Adverse Cardiovascular Events in Non-Traumatic Intracranial Hemorrhage and Ischemic Stroke Survivors
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Outcomes
2.3. Statistical Analysis
3. Results
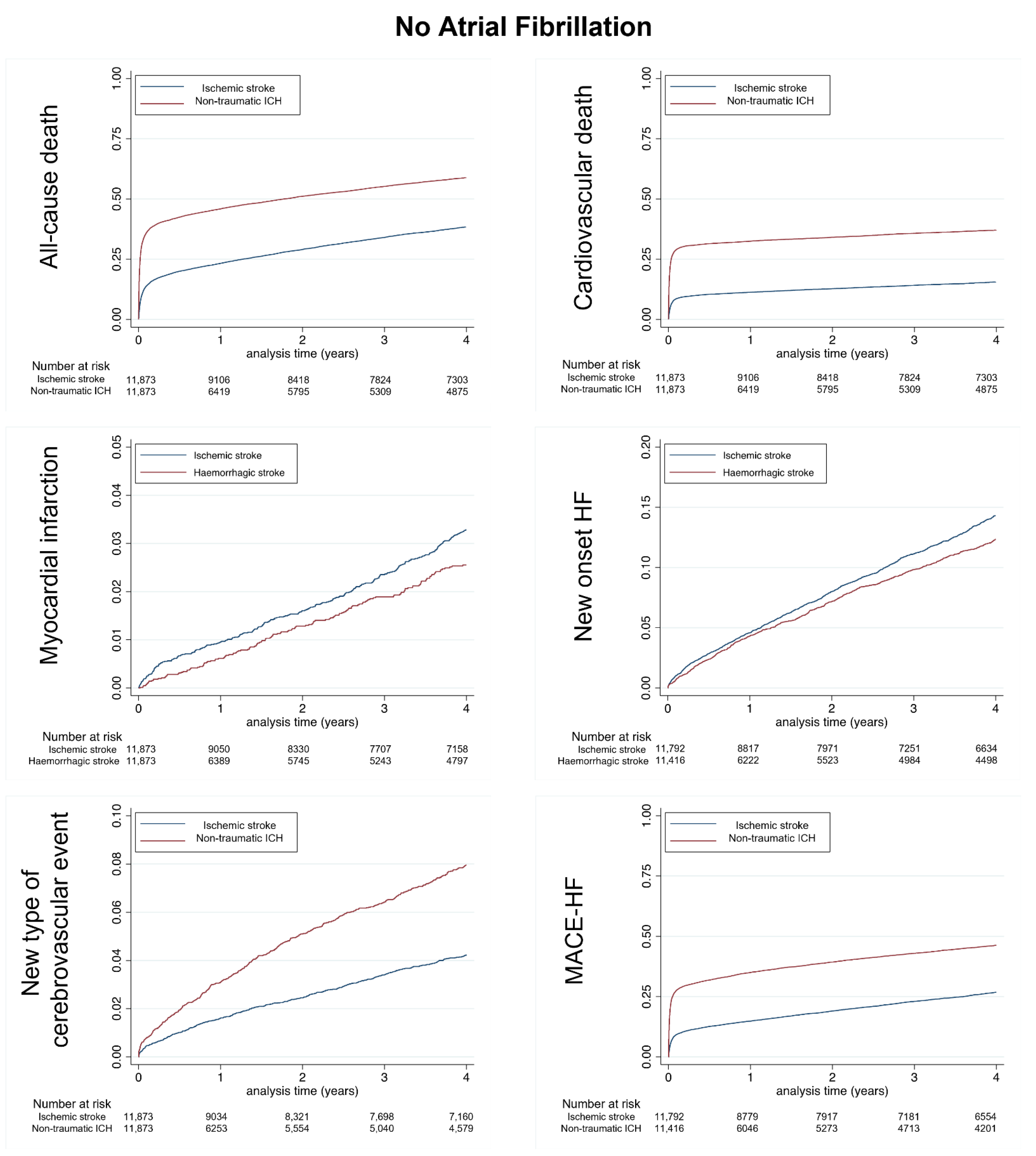
3.1. Population without AF
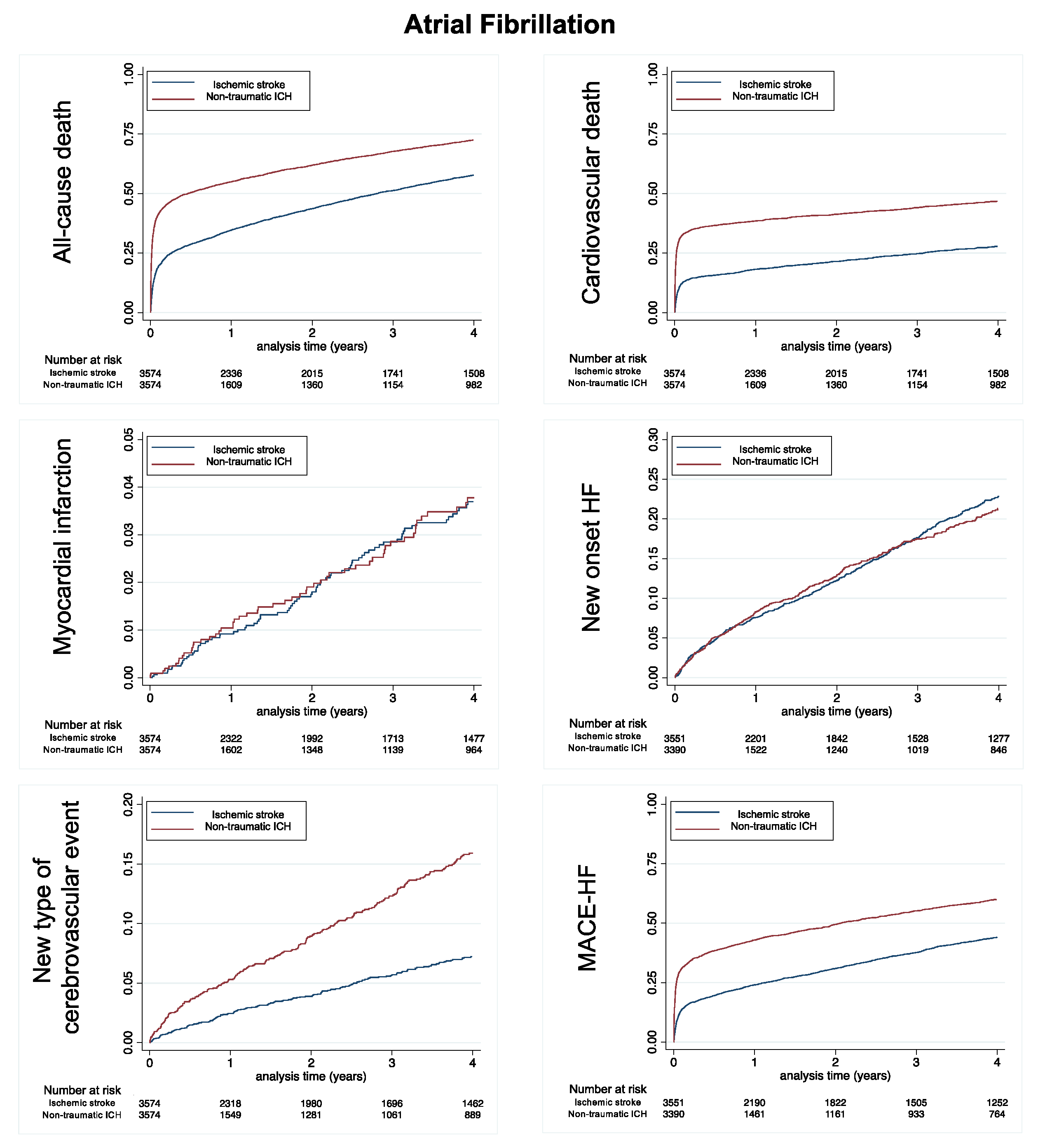
3.2. Population with AF
4. Discussion
Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Pasi, M.; Cordonnier, C. Clinical Relevance of Cerebral Small Vessel Diseases. Stroke 2020, 51, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Coull, A.J.; Lovett, J.K.; Rothwell, P.M. Population based study of early risk of stroke after transient ischemic attack or minor stroke: Implications for public education and organisation of services. BMJ 2004, 328, 326. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-J.; Kim, S.-E.; Kim, J.Y.; Kang, J.; Kim, B.J.; Han, M.-K.; Choi, K.-H.; Kim, J.-T.; Shin, D.-I.; Cha, J.-K.; et al. Five-Year Risk of Acute Myocardial Infarction After Acute Ischemic Stroke in Korea. J. Am. Heart. Assoc. 2021, 10, e018807. [Google Scholar] [CrossRef] [PubMed]
- Casolla, B.; Moulin, S.; Kyheng, M.; Hénon, H.; Labreuche, J.; Leys, D.; Bauters, C.; Cordonnier, C. Five-Year Risk of Major Ischemic and Hemorrhagic Events After Intracerebral Hemorrhage. Stroke 2019, 50, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Poon, M.T.C.; Samarasekera, N.E.; Perry, L.A.; Moullaali, T.J.; Rodrigues, M.A.; Loan, J.J.M.; Stephen, J.; Lerpiniere, C.; Tuna, M.A.; et al. Risks of recurrent stroke and all serious vascular events after spontaneous intracerebral hemorrhage: Pooled analyses of two population-based studies. Lancet Neurol. 2021, 20, 437–447. [Google Scholar] [CrossRef]
- Murthy, S.B.; Diaz, I.; Wu, X.; Merkler, A.E.; Iadecola, C.; Safford, M.M.; Sheth, K.N.; Navi, B.B.; Kamel, H. Risk of Arterial Ischemic Events After Intracerebral Hemorrhage. Stroke 2020, 51, 137–142. [Google Scholar] [CrossRef]
- Charidimou, A.; Imaizumi, T.; Moulin, S.; Biffi, A.; Samarasekera, N.; Yakushiji, Y.; Peeters, A.; Vandermeeren, Y.; Laloux, P.; Baron, J.-C.; et al. Brain hemorrhage recurrence, small vessel disease type, and cerebral microbleeds A meta-analysis. Neurology 2017, 89, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Pasi, M.; Charidimou, A.; Boulouis, G.; Auriel, E.; Ayres, A.; Schwab, K.M.; Goldstein, J.N.; Rosand, J.; Viswanathan, A.; Pantoni, L.; et al. Mixed-location cerebral hemorrhage/microbleeds: Underlying microangiopathy and recurrence risk. Neurology 2018, 90, e119–e126. [Google Scholar] [CrossRef] [PubMed]
- Fauchier, L.; Clementy, N.; Pelade, C.; Collignon, C.; Nicolle, E.; Lip, G.Y.H. Patients With Ischemic Stroke and Incident Atrial Fibrillation: A Nationwide Cohort Study. Stroke 2015, 46, 2432–2437. [Google Scholar] [CrossRef]
- Djennaoui, M.; Ficheur, G.; Beuscart, R.; Chazard, E. Improvement of the quality of medical databases: Data-mining-based prediction of diagnostic codes from previous patient codes. Stud. Health Technol. Inform. 2015, 210, 419–423. [Google Scholar]
- Pasi, M.; Casolla, B.; Kyheng, M.; Boulouis, G.; Kuchcinski, G.; Moulin, S.; Labreuche, J.; Hénon, H.; Cordonnier, C.; Leys, D. Long-term mortality in survivors of spontaneous intracerebral hemorrhage. Int. J. Stroke 2021, 16, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Odutayo, A.; Wong, C.X.; Hsiao, A.J.; Hopewell, S.; Altman, D.G.; Emdin, C.A. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: Systematic review and meta-analysis. BMJ 2016, 354, i4482. [Google Scholar] [CrossRef] [PubMed]
- Pasi, M.; Casolla, B.; Kyheng, M.; Boulouis, G.; Kuchcinski, G.; Moulin, S.; Labreuche, J.; Henon, H.; Leys, D.; Cordonnier, C. Long-term functional decline of spontaneous intracerebral hemorrhage survivors. J. Neurol. Neurosurg. Psychiatry 2021, 92, 249–254. [Google Scholar] [CrossRef] [PubMed]
- SoSTART Collaboration. Effects of oral anticoagulation for atrial fibrillation after spontaneous intracranial hemorrhage in the UK: A randomised, open-label, assessor-masked, pilot-phase, non-inferiority trial. Lancet Neurol. 2021, 20, 842–853. [Google Scholar] [CrossRef]
- Schreuder, F.H.B.M.; van Nieuwenhuizen, K.M.; Hofmeijer, J.; Vermeer, S.E.; Kerkhoff, H.; Zock, E.; Luijckx, G.-J.; Messchendorp, G.P.; van Tuijl, J.; Bienfait, H.P.; et al. Apixaban versus no anticoagulation after anticoagulation-associated intracerebral hemorrhage in patients with atrial fibrillation in the Netherlands (APACHE-AF): A randomised, open-label, phase 2 trial. Lancet Neurol. 2021, 20, 907–916. [Google Scholar] [CrossRef]
- Gisquet, E.; Aouba, A.; Aubry, R.; Jougla, E.; Rey, G. Où meurt-on en France ? Analyse des certificats de décès (1993–2008). Bull. Epidémiologique Hebd. 2012, 48, 547–551. [Google Scholar]
| No Atrial Fibrillation | Atrial Fibrillation | |||
|---|---|---|---|---|
| Ischemic Stroke N = 4023 | Non-Traumatic ICrH N = 12,028 | Ischemic Stroke N = 20,449 | Non-Traumatic ICrH N = 3574 | |
| Age (years), mean ± SD | 72.3 ± 14.1 | 68.8 ± 17.1 # | 79.9 ± 9.7 | 79.4 ± 9.4 + |
| Sex (male), n (%) | 23,193 (57.2) | 6430 (53.5) # | 10,350 (50.6) | 1941 (54.3) # |
| Hypertension, n (%) | 26,772 (66.1) | 6669 (55.4) # | 15,593 (76.3) | 2739 (76.6) NS |
| Diabetes mellitus, n (%) | 10,966 (27.1) | 1995 (16.6) # | 5497 (26.9) | 902 (25.2) NS |
| Smoker, n (%) | 5535 (13.7) | 1122 (9.3) # | 1490 (7.3) | 227 (6.4) + |
| Dyslipidemia, n (%) | 13,885 (34.3) | 2017 (16.8) # | 6820 (33.4) | 916 (25.6) NS |
| Obesity, n (%) | 5117 (12.6) | 964 (8.0) # | 2894 (14.2) | 490 (13.7) NS |
| Heart failure, n (%) | 7014 (17.3) | 1326 (11.0) # | 8810 (43.1) | 1413 (39.5) # |
| History of pulmonary edema, n (%) | 568 (1.4) | 182 (1.5) | 739 (3.6) | 100 (2.8) NS |
| Valve disease, n (%) | 2494 (6.2) | 364 (3.0) # | 3396 (16.6) | 501 (14.0) # |
| Aortic stenosis, n (%) | 1074 (2.7) | 169 (1.4) # | 1352 (6.6) | 183 (5.1) § |
| Aortic regurgitation, n (%) | 559 (1.4) | 62 (0.5) # | 676 (3.3) | 111 (3.1) # |
| Mitral regurgitation, n (%) | 931 (2.3) | 108 (0.9) # | 1612 (7.9) | 233 (6.5) # |
| Previous endocarditis, n (%) | 221 (0.5) | 61 (0.5) NS | 209 (1.0) | 57 (1.6) NS |
| Dilated cardiomyopathy, n (%) | 1571 (3.9) | 237 (2.0) # | 2024 (9.9) | 293 (8.2) # |
| Coronary artery disease, n (%) | 7945 (19.6) | 1281 (10.7) # | 6028 (29.5) | 924 (25.9) # |
| Previous MI, n (%) | 1397 (3.4) | 227 (1.9) # | 996 (4.9) | 134 (3.7) # |
| Previous PCI, n (%) | 1514 (3.7) | 243 (2.0) # | 873 (4.3) | 131 (3.7) + |
| Previous CABG, n (%) | 242 (0.6) | 26 (0.2) # | 295 (1.4) | 34 (1.0) + |
| Vascular disease, n (%) | 10,907 (26.9) | 1286 (10.7) # | 5915 (28.9) | 720 (20.1) # |
| Atrial fibrillation, n (%) | 0 (0.0) | 0 (0.0) | 20,449 (100.0) | 3574 (100.0) |
| Previous pacemaker or ICD, n (%) | 1669 (4.1) | 257 (2.1) # | 2790 (13.6) | 432 (12.1) # |
| Alcohol related diagnoses, n (%) | 3643 (9.0) | 1583 (13.2) # | 1232 (6.0) | 285 (8.0) + |
| Chronic kidney disease, n (%) | 2367 (5.8) | 527 (4.4) # | 2059 (10.1) | 355 (9.9) * |
| Lung disease, n (%) | 6853 (16.9) | 2152 (17.9) + | 4766 (23.3) | 880 (24.6) NS |
| Sleep apnea syndrome, n (%) | 2167 (5.3) | 409 (3.4) # | 1295 (6.3) | 233 (6.5) NS |
| COPD, n (%) | 3131 (7.7) | 662 (5.5) # | 2095 (10.2) | 370 (10.4) NS |
| Liver disease, n (%) | 1440 (3.6) | 748 (6.2) # | 754 (3.7) | 184 (5.1) NS |
| Gastroesophageal reflux, n (%) | 797 (2.0) | 187 (1.6) § | 363 (1.8) | 51 (1.4) * |
| Thyroid diseases, n (%) | 2817 (7.0) | 648 (5.4) # | 2711 (13.3) | 524 (14.7) * |
| Inflammatory disease, n (%) | 2370 (5.8) | 521 (4.3) # | 1611 (7.9) | 244 (6.8) * |
| Anemia, n (%) | 4737 (11.7) | 1215 (10.1) # | 3652 (17.9) | 592 (16.6) + |
| Previous cancer, n (%) | 5520 (13.6) | 1676 (13.9) NS | 3035 (14.8) | 540 (15.1) § |
| Poor nutrition, n (%) | 3232 (8.0) | 978 (8.1) NS | 2540 (12.4) | 461 (12.9) * |
| Cognitive impairment, n (%) | 5083 (12.5) | 1430 (11.9) NS | 3526 (17.2) | 581 (16.3) NS |
| Illicit drug use, n (%) | 208 (0.5) | 70 (0.6) NS | 31 (0.2) | 5 (0.1) NS |
| No Atrial Fibrillation | Ischemic Stroke (n = 11,873) | ICrH (n = 11,873) | p Value | ||
| Number of Events | Incidence, %/Yr (95% CI) | Number of Events | Incidence, %/Yr (95% CI) | ||
| All-cause death | 5758 | 12.71 (12.39–13.04) | 7868 | 25.20 (24.65–25.76) | <0.0001 |
| Cardiovascular death | 1934 | 4.27 (4.08–4.46) | 4351 | 13.94 (13.53–14.36) | <0.0001 |
| Non-cardiovascular death | 3824 | 8.44 (8.18–8.71) | 3517 | 11.26 (10.90–11.64) | <0.0001 |
| Myocardial infarction | 474 | 1.06 (0.97–1.16) | 266 | 0.86 (0.76–0.97) | 0.006 |
| New onset HF | 1995 | 4.70 (4.50–4.92) | 1171 | 3.97 (3.75–4.20) | <0.0001 |
| MACE-HF | 3713 | 8.84 (8.56–9.13) | 5450 | 19.39 (18.88–19.91) | <0.0001 |
| Major bleeding | 2463 | 5.96 (5.73–6.20) | 1595 | 5.58 (5.31–5.86) | 0.04 |
| New type of cerebrovascular event | 591 | 1.32 (1.22–1.44) | 705 | 2.36 (2.19–2.54) | <0.0001 |
| Atrial Fibrillation | Ischemic Stroke (n = 3574) | ICrH (n = 3574) | p Value | ||
| Number of Events | Incidence, %/yr (95% CI) | Number of Events | Incidence, %/yr (95% CI) | ||
| All-cause death | 2511 | 23.89 (22.97–24.84) | 2878 | 40.52 (39.06–42.03) | <0.0001 |
| Cardiovascular death | 966 | 9.19 (8.63–9.79) | 1561 | 21.98 (20.91–23.09) | <0.0001 |
| Non-cardiovascular death | 1545 | 14.70 (13.98–15.45) | 1317 | 18.54 (17.57–19.57) | <0.0001 |
| Myocardial infarction | 118 | 1.14 (0.95–1.36) | 84 | 1.20 (0.97–1.48) | 0.73 |
| New onset HF | 704 | 7.47 (6.94–8.04) | 459 | 7.12 (6.50–7.80) | 0.43 |
| MACE-HF | 1592 | 17.10 (16.28–17.97) | 1946 | 32.31 (30.90–33.77) | <0.0001 |
| Major bleeding | 899 | 9.81 (9.19–10.47) | 546 | 8.76 (8.05–9.52) | <0.0001 |
| New type of cerebrovascular event | 212 | 2.06 (1.80–2.35) | 318 | 4.78 (4.28–5.33) | <0.0001 |
| Model A | Model B | Model C | Model D | |
|---|---|---|---|---|
| All-cause death | 1.76 (1.72–1.81) | 2.05 (1.99–2.10) | 1.89 (1.85–1.95) | 1.80 (1.74–1.86) |
| Cardiovascular death | 2.79 (2.68–2.89) | 3.19 (3.08–3.32) | 2.98 (2.86–3.09) | 2.79 (2.64–2.94) |
| Non-cardiovascular death | 1.23 (1.18–1.27) | 1.43 (1.38–1.48) | 1.31 (1.26–1.36) | 1.28 (1.22–1.34) |
| Myocardial infarction | 0.64 (0.56–0.73) | 0.71 (0.62–0.81) | 0.76 (0.67–0.87) | 0.81 (0.70–0.94) |
| New onset HF | 0.71 (0.67–0.76) | 0.83 (0.78–0.888) | 0.84 (0.79–0.89) | 0.85 (0.79–0.91) |
| MACE-HF | 1.83 (1.78–1.89) | 2.11 (2.05–2.18) | 2.06 (2.00–2.13) | 1.97 (1.89–2.06) |
| Major bleeding | 0.88 (0.83–0.93) | 0.96 (0.91–1.01) | 0.93 (0.88–0.98) | 0.92 (0.87–0.98) |
| New type of cerebrovascular event | 1.63 (1.49–1.78) | 1.80 (1.65–1.95) | 1.76 (1.61–1.92) | 1.75 (1.57–1.96) |
| Model A | Model B | Model C | Model D | |
|---|---|---|---|---|
| All-cause death | 1.53(1.47–1.59) | 1.61 (1.55–1.68) | 1.57 (1.51–1.63) | 1.56 (1.47–1.64) |
| Cardiovascular death | 2.06 (1.95–2.18) | 2.19 (2.07–2.31) | 2.16 (2.04–2.29) | 2.08 (1.91–2.25) |
| Non-cardiovascular death | 1.18 (1.11–1.25) | 1.23 (1.16–1.30) | 1.18 (1.11–1.25) | 1.21 (1.13–1.31) |
| Myocardial infarction | 0.99 (0.79–1.24) | 0.99 (0.79–1.24) | 0.99 (0.79–1.24) | 1.05 (0.79–1.39) |
| New onset HF | 0.92 (0.83–1.01) | 0.95 (0.86–1.04) | 0.96 (0.87–1.06) | 0.94 (0.84–1.06) |
| MACE-HF | 1.72 (1.64–1.80) | 1.79 (1.70–1.88) | 1.78 (1.69–1.87) | 1.71 (1.60–1.83) |
| Major bleeding | 0.88 (0.80–0.96) | 0.88 (0.81–0.96) | 0.86 (0.79–0.94) | 0.89 (0.80–0.99) |
| New type of cerebrovascular event | 2.28 (2.02–2.58) | 2.32 (2.05–2.62) | 2.29 (2.02–2.59) | 2.29 (1.93–2.73) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasi, M.; Boulouis, G.; Bisson, A.; Herbert, J.; Bodin, A.; Cordonnier, C.; Lip, G.Y.H.; Fauchier, L. Adverse Cardiovascular Events in Non-Traumatic Intracranial Hemorrhage and Ischemic Stroke Survivors. J. Clin. Med. 2022, 11, 6885. https://doi.org/10.3390/jcm11236885
Pasi M, Boulouis G, Bisson A, Herbert J, Bodin A, Cordonnier C, Lip GYH, Fauchier L. Adverse Cardiovascular Events in Non-Traumatic Intracranial Hemorrhage and Ischemic Stroke Survivors. Journal of Clinical Medicine. 2022; 11(23):6885. https://doi.org/10.3390/jcm11236885
Chicago/Turabian StylePasi, Marco, Grégoire Boulouis, Arnaud Bisson, Julien Herbert, Alexandre Bodin, Charlotte Cordonnier, Gregory Y. H. Lip, and Laurent Fauchier. 2022. "Adverse Cardiovascular Events in Non-Traumatic Intracranial Hemorrhage and Ischemic Stroke Survivors" Journal of Clinical Medicine 11, no. 23: 6885. https://doi.org/10.3390/jcm11236885
APA StylePasi, M., Boulouis, G., Bisson, A., Herbert, J., Bodin, A., Cordonnier, C., Lip, G. Y. H., & Fauchier, L. (2022). Adverse Cardiovascular Events in Non-Traumatic Intracranial Hemorrhage and Ischemic Stroke Survivors. Journal of Clinical Medicine, 11(23), 6885. https://doi.org/10.3390/jcm11236885







