Novel SPEF2 Variant in a Japanese Patient with Primary Ciliary Dyskinesia: A Case Report and Literature Review
Abstract
1. Introduction
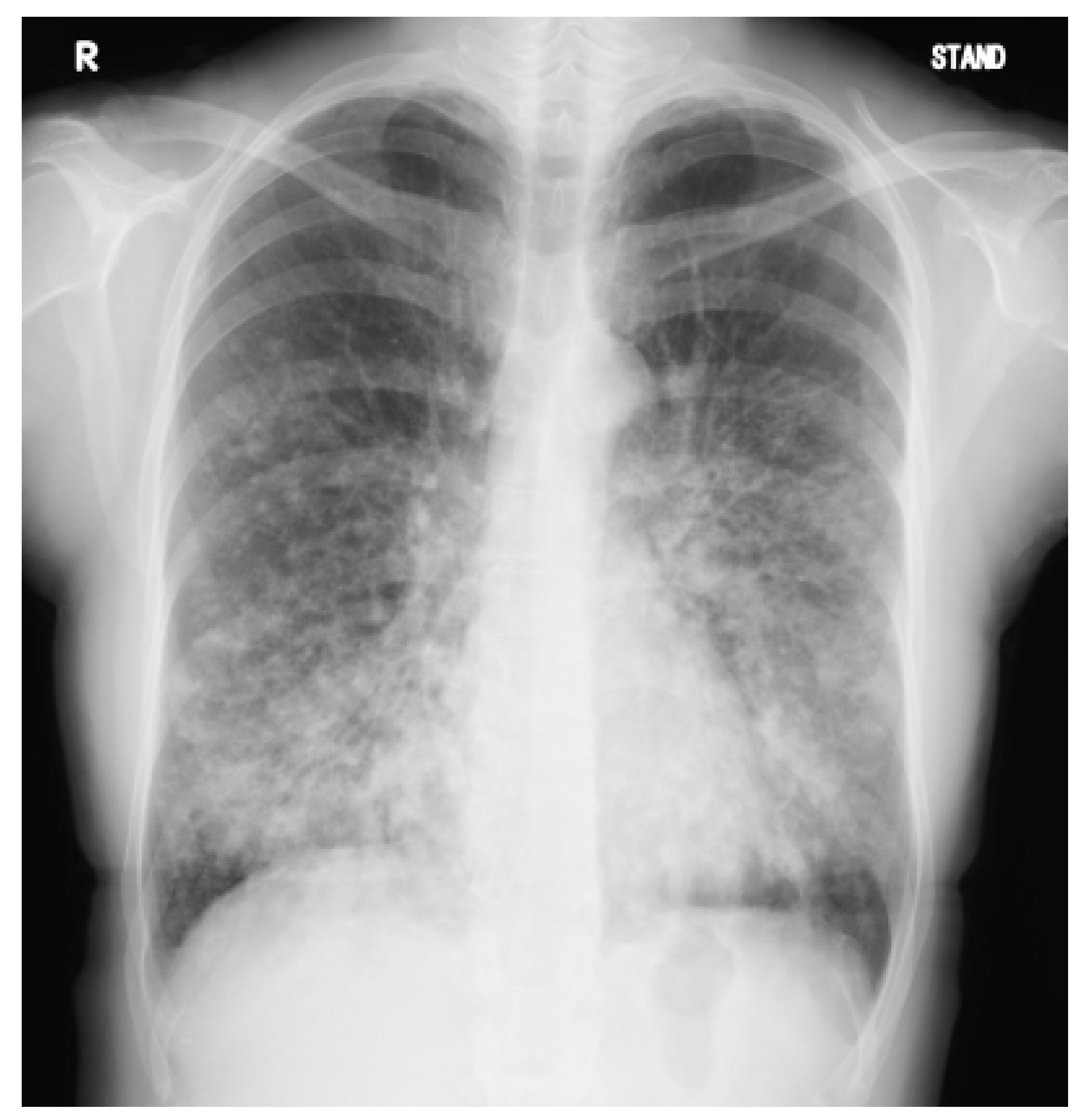
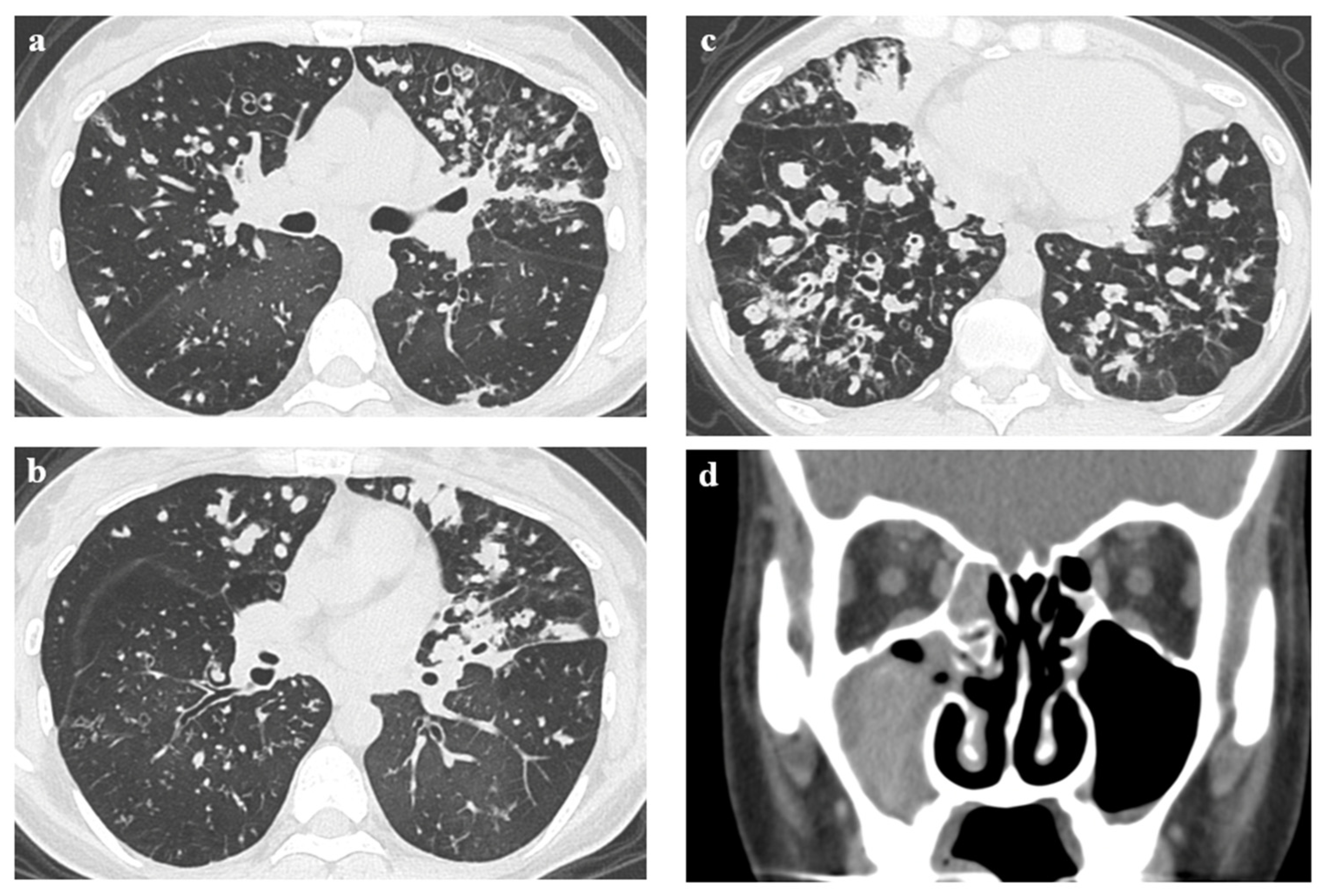
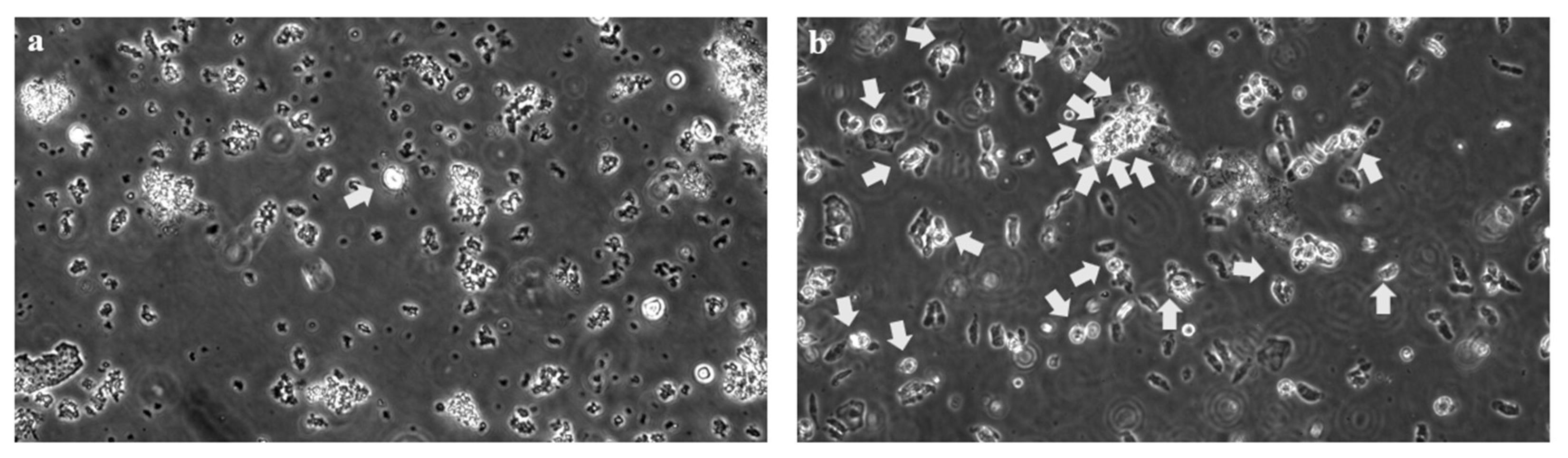
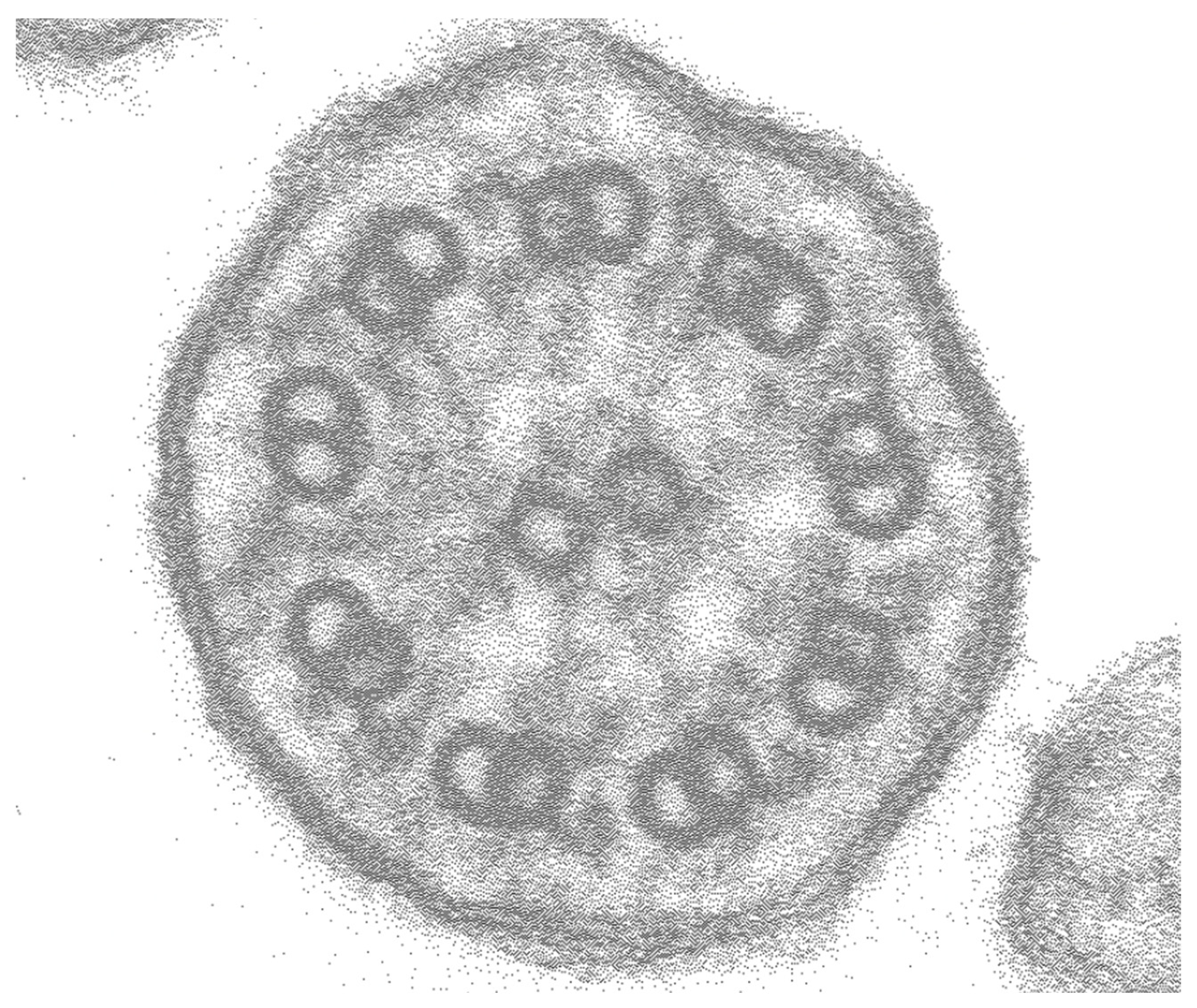
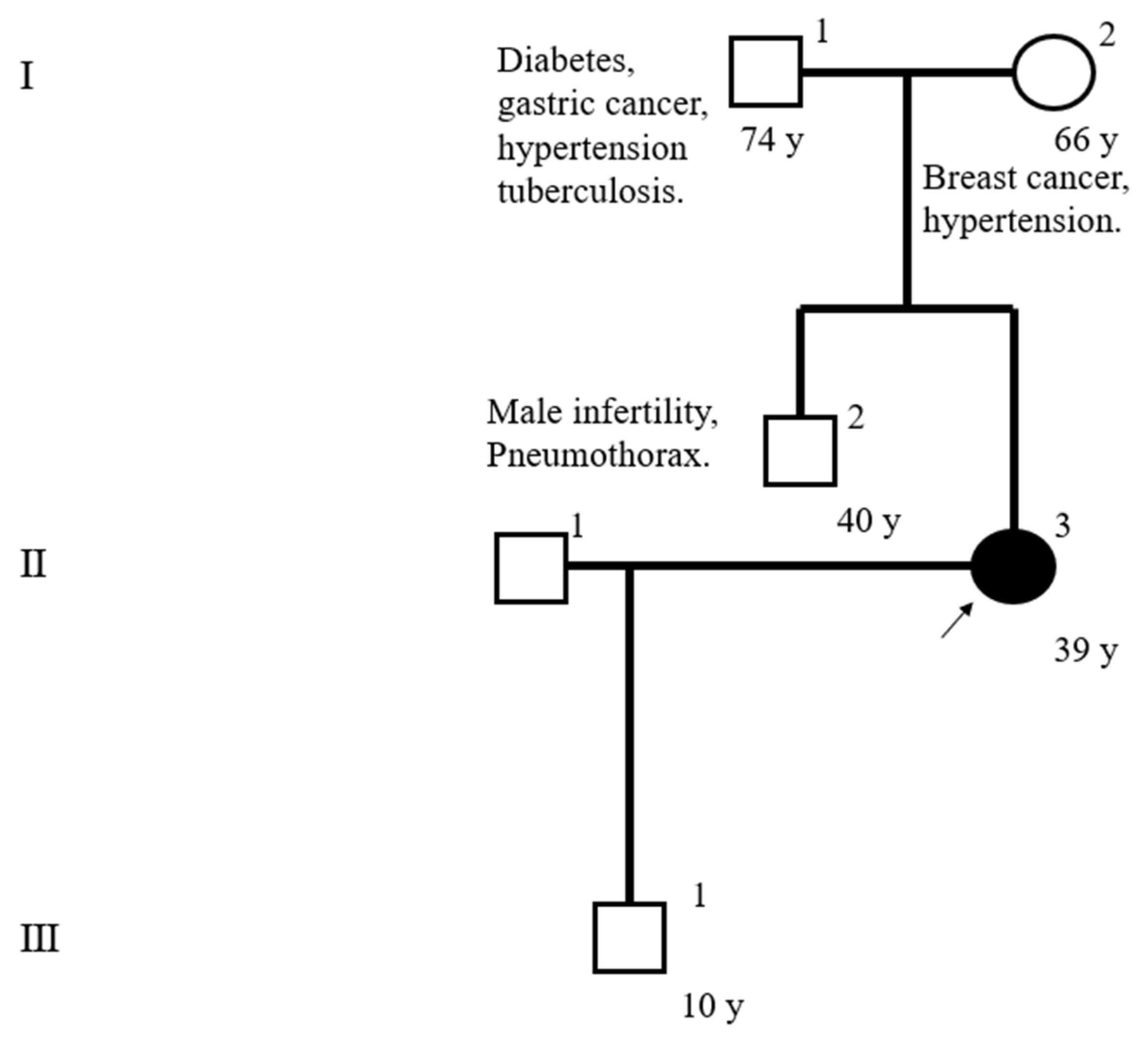
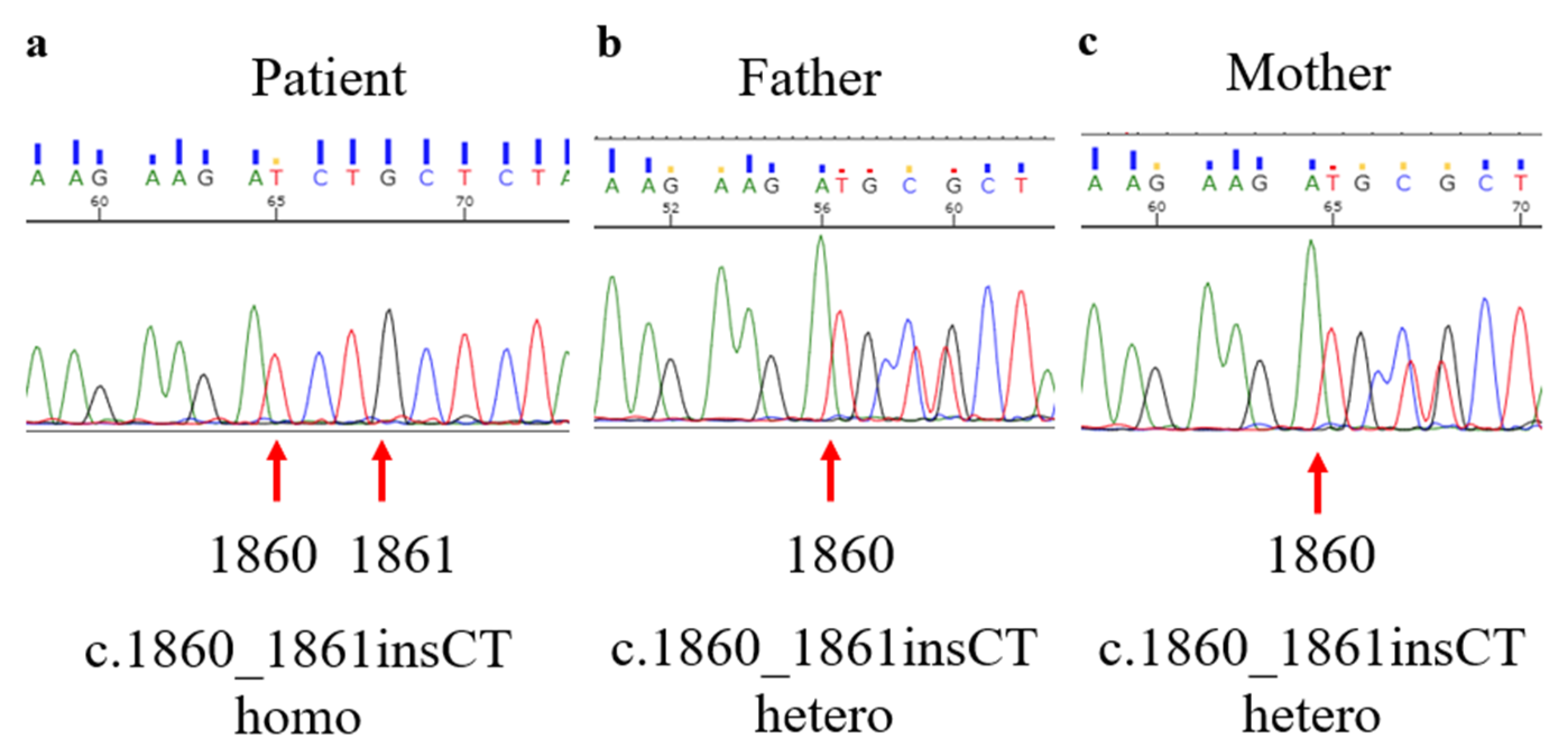
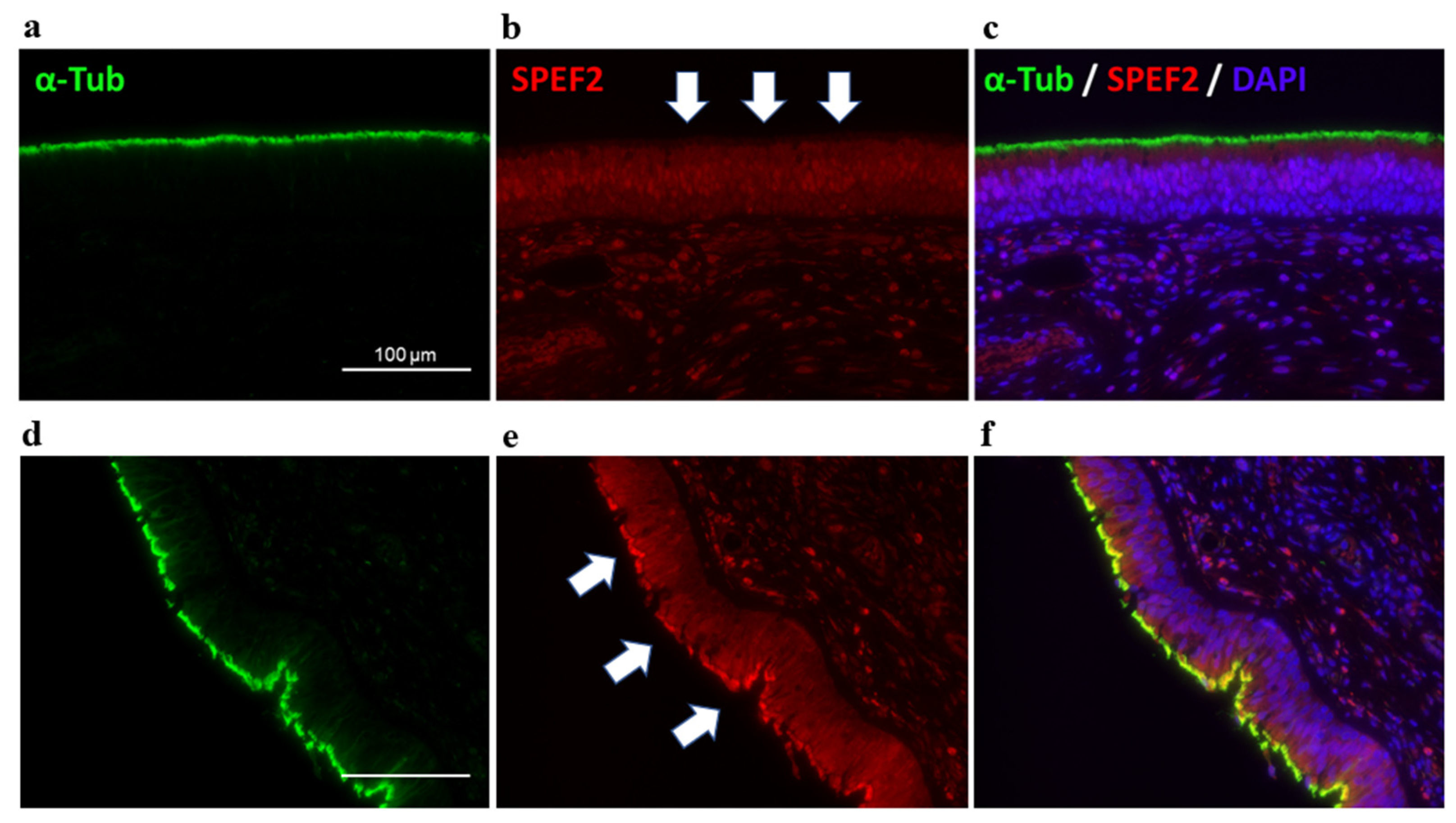
2. Case Presentation
3. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBA | ciliary beat amplitude |
| CBF | ciliary beat frequency |
| CPC | central pair complex |
| PCD | primary ciliary dyskinesia |
| HRCT | high-resolution computed tomography |
| %FEV1 | predicted value of forced expiratory volume in 1 s |
References
- Noone, P.G.; Leigh, M.W.; Sannuti, A.; Minnix, S.L.; Carson, J.L.; Hazucha, M.; Zariwala, M.A.; Knowles, M.R. Primary ciliary dyskinesia: Diagnostic and phenotypic features. Am. J. Respir. Crit. Care Med. 2004, 169, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Hogg, C. Primary ciliary dyskinesia: When to suspect the diagnosis and how to confirm it. Paediatr. Respir. Rev. 2009, 10, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Hannah, W.B.; WB Seifert, B.A.; BA Truty, R.; Zariwala, M.A.; Ameel, K.M.; Zhao, Y.; Nykamp, K.; Gaston, B. The global prevalence and ethnic heterogeneity of primary ciliary dyskinesia gene variants: A genetic database analysis. Lancet Respir. Med. 2022, 10, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Sommer, J.U.; Schäfer, K.; Omran, H.; Olbrich, H.; Wallmeier, J.; Blum, A.; Hörmann, K.; Stuck, B.A. ENT manifestations in patients with primary ciliary dyskinesia: Prevalence and significance of otorhinolaryngologic co-morbidities. Eur. Arch. Otorhinolaryngol. 2011, 268, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Behan, L.; Dunn Galvin, A.; Rubbo, B.; Masefield, S.; Copeland, F.; Manion, M.; Rindlisbacher, B.; Redfern, B.; Lucas, J.S. Diagnosing primary ciliary dyskinesia: An international patient perspective. Eur. Respir. J. 2016, 48, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Leigh, M.W.; Ferkol, T.W.; Davis, S.D.; Lee, H.S.; Rosenfeld, M.; Dell, S.D.; Sagel, S.D.; Milla, C.; Olivier, K.N.; Sullivan, K.; et al. Clinical Features and Associated Likelihood of Primary Ciliary Dyskinesia in Children and Adolescents. Ann. Am. Thorac. Soc. 2016, 13, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.D.; Ferkol, T.W.; Rosenfeld, M.; Lee, H.S.; Dell, S.D.; Sagel, S.D.; Milla, C.; Zariwala, M.A.; Pittman, J.E.; Shapiro, A.J.; et al. Clinical features of childhood primary ciliary dyskinesia by genotype and ultrastructural phenotype. Am. J. Respir. Crit. Care Med. 2015, 191, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.S.; Barbato, A.; Collins, S.A.; Goutaki, M.; Behan, L.; Caudri, D.; Dell, S.; Eber, E.; Escudier, E.; Hirst, R.A.; et al. European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia. Eur. Respir. J. 2017, 49, 1601090. [Google Scholar] [CrossRef] [PubMed]
- Raidt, J.; Wallmeier, J.; Hjeij, R.; Onnebrink, J.G.; Pennekamp, P.; Loges, N.T.; Olbrich, H.; Häffner, K.; Dougherty, G.W.; Omran, H.; et al. Ciliary beat pattern and frequency in genetic variants of primary ciliary dyskinesia. Eur. Respir. J. 2014, 44, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Hirst, R.A.; Jackson, C.L.; Coles, J.L.; Williams, G.; Rutman, A.; Goggin, P.M.; Adam, E.C.; Page, A.; Evans, H.J.; Lackie, P.M.; et al. Culture of primary ciliary dyskinesia epithelial cells at air-liquid interface can alter ciliary phenotype but remains a robust and informative diagnostic aid. PLoS ONE 2014, 9, e89675. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Xu, Y.; Kitano, M.; Chiyonobu, K.; Abo, M.; Ikegami, K.; Ogawa, S.; Ikejiri, M.; Kondo, M.; Gotoh, S.; et al. Copy number variation in DRC1 is the major cause of primary ciliary dyskinesia in the Japanese population. Mol. Genet. Genomic Med. 2020, 8, e1137. [Google Scholar] [CrossRef] [PubMed]
- Cindrić, S.; Dougherty, G.W.; Olbrich, H.; Hjeij, R.; Loges, N.T.; Amirav, I.; Philipsen, M.C.; Marthin, J.K.; Nielsen, K.G.; Sutharsan, S.; et al. SPEF2- and HYDIN-mutant cilia lack the central pair-associated protein SPEF2, aiding primary ciliary dyskinesia diagnostics. Am. J. Respir. Cell Mol. Biol. 2020, 62, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Nie, H.; Meng, L.; Wang, W.; Li, H.; Yuan, S.; Cheng, D.; He, W.; Liu, G.; Du, J.; et al. Novel mutations in SPEF2 causing different defects between flagella and cilia bridge: The phenotypic link between MMAF and PCD. Hum. Genet. 2020, 139, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lv, M.; He, X.; Zhu, Y.; Amiri-Yekta, A.; Li, W.; Wu, H.; Kherraf, Z.E.; Liu, W.; Zhang, J.; et al. Homozygous mutations in SPEF2 induce multiple morphological abnormalities of the sperm flagella and male infertility. J. Med. Genet. 2020, 57, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, L.E.; Andrews, K.; Potdar, P.; Matsuura, H.; Jetten, A.; Nettesheim, P. Cloning and characterization of KPL2, a novel gene induced during ciliogenesis of tracheal epithelial cells. Am. J. Respir. Cell Mol. Biol. 1999, 20, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Yang, X.X.; Tu, C.F.; Wang, W.L.; Meng, L.L.; Lu, G.X.; Tan, Y.Q.; Zhang, Q.J.; Du, J. Sperm flagellar 2 (SPEF2) is essential for sperm flagellar assembly in humans. Asian J. Andro. 2022, 24, 359–366. [Google Scholar] [CrossRef]
- Chilvers, M.A.; Rutman, A.; O’Callaghan, C. Ciliary beat pattern is associated with specific ultrastructural defects in primary ciliary dyskinesia. J. Allergy Clin. Immunol. 2003, 112, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.P.; Noone, P.G.; Leigh, M.W.; Zariwala, M.A.; Minnix, S.L.; Knowles, M.R.; Molina, P.L. High-resolution CT of patients with primary ciliary dyskinesia. AJR Am. J. Roentgenol. 2007, 188, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Kido, T.; Yatera, K.; Yamasaki, K.; Nagata, S.; Choujin, Y.; Yamaga, C.; Hara, K.; Ishimoto, H.; Hisaoka, M.; Mukae, H. Two cases of primary ciliary dyskinesia with different responses to macrolide treatment. Intern Med. 2012, 51, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mori, M.; Kido, T.; Sakamoto, N.; Ozasa, M.; Kido, K.; Noguchi, Y.; Tokito, T.; Okuno, D.; Yura, H.; Hara, A.; et al. Novel SPEF2 Variant in a Japanese Patient with Primary Ciliary Dyskinesia: A Case Report and Literature Review. J. Clin. Med. 2023, 12, 317. https://doi.org/10.3390/jcm12010317
Mori M, Kido T, Sakamoto N, Ozasa M, Kido K, Noguchi Y, Tokito T, Okuno D, Yura H, Hara A, et al. Novel SPEF2 Variant in a Japanese Patient with Primary Ciliary Dyskinesia: A Case Report and Literature Review. Journal of Clinical Medicine. 2023; 12(1):317. https://doi.org/10.3390/jcm12010317
Chicago/Turabian StyleMori, Mayako, Takashi Kido, Noriho Sakamoto, Mutsumi Ozasa, Kumiko Kido, Yasuko Noguchi, Takatomo Tokito, Daisuke Okuno, Hirokazu Yura, Atsuko Hara, and et al. 2023. "Novel SPEF2 Variant in a Japanese Patient with Primary Ciliary Dyskinesia: A Case Report and Literature Review" Journal of Clinical Medicine 12, no. 1: 317. https://doi.org/10.3390/jcm12010317
APA StyleMori, M., Kido, T., Sakamoto, N., Ozasa, M., Kido, K., Noguchi, Y., Tokito, T., Okuno, D., Yura, H., Hara, A., Ishimoto, H., Suematsu, T., Obase, Y., Tanaka, Y., Izumikawa, K., Takeuchi, K., & Mukae, H. (2023). Novel SPEF2 Variant in a Japanese Patient with Primary Ciliary Dyskinesia: A Case Report and Literature Review. Journal of Clinical Medicine, 12(1), 317. https://doi.org/10.3390/jcm12010317





