbar strictures, excision of the obliterative segment is typically required. If the excised segment is short such that the ends of the urethra approximate without tension, an EPA can be performed in the usual manner. If the excised segment is too long for direct suture closure, an augmented anastomotic urethroplasty (AAU) is performed in which the ventral or dorsal urethra is approximated, and the opposite side is grafted. To perform this in a non-transecting manner (NTAU), a double graft or mucosectomy is required (see below).
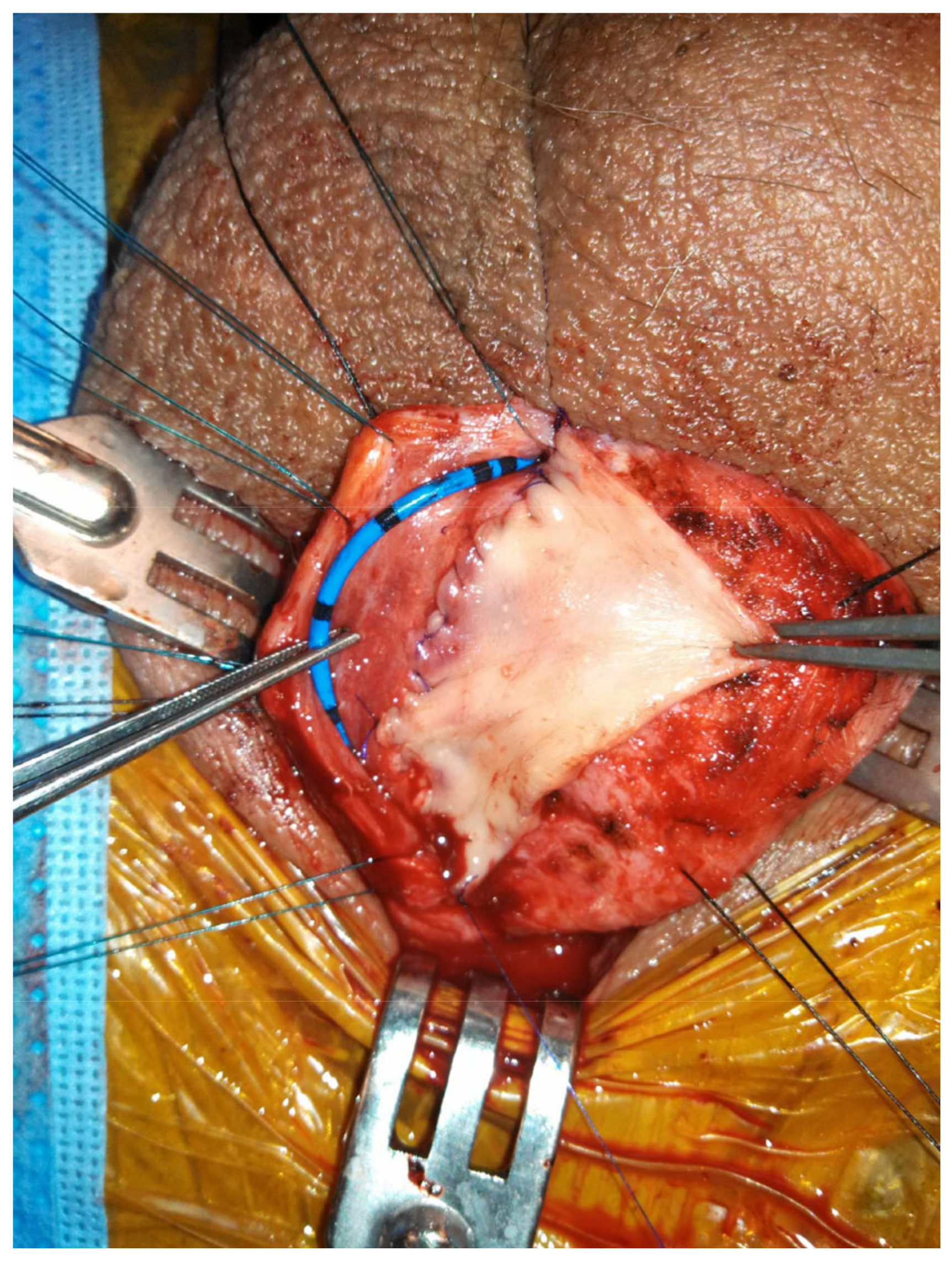
When the bulbar stricture is long (>2 cm) and non-obliterative, a dorsal onlay urethroplasty can be performed. Initially described by Barbagli in 1996, the technique has been modified over the years and has been shown to have durable success rates [
29]. Dorsal graft placement has the benefit of strong corporal fixation, thus reducing risk of sacculation (
Figure 2). There is also less operative blood loss due to the thinner bulbospongiosus dorsally. Ventral onlay described by Morey and McAnnich is also an option with durable success [
30]. This option is preferred by some when the exposure is challenging due to proximal stricture location or obesity.
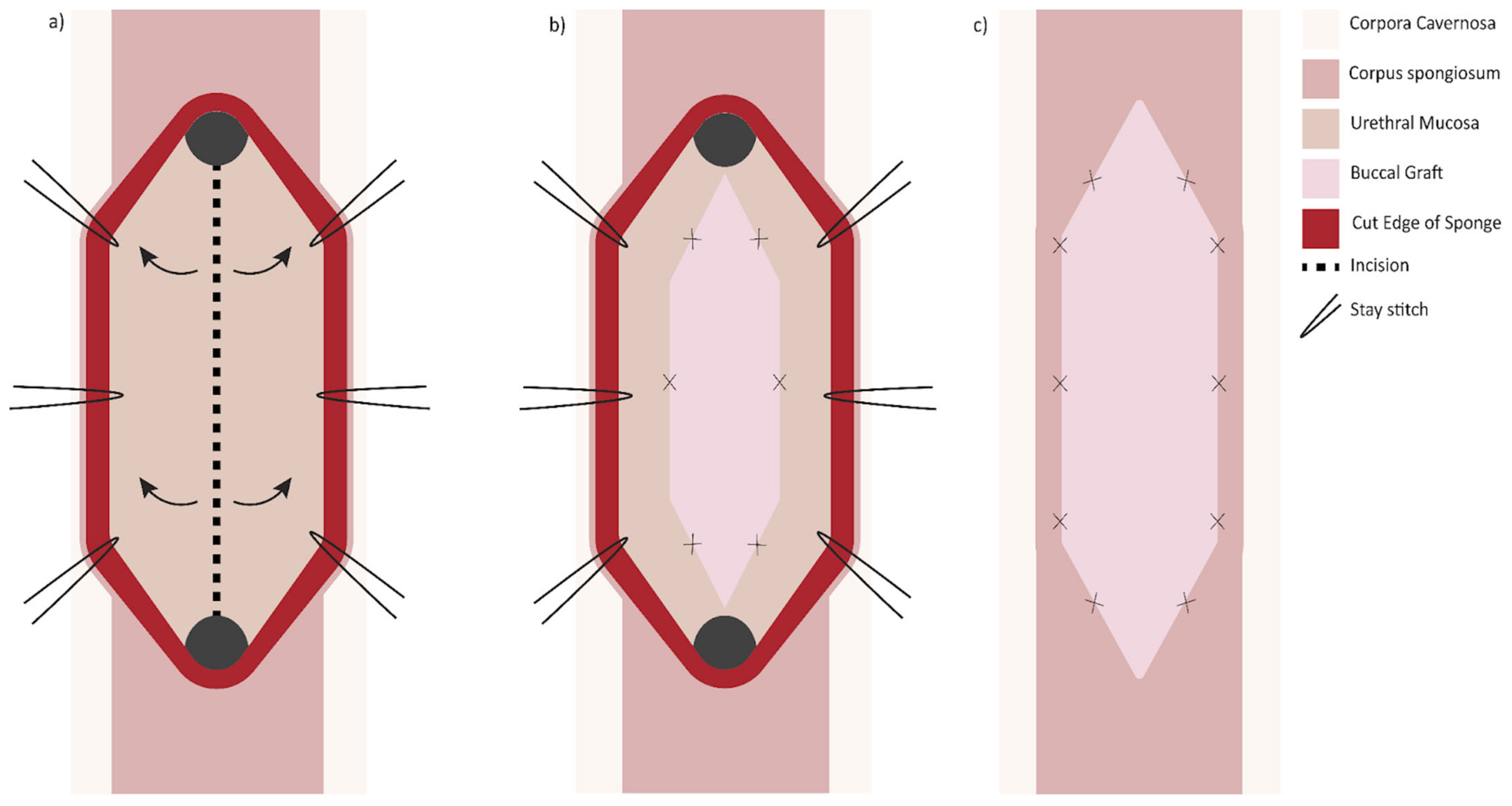
Another option when presented with a long (>2 cm) narrow (<4 Fr) stricture is two-sided (dorsal and ventral) grafting technique. The first iteration described by Palminteri in 2008 was performed by making a ventral urethrotomy through which the dorsal urethra is incised to expose tunica albuginea. A dorsal inlay BMG is then placed, followed by a ventral onlay (
Figure 3) [
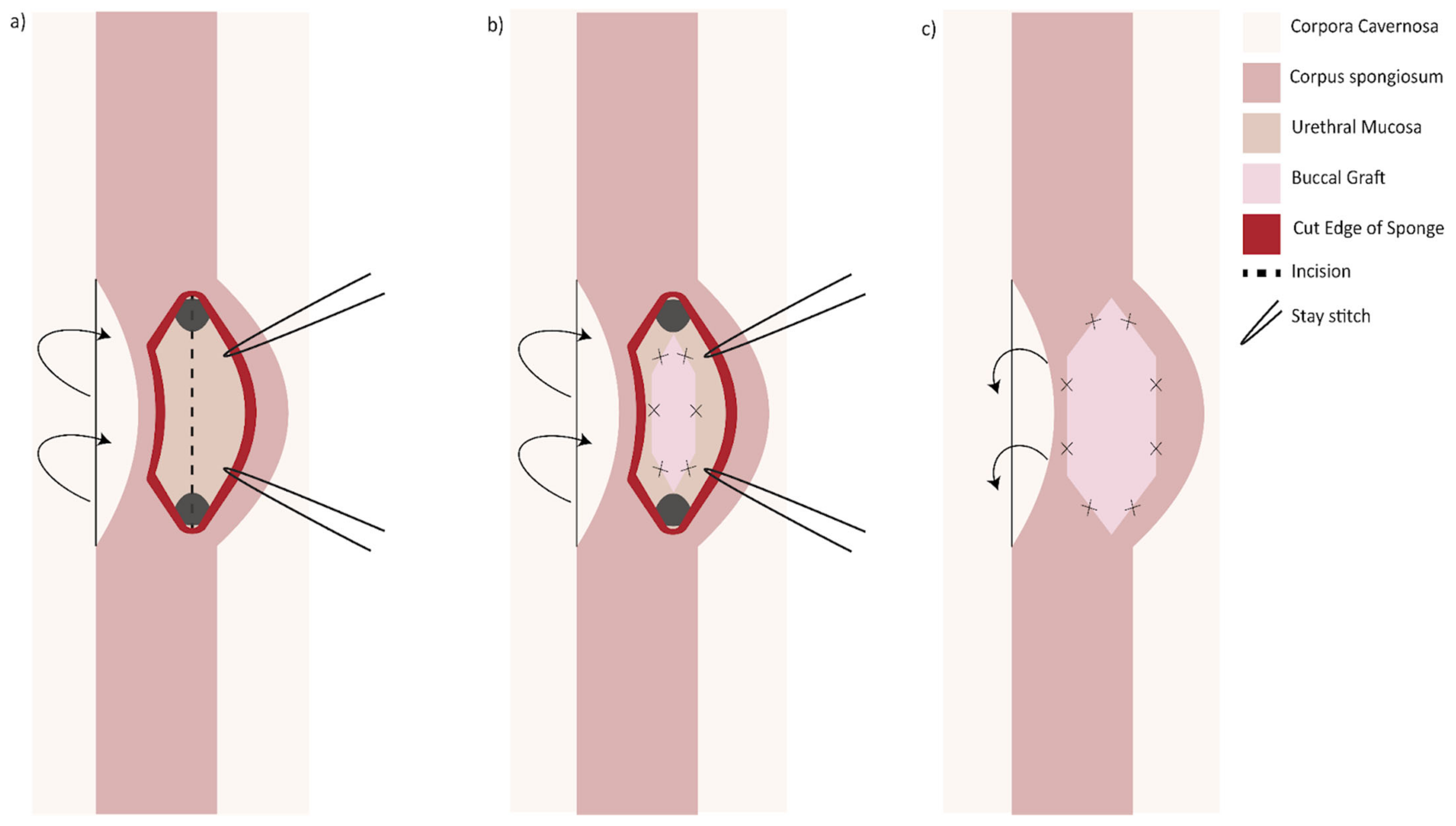
31]. Similarly, a dorsal urethrotomy is made through which the ventral urethral mucosa is incised, and a BMG may be inlayed followed by a dorsal onlay (
Figure 4). This has the proposed benefit of minimizing trauma to the thicker ventral spongiosum and providing a backing for the ventral graft [
32].
6. Discussion
Formal comparison of patency rates between urethroplasty techniques is challenging, as most published studies are retrospective cohorts and thus confounded by non-random patient selection for a given approach. The situation is further complicated by the varying definitions of “success” used and length of follow-up, which can affect the true efficacy of urethroplasty and makes comparisons between studies difficult (
Table 1) [
33]. The available data broadly suggest similar patency rates between transecting and non-transecting techniques in the bulbar urethra [
17,
18,
19]. Barbagli et al., performed a survey of seminal papers on varying bulbar urethroplasty techniques and found that transecting techniques (EPA and AAU) provided success rates ranging from 90 to 98.6% in 404 patients. The non-transecting techniques (NTAU and BMG augmented techniques) provided approximately the same success rate, ranging from 81.8 to 100% in 522 patients [
34]. Other groups have also found no significant difference in long-term stricture-free recurrence rates between the transecting and non-transecting bulbar urethroplasty [
19]. Important randomized control data awaits publication of the VeSpAR trial, a prospective, interventional, multi-center study with 1:1 randomization of patients to vessel-sparing to transecting anastomotic repair in short bulbar urethral strictures, although this will still leave unanswered questions regarding augmented techniques [
35].
Changes in sexual function following urethroplasty has been the subject of intense scrutiny, with mixed results and similar caveats regarding data quality as discussed above. When measured by IIEF-5 or Sexual Health Inventory for Men (SHIM), multiple retrospective studies have shown transient erectile dysfunction (ED) that resolves within 6 months, and little to no difference between transecting and non-transecting groups [
15,
17,
20,
36]. Nevertheless, many of these “negative” studies have shown non-significant trends favoring non-transection, and the most recent and largest of these cohorts by Chapman et al., did find significantly more de novo ED in the transecting group at the 6 month mark (14.3% vs. 4.3%,
p = 0.008) [
18]. Further, a 2022 randomized control trial published by Nilsen et al., showed significant increased complaints of penile shortening and decreased glans fullness during erections, albeit with similar IIEF-5 scores between the groups at 3 months and 12 months [
16]. This is consistent with prior retrospective data showing increased complaint of penile shortening associated with EPA [
17].
Our understanding of bulbar urethral strictures continues to evolve to vessel sparing, non-transecting techniques in efforts to avoid sexual side-effects without compromising surgical outcome. New advancements in surgical technique over the past 15 years have expanded the surgical armamentarium regarding bulbar urethroplasty. The etiology, length, location, and lumen caliber are key criteria to consider when planning surgical repair. Our algorithm provides a logical framework to guide surgical decision making, while still allowing flexibility in procedure selection based on surgical findings. In the setting of a non-traumatic, non-obliterative stricture, the technique should be non-transecting when possible, based on emerging data suggesting improved sexual outcomes with similar patency rates.
Author Contributions
Conceptualization, B.F. and N.C.; methodology, M.H.; writing—original draft preparation, N.C., M.H. and A.M.; writing—review and editing, N.C., M.H. and B.F.; visualization, N.C.; supervision, B.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AAU | augmented anastomotic urethroplasty |
| BMG | buccal mucosa graft |
| DVIU | direct vision internal urethrotomy |
| ED | erectile dysfunction |
| EPA | excision and primary anastomosis |
| HM | Heineke-Mickulicz |
| IIEF-5 | international index of erectile function |
| NTAU | non-transecting anastomotic urethroplasty |
| NTU | non-transecting urethroplasty |
| RUG | retrograde urethrogram |
| SPT | suprapubic tube |
| VCUG | voiding cystourethrogram |
| VS-EPA | vessel sparing excision and primary anastomosis |
References
- Santucci, R.A.; Joyce, G.F.; Wise, M. Male urethral stricture disease. J. Urol. 2007, 177, 1667–1674. [Google Scholar] [CrossRef]
- Mundy, A.R.; Andrich, D.E. Urethral strictures. BJU Int. 2011, 107, 6–26. [Google Scholar] [CrossRef]
- Palminteri, E.; Berdondini, E.; Verze, P.; De Nunzio, C.; Vitarelli, A.; Carmignani, L. Contemporary urethral stricture characteristics in the developed world. Urology 2013, 81, 191–196. [Google Scholar] [CrossRef]
- Campos-Juanatey, F.; Osman, N.I.; Greenwell, T.; Martins, F.E.; Riechardt, S.; Waterloos, M.; Barratt, R.; Chan, G.; Esperto, F.; Ploumidis, A.; et al. European Association of Urology Guidelines on Urethral Stricture Disease (Part 2): Diagnosis, Perioperative Management, and Follow-up in Males. Eur. Urol. 2021, 80, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Heyns, C.F.; Steenkamp, J.W.; De Kock, M.L.; Whitaker, P. Treatment of male urethral strictures: Is repeated dilation or internal urethrotomy useful? J. Urol. 1998, 160, 356–358. [Google Scholar] [CrossRef]
- Santucci, R.; Eisenberg, L. Urethrotomy has a much lower success rate than previously reported. J. Urol. 2010, 183, 1859–1862. [Google Scholar] [CrossRef] [PubMed]
- Furr, J.; Gelman, J. Endoscopic Management of Urethral Stricture Disease and Bladder Neck Contractures. J. Endourol. 2020, 34, S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.K.; Kumar, S.; Ghosh, B. Direct visual internal urethrotomy: Is it a durable treatment option? Urol. Ann. 2017, 9, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Wessells, H.; Angermeier, K.W.; Elliott, S.; Gonzalez, C.M.; Kodama, R.; Peterson, A.C.; Reston, J.; Rourke, K.; Stoffel, J.T.; Vanni, A.J.; et al. Male Urethral Stricture: American Urological Association Guideline. J. Urol. 2017, 197, 182–190. [Google Scholar] [CrossRef]
- Barbagli, G.; Kulkarni, S.B.; Fossati, N.; Larcher, A.; Sansalone, S.; Guazzoni, G.; Romano, G.; Pankaj, J.M.; Dell’Acqua, V.; Lazzeri, M. Long-term followup and deterioration rate of anterior substitution urethroplasty. J. Urol. 2014, 192, 808–813. [Google Scholar] [CrossRef]
- Morey, A.F.; Watkin, N.; Shenfeld, O.; Eltahawy, E.; Giudice, C. SIU/ICUD Consultation on Urethral Strictures: Anterior urethra—Primary anastomosis. Urology 2014, 83 (Suppl. 3), S23–S26. [Google Scholar] [CrossRef] [PubMed]
- Warncke, J.; Marks, J.; Koslov, D.; Villarreal, H.; Parrillo, L.; Flynn, B. Mp67-10—A simplified surgical algorithm for the management of bulbar urethral strictures: Comparison of transecting versus non-transecting urethroplasty at a single institution. J. Urol. 2019, 201 (Suppl. 4), e971. [Google Scholar] [CrossRef]
- Eltahawy, E.A.; Virasoro, R.; Schlossberg, S.M.; McCammon, K.A.; Jordan, G.H. Long-term followup for excision and primary anastomosis for anterior urethral strictures. J. Urol. 2007, 177, 1803–1806. [Google Scholar] [CrossRef]
- Barbagli, G.; De Angelis, M.; Romano, G.; Lazzeri, M. Long-term followup of bulbar end-to-end anastomosis: A retrospective analysis of 153 patients in a single center experience. J. Urol. 2007, 178, 2470–2473. [Google Scholar] [CrossRef] [PubMed]
- Calleja Hermosa, P.; Campos-Juanatey, F.; Varea Malo, R.; Correas Gómez, M.Á.; Gutiérrez Baños, J.L. Trauma and Reconstructive Urology Working Party of the European Association of Urology Young Academic Urologists. Sexual function after anterior urethroplasty: A systematic review. Transl. Androl. Urol. 2021, 10, 2554–2573. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, O.J.; Holm, H.V.; Ekerhult, T.O.; Lindqvist, K.; Grabowska, B.; Persson, B.; Sairanen, J. To Transect or Not Transect: Results from the Scandinavian Urethroplasty Study, A Multicentre Randomised Study of Bulbar Urethroplasty Comparing Excision and Primary Anastomosis Versus Buccal Mucosal Grafting. Eur. Urol. 2022, 81, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Furr, J.R.; Wisenbaugh, E.S.; Gelman, J. Urinary and Sexual Outcomes Following Bulbar Urethroplasty—An Analysis of 2 Common Approaches. Urology 2019, 130, 162–166. [Google Scholar] [CrossRef]
- Chapman, D.W.; Cotter, K.; Johnsen, N.V.; Patel, S.; Kinnaird, A.; Erickson, B.A.; Voelzke, B.; Buckley, J.; Rourke, K. Nontransecting Techniques Reduce Sexual Dysfunction after Anastomotic Bulbar Urethroplasty: Results of a Multi-Institutional Comparative Analysis. J. Urol. 2019, 201, 364–370. [Google Scholar] [CrossRef]
- Anderson, K.M.; Blakely, S.A.; O’Donnell, C.I.; Nikolavsky, D.; Flynn, B.J. Primary non-transecting bulbar urethroplasty long-term success rates are similar to transecting urethroplasty. Int. Urol. Nephrol. 2017, 49, 83–88. [Google Scholar] [CrossRef]
- Haines, T.; Rourke, K.F. The effect of urethral transection on erectile function after anterior urethroplasty. World J. Urol. 2017, 35, 839–845. [Google Scholar] [CrossRef]
- Chen, M.L.; Correa, A.F.; Santucci, R.A. Urethral Strictures and Stenoses Caused by Prostate Therapy. Rev. Urol. 2016, 18, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Voelzke, B.B.; Leddy, L.S.; Myers, J.B.; Breyer, B.N.; Alsikafi, N.F.; Broghammer, J.A.; Elliott, S.P.; Vanni, A.J.; Erickson, B.A.; Buckley, J.C.; et al. Multi-institutional Outcomes and Associations after Excision and Primary Anastomosis for Radiotherapy-associated Bulbomembranous Urethral Stenoses Following Prostate Cancer Treatment. Urology 2021, 152, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Andrich, D.E.; Mundy, A.R. Non-transecting anastomotic bulbar urethroplasty: A preliminary report. BJU Int. 2012, 109, 1090–1094. [Google Scholar] [CrossRef]
- El-Kasaby, A.W.; Fath-Alla, M.; Noweir, A.M.; el-Halaby, M.R.; Zakaria, W.; el-Beialy, M.H. The use of buccal mucosa patch graft in the management of anterior urethral strictures. J. Urol. 1993, 149, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Jordan, G.H.; Eltahawy, E.A.; Virasoro, R. The technique of vessel sparing excision and primary anastomosis for proximal bulbous urethral reconstruction. J. Urol. 2007, 177, 1799–1802. [Google Scholar] [CrossRef]
- Virasoro, R.; Zuckerman, J.M.; McCammon, K.A.; DeLong, J.M.; Tonkin, J.B.; Capiel, L.; Rovegno, A.R.; Favre, G.; Giudice, C.R.; Eltahawy, E.A.; et al. International multi-institutional experience with the vessel-sparing technique to reconstruct the proximal bulbar urethra: Mid-term results. World J. Urol. 2015, 33, 2153–2157. [Google Scholar] [CrossRef]
- Lumen, N.; Poelaert, F.; Oosterlinck, W.; Lambert, E.; Decaestecker, K.; Tailly, T.; Hoebeke, P.; Spinoit, A.-F. Nontransecting Anastomotic Repair in Urethral Reconstruction: Surgical and Functional Outcomes. J. Urol. 2016, 196, 1679–1684. [Google Scholar] [CrossRef]
- Ivaz, S.; Bugeja, S.; Frost, A.; Andrich, D.; Mundy, A.R. The Nontransecting Approach to Bulbar Urethroplasty. Urol. Clin. N. Am. 2017, 44, 57–66. [Google Scholar] [CrossRef]
- Barbagli, G.; Selli, C.; di Cello, V.; Mottola, A. A one-stage dorsal free-graft urethroplasty for bulbar urethral strictures. Br. J. Urol. 1996, 78, 929–932. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.P.; Metro, M.J.; McAninch, J.W. Long-term followup of the ventrally placed buccal mucosa onlay graft in bulbar urethral reconstruction. J. Urol. 2003, 169, 1754–1757. [Google Scholar] [CrossRef] [PubMed]
- Palminteri, E.; Manzoni, G.; Berdondini, E.; di Fiore, F.; Testa, G.; Poluzzi, M.; Molon, A. Combined dorsal plus ventral double buccal mucosa graft in bulbar urethral reconstruction. Eur. Urol. 2008, 53, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Barroso, U.; Prado, F. A new double graft technique in urethroplasty for complex urethral stenosis: Preliminary findings. Int. Braz. J. Urol. 2021, 47, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.T.; Vanni, A.J.; Erickson, B.A.; Myers, J.B.; Voelzke, B.; Breyer, B.N.; Broghammer, J.A.; Buckley, J.C.; Zhao, L.C.; Smith, T.G., 3rd; et al. Defining Success after Anterior Urethroplasty: An Argument for a Universal Definition and Surveillance Protocol. J. Urol. 2022, 208, 135–143. [Google Scholar] [CrossRef]
- Barbagli, G.; Sansalone, S.; Romano, G.; Lazzeri, M. Bulbar urethroplasty: Transecting vs. nontransecting techniques. Curr. Opin. Urol. 2012, 22, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Verla, W.; Waterloos, M.; Waterschoot, M.; Van Parys, B.; Spinoit, A.F.; Lumen, N. VeSpAR trial: A randomized controlled trial comparing vessel-sparing anastomotic repair and transecting anastomotic repair in isolated short bulbar urethral strictures. Trials 2020, 21, 782. [Google Scholar] [CrossRef] [PubMed]
- Beysens, M.; Palminteri, E.; Oosterlinck, W.; Spinoit, A.-F.; Hoebeke, P.; François, P.; Decaestecker, K.; Lumen, N. Anastomotic Repair versus Free Graft Urethroplasty for Bulbar Strictures: A Focus on the Impact on Sexual Function. Adv. Urol. 2015, 2015, 912438. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).