Is There an Added Neonatal Risk in Vacuum-Assisted Deliveries with Nuchal Cord?
Abstract
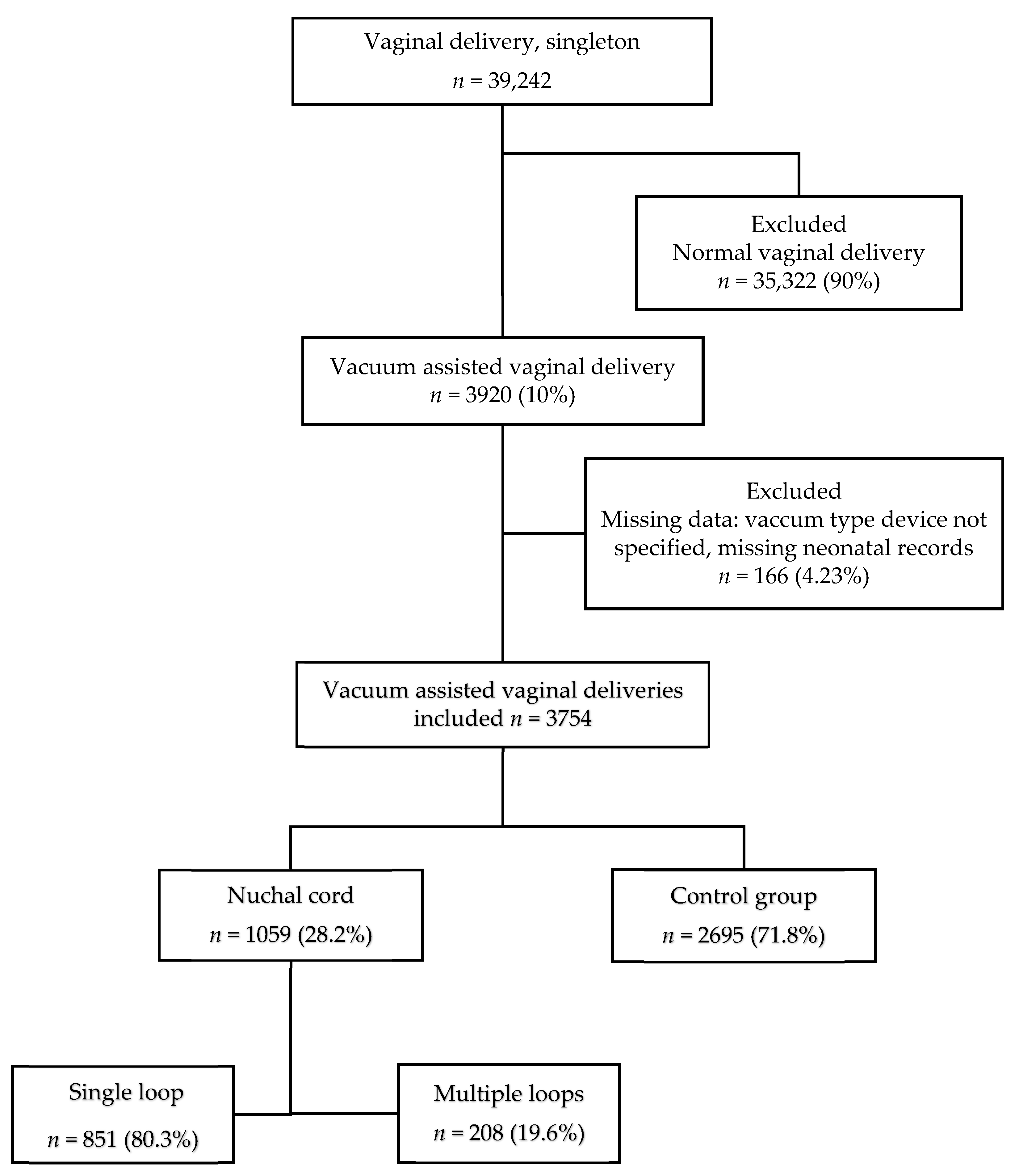
:1. Introduction
2. Materials and Methods
2.1. Data
2.2. Vacuum-Assisted Delivery (VAD)
2.3. Statistical Analysis
2.4. Power Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayes, D.J.L.; Warland, J.; Parast, M.M.; Bendon, R.W.; Hasegawa, J.; Banks, J.; Clapham, L.; Heazell, A.E.P. Umbilical cord characteristics and their association with adverse pregnancy outcomes: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0239630. [Google Scholar] [CrossRef] [PubMed]
- Zahedi-Spung, L.D.; Raghuraman, N.; Carter, E.; Cahill, A.G.; Rosenbloom, J.I. Umbilical artery cord gas abnormalities in the presence of a nuchal cord in term singleton pregnancies: A cohort study. Am. J. Perinatol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Carter, E.B.; Chu, C.S.; Thompson, Z.; Tuuli, M.G.; Macones, G.A.; Cahill, A.G. Electronic Fetal Monitoring and Neonatal Outcomes when a Nuchal Cord Is Present at Delivery. Am. J. Perinatol. 2020, 37, 378–383. [Google Scholar] [CrossRef]
- Gursoy, A.Y.; Ozgu, B.; Tasci, Y.; Candar, T. The impact of nuchal cord on umbilical cord blood gas analysis and ischaemia-modified albumin levels in elective C-section. J. Obstet. Gynecol. 2018, 38, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.-J.; Chen, Y.-H.; Zheng, H.-Y.; Xu, C.-M.; Liu, X.; Yan, S.-P. Standardized ultrasound diagnosis of nuchal cord. Int. J. Gen. Med. 2021, 14, 5825–5834. [Google Scholar] [CrossRef] [PubMed]
- Assimakopoulos, E.; Zafrakas, M.; Garmiris, P.; Goulis, D.G.; Athanasiadis, A.P.; Dragoumis, K.; Bontis, J. Nuchal cord detected by ultrasound at term is associated with mode of delivery and perinatal outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 123, 188–192. [Google Scholar] [CrossRef] [PubMed]
- American Institute of Ultrasound in Medicine. AIUM practice guideline for the performance of obstetric ultrasound examinations. J. Ultrasound Med. 2013, 32, 1083–1101. [Google Scholar] [CrossRef]
- Salomon, L.J.; Alfirevic, Z.; Berghella, V.; Bilardo, C.; Hernandez-Andrade, E.; Johnsen, S.L.; Kalache, K.; Leung, K.Y.; Malinger, G.; Munoz, H.; et al. Practice guidelines for performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet. Gynecol. 2011, 37, 116–126. [Google Scholar] [CrossRef]
- Kesrouani, A.; Daher, A.; Maoula, A.; Attieh, E.; Richa, S. Impact of a prenatally diagnosed nuchal cord on obstetrical outcome in an unselected population. J. Matern. Fetal Neonatal Med. 2017, 30, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Masad, R.; Gutvirtz, G.; Wainstock, T.; Sheiner, E. The effect of nuchal cord on perinatal mortality and long-term offspring morbidity. J. Perinatol. 2020, 40, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Ogueh, O.; Al-Tarkait, A.; Vallerand, D.; Rouah, F.; Morin, L.; Benjamin, A.; Usher, R.H. Obstetrical factors related to nuchal cord. Acta Obstet. Gynecol. Scand. 2006, 85, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Sherer, D.M.; Roach, C.; Soyemi, S.; Dalloul, M. Current perspectives of prenatal sonographic diagnosis and clinical management challenges of complex umbilical cord entanglement. Int. J. Womens Health 2021, 13, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, W. Antenatal course and perinatal outcome after ultrasound detection of triple nuchal cord: A case series. J. Matern. Fetal Neonatal Med. 2021, 34, 3246–3251. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, H.; Daykan, Y.; Arbib, N.; Markovitch, O.; Berkovitz, A.; Biron-Shental, T. Adverse pregnancy outcomes and multiple nuchal cord loops. Arch. Gynecol. Obstet. 2019, 300, 279–283. [Google Scholar] [CrossRef]
- Dollberg, S.; Haklai, Z.; Mimouni, F.B.; Gorfein, I.; Gordon, E.-S. Birth weight standards in the live-born population in Israel. Isr. Med. Assoc. J. 2005, 7, 311–314. [Google Scholar] [PubMed]
- Larson, J.D.; Rayburn, W.F.; Crosby, S.; Thurnau, G.R. Multiple nuchal cord entanglements and intrapartum complications. Am. J. Obstet. Gynecol. 1995, 173, 1228–1231. [Google Scholar] [CrossRef] [PubMed]
- Sheiner, E.; Abramowicz, J.S.; Levy, A.; Silberstein, T.; Mazor, M.; Hershkovitz, R. Nuchal cord is not associated with adverse perinatal outcome. Arch. Gynecol. Obstet. 2006, 274, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.W.; Chan, L.W.; To, W.W.K. Neonatal outcome and mode of delivery in the presence of nuchal cord loops: Implications on patient counselling and the mode of delivery. Arch. Gynecol. Obstet. 2015, 292, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Młodawska, M.; Młodawski, J.; Świercz, G.; Zieliński, R. The Relationship between Nuchal Cord and Adverse Obstetric and Neonatal Outcomes: Retrospective Cohort Study. Pediatr. Rep. 2022, 14, 40–47. [Google Scholar] [CrossRef]
- Mariya, T.; Fujibe, Y.; Shinkai, S.; Sugita, N. Multiple part umbilical cord entanglement and neonatal outcomes. Taiwan J. Obstet. Gynecol. 2018, 57, 672–676. [Google Scholar] [CrossRef] [PubMed]
| Variable | Nuchal Cord Group (n = 1059) | Control Group (n = 2695) | p-Value |
|---|---|---|---|
| Maternal age (years ± SD) | 30.12 ± 5.4 | 37.8 ± 2.6 | 0.427 |
| Gestational age (weeks + days ± SD) | 39 + 3 ± 1.3 | 39 + 3 ± 1.4 | 0.685 |
| Nullipara, n (%) | 729 (68.8) | 2004 (74.4) | 0.001 |
| Body mass index (kg/m2) | 23.6 ± 4.3 | 23.1 ± 4.5 | 0.017 |
| DM, n (%) | 74 (7.0) | 37 (10.6) | 0.735 |
| Smoking, n (%) | 53 (5) | 177 50.9) | 0.884 |
| Vaginal birth after cesarean, n (%) | 77 (7.3) | 208 (7.7) | 0.640 |
| Hypertension/Preeclampsia, n (%) | 27 (2.5) | 52 (1.9) | 0.234 |
| Variable | Nuchal Cord Group (n = 1059) | Control Group (n = 2695) | p-Value |
|---|---|---|---|
| Induction, n (%) | 207 (19.5) | 431 (16) | 0.009 |
| Meconium, n (%) | 203 (18.5) | 458 (17.0) | 0.115 |
| Epidural, n (%) | 944 (89.1) | 2695 (90.6) | 0.93 |
| 2nd-stage duration (min ± SD) | 128 ± 81 | 141 ± 80 | <0.001 |
| Head position-OA (n, %) | 809 (76.4) | 2095 (77.7) | 0.617 |
| Head station | 0.617 | ||
| S + 1 (n, %) | 567 (55.6) | 1502 (57.8) | |
| S + 2 (n, %) | 422 (41.4) | 1013 (39.0) | |
| S + 3 (n, %) | 22 (2.2) | 60 (2.3) | |
| Missing data | 48 (4.5) | 120 (4.4) | |
| Vacuum indication | 0.004 | ||
| NRFHR, n (%) | 830 (80.7) | 1989 (75.6) | |
| Prolonged 2nd stage, n (%) | 186 (18.1) | 607 (23.1) | |
| Other, n (%) | 43 (4.1) | 99 (3.7) | |
| Cup type | 0.522 | ||
| Kiwi, n (%) | 690 (65.2) | 1726 (64.0) | |
| Sylastic, n (%) | 369 (34.8) | 969 (36.0) | |
| Cup detachment, n (%) | 212 (20.1) | 566 (21.0) | 0.514 |
| True knot, n (%) | 10 (0.9) | 25 (0.9) | 0.962 |
| Episiotomy, n (%) | 1573 (76.1) | 250 (71.8) | 0.087 |
| Birthweight, (g ± SD) | 3185 ± 413 | 3223 ± 436 | 0.013 |
| Small for gestational age, n (%) | 113 (10.7) | 255 (9.6) | 0.262 |
| Variable | Nuchal Cord Group (n = 1059) | Control Group (n = 2695) | p-Value |
|---|---|---|---|
| 5 min Apgar ≤ 7, n (%) | 11 (1.0) | 25 (0.9) | 0.751 |
| Cord pH ≤ 7.15, n (%) | 103 (9.7) | 249 (9.2) | 0.890 |
| Cord pH ≤ 7.1, n (%) | 54 (5.1) | 107 (4.0) | 0.188 |
| Neonatal intensive care unit admission, n (%) | 18 (1.7) | 41 (1.5) | 0.693 |
| Mechanical ventilation, n (%) | 6 (0.6) | 12 (0.45) | 0.628 |
| Phototherapy, n (%) | 77 (7.3) | 192 (7.1) | 0.875 |
| Subgaleal hematoma, n (%) | 45 (4.2) | 141 (5.2) | 0.228 |
| Shoulder dystocia, n (%) | 17 (1.6) | 42 (1.6) | 0.917 |
| Clavicular fracture, n (%) | 8 (0.8) | 22 (0.8) | 0.850 |
| Third/fourth-degree perineal tear, n (%) | 20 (1.9) | 60 (2.2) | 0.519 |
| Variable | Multiple Nuchal Cord (n = 208) | No Nuchal Cord (n = 2695) | p-Value |
|---|---|---|---|
| 5 min Apgar ≤ 7, n (%) | 2 (1) | 25 (0.9) | 0.999 |
| pH ≤ 7.15, n (%) | 27 (13.0) | 249 (9.2) | 0.106 |
| pH ≤ 7.1, n (%) | 14 (6.7) | 107 (4.0) | 0.068 |
| Neonatal intensive care unit admission, n (%) | 0 (0) | 41 (1.5) | 0.115 |
| Subgaleal hematoma, n (%) | 6 (2.9) | 141 (5.2) | 0.137 |
| Shoulder dystocia, n (%) | 1 (0.5) | 42 (1.6) | 0.366 |
| Clavicular fracture, n (%) | 1 (0.5) | 22 (0.8) | 0.999 |
| Third/fourth-degree perineal tear, n (%) | 4 (2.0) | 60 (2.2) | 0.699 |
| Variable | p-Value | OR | 95% Confidence Interval | |
|---|---|---|---|---|
| Lower | Upper | |||
| Maternal BMI | 0.087 | 1.02 | 0.997 | 1.044 |
| Parous | <0.001 | 1.64 | 1.26 | 2.14 |
| Labor induction | <0.001 | 0.66 | 0.53 | 0.81 |
| Second-stage duration | 0.713 | 1.00 | 1.00 | 1.00 |
| NRFHR | 0.396 | 1.14 | 0.85 | 1.53 |
| Birthweight | 0.002 | 1.00 | 0.99 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schreiber, H.; Cohen, G.; Mevorach, N.; Shavit, M.; Kovo, M.; Biron-Shental, T.; Markovitch, O. Is There an Added Neonatal Risk in Vacuum-Assisted Deliveries with Nuchal Cord? J. Clin. Med. 2022, 11, 6970. https://doi.org/10.3390/jcm11236970
Schreiber H, Cohen G, Mevorach N, Shavit M, Kovo M, Biron-Shental T, Markovitch O. Is There an Added Neonatal Risk in Vacuum-Assisted Deliveries with Nuchal Cord? Journal of Clinical Medicine. 2022; 11(23):6970. https://doi.org/10.3390/jcm11236970
Chicago/Turabian StyleSchreiber, Hanoch, Gal Cohen, Nir Mevorach, Maya Shavit, Michal Kovo, Tal Biron-Shental, and Ofer Markovitch. 2022. "Is There an Added Neonatal Risk in Vacuum-Assisted Deliveries with Nuchal Cord?" Journal of Clinical Medicine 11, no. 23: 6970. https://doi.org/10.3390/jcm11236970
APA StyleSchreiber, H., Cohen, G., Mevorach, N., Shavit, M., Kovo, M., Biron-Shental, T., & Markovitch, O. (2022). Is There an Added Neonatal Risk in Vacuum-Assisted Deliveries with Nuchal Cord? Journal of Clinical Medicine, 11(23), 6970. https://doi.org/10.3390/jcm11236970








