Idiopathic Pulmonary Fibrosis and Telomeres
Abstract
1. Introduction
2. Etiopathogenesis
2.1. Pulmonary Toxics
2.2. Gastroesophageal Reflux
2.3. Genetic Factors
2.4. Short Telomeres
3. What Are Telomeres?
4. Genetics in IPF
- SFTPC
- SFTPA2
Familial Pulmonary Fibrosis
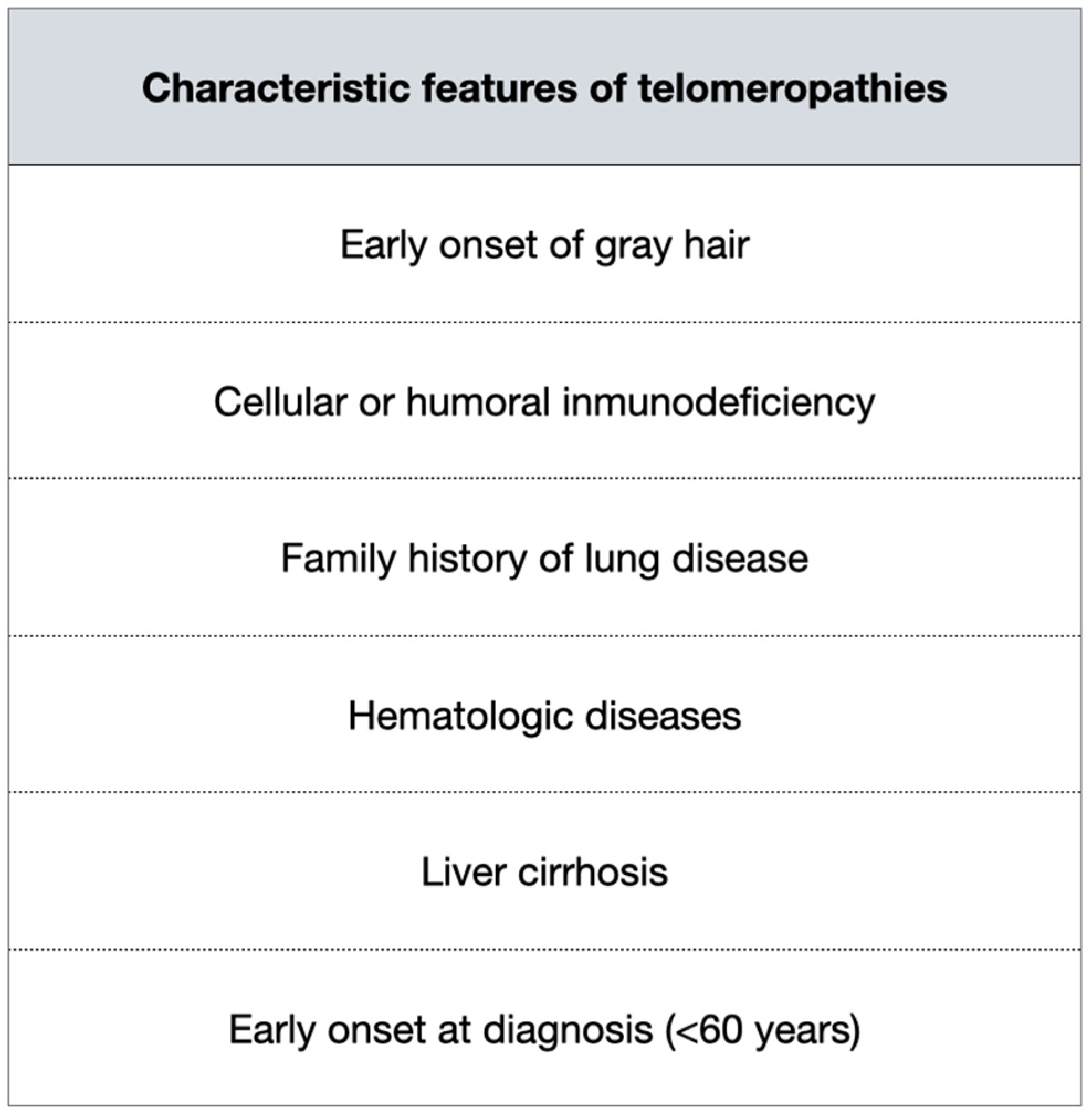
5. When to Ask for Telomere Length?
Screening for Relatives
6. Telomeres Relevance in Treatment
7. Prognostic Biomarkers
8. Telomeres in Others Interstitial Diseases
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Glass, D.S.; Grossfeld, D.; Renna, H.A.; Agarwala, P.; Spiegler, P.; Kasselman, L.J.; Glass, A.D.; DeLeon, J.; Reiss, A.B. Idiopathic pulmonary fibrosis: Molecular mechanisms and potential treatment approaches. Respir. Investig. 2020, 58, 320–335. [Google Scholar] [CrossRef] [PubMed]
- Navaratnam, V.; Fleming, K.M.; West, J.; Smith, C.J.; Jenkins, R.G.; Fogarty, A.; Hubbard, R.B. The rising incidence of idiopathic pulmonary fibrosis in the U.K. Thorax 2011, 66, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Lederer, D.J.; Martinez, F.J. Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2018, 378, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Taskar, V.S.; Coultas, D.B. Is idiopathic pulmonary fibrosis an environmental disease? Proc. Am. Thorac. Soc. 2006, 3, 293–298. [Google Scholar] [CrossRef]
- Oh, C.K.; Murray, L.A.; Molfino, N.A. Smoking and idiopathic pulmonary fibrosis. Pulm. Med. 2012, 2012, 808260. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, Z. Fibroblast Senescence in Idiopathic Pulmonary Fibrosis. Front. Cell Dev. Biol. 2020, 8, 593283. [Google Scholar] [CrossRef]
- Hutchinson, J.P.; McKeever, T.M.; Fogarty, A.W.; Navaratnam, V.; Hubbard, R.B. Increasing global mortality from idiopathic pulmonary fibrosis in the twenty-first century. Ann. Am. Thorac. Soc. 2014, 11, 1176–1185. [Google Scholar] [CrossRef]
- Esposito, D.B.; Lanes, S.; Donneyong, M.; Holick, C.N.; Lasky, J.A.; Lederer, D.; Nathan, S.D.; O’Quinn, S.; Parker, J.; Tran, T.N. Idiopathic Pulmonary Fibrosis in United States Automated Claims. Incidence, Prevalence, and Algorithm Validation. Am. J. Respir. Crit. Care Med. 2015, 192, 1200–1207. [Google Scholar] [CrossRef]
- Hutchinson, J.; Fogarty, A.; Hubbard, R.; McKeever, T. Global incidence and mortality of idiopathic pulmonary fibrosis: A systematic review. Eur. Respir. J. 2015, 46, 795–806. [Google Scholar] [CrossRef]
- Martinez, F.J.; Collard, H.R.; Pardo, A.; Raghu, G.; Richeldi, L.; Selman, M.; Swigris, J.J.; Taniguchi, H.; Wells, A.U. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Primers 2017, 3, 17074. [Google Scholar] [CrossRef]
- Meyer, K.C. Pulmonary fibrosis, part II: State-of-the-art patient management. Expert Rev. Respir. Med. 2017, 11, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Chen, S.Y.; Yeh, W.S.; Maroni, B.; Li, Q.; Lee, Y.C.; Collard, H.R. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: Incidence, prevalence, and survival, 2001–2011. Lancet Respir. Med. 2014, 2, 566–572, Erratum in Lancet Respir. Med. 2014, 2, e12. [Google Scholar] [CrossRef]
- Dressen, A.; Abbas, A.R.; Cabanski, C.; Reeder, J.; Ramalingam, T.R.; Neighbors, M.; Bhangale, T.R.; Brauer, M.J.; Hunkapiller, J.; Reeder, J.; et al. Analysis of protein-altering variants in telomerase genes and their association with MUC5B common variant status in patients with idiopathic pulmonary fibrosis: A candidate gene sequencing study. Lancet Respir. Med. 2018, 6, 603–614. [Google Scholar] [CrossRef]
- Alder, J.K.; Chen, J.J.; Lancaster, L.; Danoff, S.; Su, S.C.; Cogan, J.D.; Vulto, I.; Xie, M.; Qi, X.; Tuder, R.M.; et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc. Natl. Acad. Sci. USA 2008, 105, 13051–13056. [Google Scholar] [CrossRef]
- Hastie, N.D.; Dempster, M.; Dunlop, M.G.; Thompson, A.M.; Green, D.K.; Allshire, R.C. Telomere reduction in human colorectal carcinoma and with ageing. Nature 1990, 346, 866–868. [Google Scholar] [CrossRef]
- Harley, C.B.; Futcher, A.B.; Greider, C.W. Telomeres shorten during ageing of human fibroblasts. Nature 1990, 345, 458–460. [Google Scholar] [CrossRef]
- Hadjicharalambous, M.R.; Lindsay, M.A. Idiopathic Pulmonary Fibrosis: Pathogenesis and the Emerging Role of Long Non-Coding RNAs. Int. J. Mol. Sci. 2020, 21, 524. [Google Scholar] [CrossRef]
- Abramson, M.J.; Murambadoro, T.; Alif, S.M.; Benke, G.P.; Dharmage, S.C.; Glaspole, I.; Hopkins, P.; Hoy, R.F.; Klebe, S.; Moodley, Y.; et al. Occupational and environmental risk factors for idiopathic pulmonary fibrosis in Australia: Case-control study. Thorax 2020, 75, 864–869. [Google Scholar] [CrossRef]
- Baumgartner, K.B.; Samet, J.M.; Coultas, D.B.; Stidley, C.A.; Hunt, W.C.; Colby, T.V.; Waldron, J.A. Occupational and environmental risk factors for idiopathic pulmonary fibrosis: A multicenter case-control study. Collaborating Centers. Am. J. Epidemiol. 2000, 152, 307–315. [Google Scholar] [CrossRef]
- Nett, R.J.; Cummings, K.J.; Cannon, B.; Cox-Ganser, J.; Nathan, S.D. Dental Personnel Treated for Idiopathic Pulmonary Fibrosis at a Tertiary Care Center—Virginia, 2000–2015. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 270–273. [Google Scholar] [CrossRef]
- Marshall, R.P.; Puddicombe, A.; Cookson, W.O.; Laurent, G.J. Adult familial cryptogenic fibrosing alveolitis in the United Kingdom. Thorax 2000, 55, 143–146. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hodgson, U.; Laitinen, T.; Tukiainen, P. Nationwide prevalence of sporadic and familial idiopathic pulmonary fibrosis: Evidence of founder effect among multiplex families in Finland. Thorax 2002, 57, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Loyd, J.E. Pulmonary fibrosis in families. Am. J. Respir. Cell Mol. Biol. 2003, 29 (Suppl. S3), S47–S50. [Google Scholar] [PubMed]
- García-Sancho, C.; Buendía-Roldán, I.; Fernández-Plata, M.R.; Navarro, C.; Pérez-Padilla, R.; Vargas, M.H.; Loyd, J.E.; Selman, M. Familial pulmonary fibrosis is the strongest risk factor for idiopathic pulmonary fibrosis. Respir. Med. 2011, 105, 1902–1907. [Google Scholar] [CrossRef] [PubMed]
- Diaz de Leon, A.; Cronkhite, J.T.; Katzenstein, A.L.; Godwin, J.D.; Raghu, G.; Glazer, C.S.; Rosenblatt, R.L.; Girod, C.E.; Garrity, E.R.; Xing, C.; et al. Telomere lengths, pulmonary fibrosis and telomerase (TERT) mutations. PLoS ONE 2010, 5, e10680. [Google Scholar] [CrossRef] [PubMed]
- Cronkhite, J.T.; Xing, C.; Raghu, G.; Chin, K.M.; Torres, F.; Rosenblatt, R.L.; Garcia, C.K. Telomere shortening in familial and sporadic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2008, 178, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Snetselaar, R.; van Moorsel, C.H.M.; Kazemier, K.M.; van der Vis, J.J.; Zanen, P.; van Oosterhout, M.F.M.; Grutters, J.C. Telomere length in interstitial lung diseases. Chest 2015, 148, 1011–1018. [Google Scholar] [CrossRef]
- Valdes, A.M.; Andrew, T.; Gardner, J.P.; Kimura, M.; Oelsner, E.; Cherkas, L.F.; Aviv, A.; Spector, T.D. Obesity, cigarette smoking, and telomere length in women. Lancet 2005, 366, 662–664. [Google Scholar] [CrossRef]
- Plunkett, F.J.; Franzese, O.; Belaramani, L.L.; Fletcher, J.M.; Gilmour, K.C.; Sharifi, R.; Khan, N.; Hislop, A.D.; Cara, A.; Salmon, M.; et al. The impact of telomere erosion on memory CD8+ T cells in patients with X-linked lymphoproliferative syndrome. Mech. Ageing Dev. 2005, 126, 855–865. [Google Scholar] [CrossRef]
- McDonough, J.E.; Martens, D.S.; Tanabe, N.; Ahangari, F.; Verleden, S.E.; Maes, K.; Verleden, G.M.; Kaminski, N.; Hogg, J.C.; Nawrot, T.S.; et al. A role for telomere length and chromosomal damage in idiopathic pulmonary fibrosis. Respir. Res. 2018, 19, 132. [Google Scholar] [CrossRef]
- Martínez, P.; Blasco, M.A. Telomere-driven diseases and telomere-targeting therapies. J. Cell Biol. 2017, 216, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Greider, C.W.; Blackburn, E.H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 1985, 43, 405–413. [Google Scholar] [CrossRef]
- d’Adda di Fagagna, F.; Reaper, P.M.; Clay-Farrace, L.; Fiegler, H.; Carr, P.; Von Zglinicki, T.; Saretzki, G.; Carter, N.P.; Jackson, S.P. A DNA damage checkpoint response in telomere-initiated senescence. Nature 2003, 426, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Hemann, M.T.; Strong, M.A.; Hao, L.Y.; Greider, C.W. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell 2001, 107, 67–77. [Google Scholar] [CrossRef]
- Lee, H.W.; Blasco, M.A.; Gottlieb, G.J.; Horner, J.W., 2nd; Greider, C.W.; DePinho, R.A. Essential role of mouse telomerase in highly proliferative organs. Nature 1998, 392, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Strong, M.A.; Vidal-Cardenas, S.L.; Karim, B.; Yu, H.; Guo, N.; Greider, C.W. Phenotypes in mTERT+/− and mTERT−/− mice are due to short telomeres, not telomere-independent functions of telomerase reverse transcriptase. Mol. Cell. Biol. 2011, 31, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Njajou, O.T.; Cawthon, R.M.; Damcott, C.M.; Wu, S.H.; Ott, S.; Garant, M.J.; Blackburn, E.H.; Mitchell, B.D.; Shuldiner, A.R.; Hsueh, W.C. Telomere length is paternally inherited and is associated with parental lifespan. Proc. Natl. Acad. Sci. USA 2007, 104, 12135–12139. [Google Scholar] [CrossRef]
- Tsakiri, K.D.; Cronkhite, J.T.; Kuan, P.J.; Xing, C.; Raghu, G.; Weissler, J.C.; Rosenblatt, R.L.; Shay, J.W.; Garcia, C.K. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc. Natl. Acad. Sci. USA 2007, 104, 7552–7557. [Google Scholar] [CrossRef]
- Petrovski, S.; Todd, J.L.; Durheim, M.T.; Wang, Q.; Chien, J.W.; Kelly, F.L.; Frankel, C.; Mebane, C.M.; Ren, Z.; Bridgers, J.; et al. An Exome Sequencing Study to Assess the Role of Rare Genetic Variation in Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2017, 196, 82–93. [Google Scholar] [CrossRef]
- Lawson, W.E.; Loyd, J.E. The genetic approach in pulmonary fibrosis: Can it provide clues to this complex disease? Proc. Am. Thorac. Soc. 2006, 3, 345–349. [Google Scholar] [CrossRef][Green Version]
- Fernandez, B.A.; Fox, G.; Bhatia, R.; Sala, E.; Noble, B.; Denic, N.; Fernandez, D.; Duguid, N.; Dohey, A.; Kamel, F.; et al. A Newfoundland cohort of familial and sporadic idiopathic pulmonary fibrosis patients: Clinical and genetic features. Respir. Res. 2012, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Newton, C.A. Familial Pulmonary Fibrosis: Genetic Features and Clinical Implications. Chest 2021, 160, 1764–1773. [Google Scholar] [CrossRef] [PubMed]
- van Moorsel, C.H.; van Oosterhout, M.F.; Barlo, N.P.; de Jong, P.A.; van der Vis, J.J.; Ruven, H.J.; van Es, H.W.; van den Bosch, J.M.; Grutters, J.C. Surfactant protein C mutations are the basis of a significant portion of adult familial pulmonary fibrosis in a dutch cohort. Am. J. Respir. Crit. Care Med. 2010, 182, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Ryu, J.H.; Wittmer, M.H.; Hartman, T.E.; Lymp, J.F.; Tazelaar, H.D.; Limper, A.H. Familial idiopathic pulmonary fibrosis: Clinical features and outcome. Chest 2005, 127, 2034–2041. [Google Scholar] [CrossRef]
- Lai, T.P.; Wright, W.E.; Shay, J.W. Comparison of telomere length measurement methods. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20160451. [Google Scholar] [CrossRef]
- Lee, H.Y.; Seo, J.B.; Steele, M.P.; Schwarz, M.I.; Brown, K.K.; Loyd, J.E.; Talbert, J.L.; Schwartz, D.A.; Lynch, D.A. High-resolution CT scan findings in familial interstitial pneumonia do not conform to those of idiopathic interstitial pneumonia. Chest 2012, 142, 1577–1583. [Google Scholar] [CrossRef]
- van Batenburg, A.A.; Kazemier, K.M.; van Oosterhout, M.F.M.; van der Vis, J.J.; van Es, H.W.; Grutters, J.C.; Goldschmeding, R.; van Moorsel, C.H.M. From organ to cell: Multi-level telomere length assessment in patients with idiopathic pulmonary fibrosis. PLoS ONE 2020, 15, e0226785. [Google Scholar] [CrossRef] [PubMed]
- Planas-Cerezales, L.; Arias-Salgado, E.G.; Buendia-Roldán, I.; Montes-Worboys, A.; López, C.E.; Vicens-Zygmunt, V.; Hernaiz, P.L.; Sanuy, R.L.; Leiro-Fernandez, V.; Vilarnau, E.B.; et al. Predictive factors and prognostic effect of telomere shortening in pulmonary fibrosis. Respirology 2019, 24, 146–153. [Google Scholar] [CrossRef]
- Steele, M.P.; Speer, M.C.; Loyd, J.E.; Brown, K.K.; Herron, A.; Slifer, S.H.; Burch, L.H.; Wahidi, M.M.; Phillips, J.A., 3rd; Sporn, T.A.; et al. Clinical and pathologic features of familial interstitial pneumonia. Am. J. Respir. Crit. Care Med. 2005, 172, 1146–1152. [Google Scholar] [CrossRef]
- Reddy, T.L.; Tominaga, M.; Hansell, D.M.; von der Thusen, J.; Rassl, D.; Parfrey, H.; Guy, S.; Twentyman, O.; Rice, A.; Maher, T.M.; et al. Pleuroparenchymal fibroelastosis: A spectrum of histopathological and imaging phenotypes. Eur. Respir. J. 2012, 40, 377–385. [Google Scholar] [CrossRef]
- Scholand, M.B.; Coon, H.; Wolff, R.; Cannon-Albright, L. Use of a genealogical database demonstrates heritability of pulmonary fibrosis. Lung 2013, 191, 475–481. [Google Scholar] [CrossRef]
- Okamoto, T.; Miyazaki, Y.; Tomita, M.; Tamaoka, M.; Inase, N. A familial history of pulmonary fibrosis in patients with chronic hypersensitivity pneumonitis. Respiration 2013, 85, 384–390. [Google Scholar] [CrossRef]
- Rosas, I.O.; Ren, P.; Avila, N.A.; Chow, C.K.; Franks, T.J.; Travis, W.D.; McCoy, J.P., Jr.; May, R.M.; Wu, H.P.; Nguyen, D.M.; et al. Early interstitial lung disease in familial pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2007, 176, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Goldman, F.; Bouarich, R.; Kulkarni, S.; Freeman, S.; Du, H.Y.; Harrington, L.; Mason, P.J.; Londoño-Vallejo, A.; Bessler, M. The effect of TERC haploinsufficiency on the inheritance of telomere length. Proc. Natl. Acad. Sci. USA 2005, 102, 17119–17124. [Google Scholar] [CrossRef] [PubMed]
- Šelb, J.; Osolnik, K.; Kern, I.; Korošec, P.; Rijavec, M. Utility of Telomerase Gene Mutation Testing in Patients with Idiopathic Pulmonary Fibrosis in Routine Practice. Cells 2022, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Newton, C.A.; Zhang, D.; Oldham, J.M.; Kozlitina, J.; Ma, S.F.; Martinez, F.J.; Raghu, G.; Noth, I.; Garcia, C.K. Telomere Length and Use of Immunosuppressive Medications in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 200, 336–347. [Google Scholar] [CrossRef]
- Newton, C.A.; Kozlitina, J.; Lines, J.R.; Kaza, V.; Torres, F.; Garcia, C.K. Telomere length in patients with pulmonary fibrosis associated with chronic lung allograft dysfunction and post-lung transplantation survival. J. Heart Lung Transplant. 2017, 36, 845–853. [Google Scholar] [CrossRef]
- Townsley, D.M.; Dumitriu, B.; Liu, D.; Biancotto, A.; Weinstein, B.; Chen, C.; Hardy, N.; Mihalek, A.D.; Lingala, S.; Kim, Y.J.; et al. Danazol Treatment for Telomere Diseases. N. Engl. J. Med. 2016, 374, 1922–1931. [Google Scholar] [CrossRef]
- Lanna, A.; Vaz, B.; D’Ambra, C.; Valvo, S.; Vuotto, C.; Chiurchiù, V.; Devine, O.; Sanchez, M.; Borsellino, G.; Akbar, A.N.; et al. An intercellular transfer of telomeres rescues T cells from senescence and promotes long-term immunological memory. Nat. Cell Biol. 2022, 24, 1461–1474. [Google Scholar] [CrossRef]
- Stuart, B.D.; Lee, J.S.; Kozlitina, J.; Noth, I.; Devine, M.S.; Glazer, C.S.; Torres, F.; Kaza, V.; Girod, C.E.; Jones, K.D.; et al. Effect of telomere length on survival in patients with idiopathic pulmonary fibrosis: An observational cohort study with independent validation. Lancet Respir. Med. 2014, 2, 557–565. [Google Scholar] [CrossRef]
- Dai, J.; Cai, H.; Li, H.; Zhuang, Y.; Min, H.; Wen, Y.; Yang, J.; Gao, Q.; Shi, Y.; Yi, L. Association between telomere length and survival in patients with idiopathic pulmonary fibrosis. Respirology 2015, 20, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Janicki-Deverts, D.; Turner, R.B.; Casselbrant, M.L.; Li-Korotky, H.S.; Epel, E.S.; Doyle, W.J. Association between telomere length and experimentally induced upper respiratory viral infection in healthy adults. JAMA 2013, 309, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, R.M.; Smith, K.R.; O’Brien, E.; Sivatchenko, A.; Kerber, R.A. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 2003, 361, 393–395. [Google Scholar] [CrossRef]
- Guan, J.Z.; Maeda, T.; Sugano, M.; Oyama, J.; Higuchi, Y.; Suzuki, T.; Makino, N. An analysis of telomere length in sarcoidosis. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
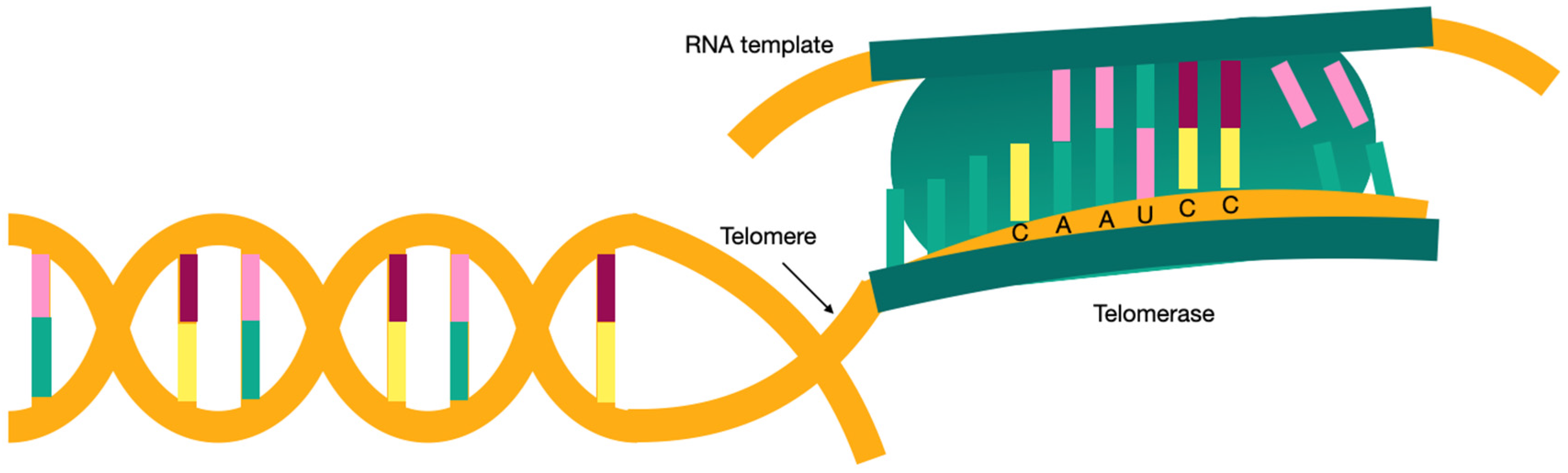
| Telomerase reverse transcriptase (TERT) | The coding gene for the telomerase reverse transcriptase. It is responsible for 15% of familial pulmonary fibrosis (FPF). There are gender differences in this mutation, with females being 11.9 years older at diagnosis than males. |
| Telomerase RNA Component (TERC) | The gene encoding the telomerase RNA component. |
| Regulator Of Telomere Elongation Helicase 1 (RTEL1) | Regulator of Telomere Elongation Helicase 1. The established locus for dyskeratosis congenita. |
| Poly(A)-Specific Ribonuclease (PARN) | Poly(A)-specific Ribonuclease Deadenylation Nuclease. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulet, A.; Signes-Costa, J. Idiopathic Pulmonary Fibrosis and Telomeres. J. Clin. Med. 2022, 11, 6893. https://doi.org/10.3390/jcm11236893
Mulet A, Signes-Costa J. Idiopathic Pulmonary Fibrosis and Telomeres. Journal of Clinical Medicine. 2022; 11(23):6893. https://doi.org/10.3390/jcm11236893
Chicago/Turabian StyleMulet, Alba, and Jaime Signes-Costa. 2022. "Idiopathic Pulmonary Fibrosis and Telomeres" Journal of Clinical Medicine 11, no. 23: 6893. https://doi.org/10.3390/jcm11236893
APA StyleMulet, A., & Signes-Costa, J. (2022). Idiopathic Pulmonary Fibrosis and Telomeres. Journal of Clinical Medicine, 11(23), 6893. https://doi.org/10.3390/jcm11236893





