Abstract
The rate of abdominal surgical interventions and associated postoperative complications in inflammatory bowel disease (IBD) patients is still substantially high. There is an ongoing debate as to whether or not patients who undergo treatment with anti-tumor necrosis factor-alpha (TNF-α) agents may have an increased risk for general and surgical postoperative complications. Therefore, a systematic review and meta-analysis was conducted in order to assess the effect of anti-TNF-α treatment within 12 weeks (washout period) prior to abdominal surgery on 30-day postoperative complications in patients with IBD. The results of previously published meta-analyses examining the effect of preoperative anti-TNF-α treatment on postoperative complications reported conflicting findings which is why we specifically focus on the effect of anti-TNF-α treatment within 12 weeks prior to surgery. PubMed, Cochrane, Scopus, Web of Science, World Health Organization Trial Registry, ClinicalTrials.gov and reference lists were searched (June 1995–February 2022) to identify studies, investigating effects of anti-TNF-α treatment prior to abdominal surgery on postoperative complications in IBD patients. Pooled odds ratios (OR) with 95% confidence intervals (CI) were calculated and subgroup analyses were performed. In this case, 55 cohort studies (22,714 patients) were included. Overall, postoperative complications (OR, 1.23; 95% CI, 1.04–1.45; p = 0.02), readmission (OR, 1.39; 95% CI, 1.11–1.73; p = 0.004), and intra-abdominal septic complications (OR, 1.89; 95% CI, 1.44–2.49; p < 0.00001) were significantly higher for anti-TNF-α-treated patients. Significantly higher intra-abdominal abscesses and readmission were found for anti-TNF-α-treated CD patients (p = 0.05; p = 0.002). Concomitant treatment with immunosuppressives in <50% of anti-TNF-α-treated patients was associated with significantly lower mortality rates (OR, 0.32; 95% CI, 0.12–0.83; p = 0.02). Anti-TNF-α treatment within 12 weeks prior to surgery is associated with higher short-term postoperative complication rates (general and surgical) for patients with IBD, especially CD.
1. Introduction
With worldwide increasing incidence and prevalence, inflammatory bowel diseases (IBD), encompassing Crohn’s Disease (CD) and Ulcerative Colitis (UC), belong to a group of diseases with substantial social and economic burden on health care systems and governments. [1] Compared to the 1990s, the life-time risk of undergoing a major abdominal surgery for IBD has decreased in the last decade. [2] Consequently, the treatment with biological drugs has continuously increased over these years and has become an established practice in the treatment of IBD nowadays. [2] Tumor necrosis factor-alpha (TNF-α) inhibitors were among the first FDA approved biologic drugs in the treatment for IBD. Today, up to 40% of CD patients and up to 16% of UC patients are under treatment with anti-TNF-α biologics such as Adalimumab, Infliximab, Golimumab, Certolizumab (pegol) or one of its biosimilars. However, in comparison with the general population, the 5-year surgery rates with its accompanying potential postoperative complications remain substantial for IBD patients. [2] Previously conducted meta-analyses, analyzing the effect of preoperative anti-TNF-α treatment on postoperative complications has reported conflicting findings [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17].
The latter is mostly the result of including different preoperative drug withdrawal periods in the analyses. [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17] In order to find consistent values regarding the effect of preoperative anti-TNF-α treatment on postoperative complications and to ensure better comparisons of data, we chose the objectifiable anti-TNF-α drug washout period of 12 weeks [18] as the preoperative cut-off value in this analysis.
Therefore, the aim of this study was to evaluate the effect of anti-TNF-α treatment exclusively within the washout period of 12 weeks (3 months) [18] prior to abdominal surgery, on a 30-day general and surgical postoperative complication rate in IBD patients. A systematic review and meta-analysis were conducted, comparing summary effect sizes, calculating the pooled odds ratio (OR) with 95% confidence intervals (CI) and performing subgroup analyses with subgroups stratified by IBD subtype, surgical approach (open versus (vs.) laparoscopic), elective vs. emergency surgery, protective ileostomy use and concomitant use of corticosteroids and/or immunomodulatory drugs.
2. Methods
This systematic review and meta-analysis were conducted and reported according to the recommendations in the Cochrane Handbook for Reviews of Interventions [19] and the Preferred Reporting Items for Systematic review and Meta-Analyses (PRISMA) statement 2020 [20].
2.1. Eligibility Criteria
All observational studies (prospective or retrospective comparative cohort or case-control studies), nested case-control studies, non-randomized controlled trials, randomized controlled trials and cross-sectional studies were included based on the following criteria: examined humans (of which the majority were ≥18 years old), being published in English or German language, being available as full-text article in the electronic medical databases between 1 June 1995 and 17 February 2022, patients undergoing any intestinal surgical procedures for IBD (CD/UC/Indeterminate Colitis (IC)), an intervention group including patients who either received anti-TNF-α biologics within the washout period of 12 weeks (3 months) [18] prior to surgery or had a detectable serum concentrations (≥0.98 µg/mL) [21] of these drugs at surgery (regardless of anti-TNF-α biologic preparation and dose), a control group including patients who either did not received any biologic therapy before surgery or had no detectable serum concentrations of an anti-TNF-α biologic (<0.98 µg/mL) [21] at surgery, and a reported exactly 30-day postoperative complication rate. If studies reported two or more discrete data sets, these sets were separately included for the analysis.
The exclusion criteria comprised studies without a control group, abstracts and conference proceedings, reviews and meta-analyses, case-reports and case-series, in-vivo, ex-vivo and in-vitro studies, animal studies, predominantly pediatric patients being studied, concomitant use of any biologic drug other than anti-TNF-α biologics, the intervention groups’ last exposure to anti-TNF-α biologics more than 12 weeks (3 months) [18] prior to intestinal surgery, and a follow-up period below or above 30 days after surgery for both the intervention and control groups.
2.2. Search Strategy
A systematic literature search was performed for studies published in the electronic medical databases PubMed (MEDLINE), Web of Science, Cochrane Library and Cochrane central register of controlled trials, Scopus, ClinicalTrials.gov and the World Health Organization Trials Registry, using predefined search items for each database (Supplementary Tables S1.1.–S1.6.). The reference lists of the included studies were examined manually, and an additional web search was conducted to ensure that potentially relevant studies were not missed (Supplementary Table S1.7.) [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. In the case of insufficient or inadequate data presentation, authors were contacted to provide the required information. The final search was conducted on 17 February 2022.
2.3. Selection Process
All studies were assessed manually and independently by two investigators (surgical resident: K.C., radiology resident: S.R.) and were exported to the reference management tool EndNote X9 (EndNote X9; The EndNote Team, Clarivate 2013; Philadelphia, PA, USA). According to this studies’ predefined eligibility criteria, titles and abstracts were assessed, excluding both duplicates and studies not coinciding with the eligibility criteria. Finally, the remaining full-text articles were retrieved and evaluated for eligibility. The disagreements concerning eligibility were resolved in consensus with a third investigator (surgical specialist: P-A.N.), who independently assessed the accuracy of search results.
2.4. Data Collection Process
The data were collected and analyzed independently by two investigators (K.C., S.R.) onto a Microsoft Excel spreadsheet (Home and Student 2019 edition; Microsoft, Redmond, WA) and was assessed independently for accuracy by a third investigator (P-A.N.). The emerging discrepancies in relation to data extracted were discussed and resolved by consensus of all three investigators.
2.5. Data Items
The following data were collected for each study if available: author, year, and country of publication, study design and inclusion period, medical treatment and time frame in which drugs were used preoperatively, number of patients in the intervention and control group, patients’ baseline and surgical characteristics, and postoperative follow-up period and postoperative complication rates.
In order to capture all complications while ensuring comparability of data, postoperative complication rates were analyzed according to the definitions used in the included studies.
For this review, 30-day postoperative complication rates were quantified and analyzed, including:
- (1)
- general overall complication rates:
- overall postoperative complications
- overall infectious postoperative complications
- overall Clavien-Dindo minor and major complications
- readmission rates, reoperation rates, mortality rates
- (2)
- surgical-site complication rates:
- overall infectious surgical complications
- intra-abdominal septic complications, anastomotic leakages (AL), intra-abdominal abscesses (without drainage)
- surgical-site infections (SSI) (incisional- and deep or organ space)
- postoperative hemorrhages, ileus, small bowel obstructions, fistula formations
- (3)
- non-surgical-site complication rates:
- overall infectious and non-infectious non-surgical-site complications
- thrombosis, cardiovascular complications
- pneumonia, urinary tract infections and sepsis
In order to serve as an orientation for surgeons and provide an implication into surgical practice, this review and the resulting meta-analysis primarily focused on 30-day postoperative general and surgical complication rates.
2.6. Assessment of Risk of Bias and Quality of Included Studies
The qualities of the included studies were assessed independently by two investigators (K.C., S.R.) using the Newcastle-Ottawa scale (NOS) for cohort studies [43], a valid and commonly used score applied for observational studies. [4,5,6,7,9,16,17,43] The investigators’ (K.C., S.R.) judgements were conclusively justified (Supplementary Tables S2.1.–S2.51.) and any disagreements were resolved in consensus with a third investigator (P-A.N.). A NOS score of >7 was defined as high-quality, 5–7 as moderate-quality and <5 as low-quality study.
Potential risk factors for postoperative complications within each study group were defined a priori as: tobacco use in >50% of patients, open surgical approach and/or conversion to open surgery in >50% of patients (OA), elective and emergency surgery (ELEMS) vs. exclusively elective surgery (EL), performance of a temporary protective ileostomy in < 50% of patients or no use at all (PRI), Body-Mass-Index (BMI) of >25 kg/m2 or <18.5 kg/m2, dysplasia or malignancy in >50% of patients; perforating or penetrating disease as indication for surgery in >50% of patients, concomitant corticosteroid and/or immunomodulatory agent use in >50% of patient (CSIM), laboratory values (median or mean C-reactive protein concentration of >10 mg/L, white blood cell count > 11 × 109 cells/L or <4 × 109 cells/L, hemoglobin value of <10 g/dL, and albumin value of <3 g/dL and platelet count of <150 × 103/µL).
2.7. Synthesis Methods
The heterogeneity between studies was analyzed using the statistical I2 test [44], considering a I2 of ≥50% as substantial heterogeneity. [44] In case of significant or substantial heterogeneity among the included studies, sensitivity analysis was conducted by evaluating the effect of excluding one study at a time on the pooled OR. According to the recommendations of the Cochrane Handbook for Reviews of Interventions [19], potential publication biases were assessed for outcomes reported by ≥10 studies by applying the Egger’s test [45] for funnel plot asymmetry.
The prespecified subgroup analyses were performed for outcomes reported by ≥ 5 studies to evaluate the influence of IBD subtypes and potential risk factors on the 30-day postoperative complications. The differences in the outcomes between these subgroups were assessed and reported using the test for subgroup differences (TSD). For subgroups stratified by IBD subtypes, sensitivity analysis was conducted by excluding IBD mixed populations, for which data of the separate IBD subtypes could not be retrieved. Further sensitivity analysis was conducted for subgroups stratified by prespecified risk factors by excluding studies, for which data of studied risk factor were not available.
The results with a p-value of <0.05 were considered significant. All statistical analyses were carried out using the Review Manager software version 5.3 (Nordic Cochrane Centre, Copenhagen, Denmark) and JASP Team (2021; JASP (Version 0.16) [Computer software]). In the case of significant or substantial heterogeneity (I2 ≥ 50%) of included studies, random-effects model (RE) was used to conduct the meta-analysis. With a non-significant I2 < 50% of included studies, fixed-effects model (FE) was used to conduct the meta-analysis. Using either RE or FE meta-analysis, pooled OR with 95% CI were summarized and depicted in a forest plot.
3. Results
3.1. Study Selection
As depicted in Figure 1, 914 studies were identified through electronic medical database search and another 21 studies through manual search (other methods). After removing 181 duplicates, titles and abstracts of 733 articles were screened manually of which 590 were excluded. For the full-text review 137 out of 143 studies (electronic medical database search) and 9 out of 21 studies (other methods) could be retrieved.
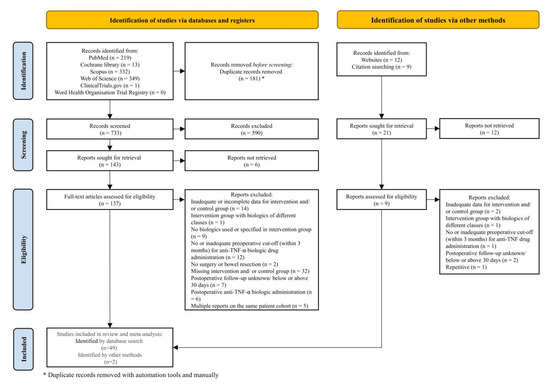
Figure 1.
The study flow diagram according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) Statement 2020 [20].
Finally, 51 cohort studies [21,22,23,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93] fulfilled the inclusion criteria, three [21,55,89] of which included at least two discrete data sets and were subjected to qualitative and quantitative analysis (Figure 1).
3.2. Study Characteristics
The systematic review and meta-analysis investigate 47 retrospective [21,22,23,46,47,48,50,51,53,54,55,56,57,58,60,61,62,63,64,65,66,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93] and four prospective [49,52,59,67] studies.
Overall, 22,714 patients were included in the 51 studies (54 discrete data sets) [21,22,23,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93], 4417 patients were treated with an anti-TNF-α agent within 12 weeks prior to abdominal surgery (intervention group) and 18,297 patients did not receive anti-TNF-α treatment (control group). TNF-α inhibitors that were investigated included: Adalimumab, Certolizumab (pegol), Golimumab, Infliximab and/or its biosimilars [21,22,23,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93].
The most commonly studied IBD subtype was CD, including 28 studies (30 discrete data sets) [21,22,23,46,47,49,50,51,56,57,58,60,61,64,65,66,68,69,70,73,77,79,80,81,84,88,89,90,91,92] investigating 2272 patients in the intervention and 8039 patients in the control group. Another 13 studies (16 discrete data sets) [21,48,53,54,55,59,71,72,76,82,83,85,86,89,93] investigated 1600 UC (and IC) patients in the intervention and 8734 UC (and IC) patients in the control group. Ten studies [21,52,62,63,67,74,75,78,87,89] examined a mixed IBD patient group, out of which eight studies [52,62,63,67,74,75,78,87] did not further specify the underlying disease (545 patients in the intervention and 1524 in the control group) (Table 1).

Table 1.
Study and patient characteristics.
All included studies analyzed the short-term postoperative complication and 30-day complication rates (Table 2 and Supplementary Tables S2.1.–S2.51. and S3.1.–S3.2.).

Table 2.
30-day general and surgical-site postoperative complications.
3.3. Results of Synthesis
3.3.1. Analysis of General Postoperative Complications
Overall Postoperative Complications
Overall, 27 studies (30 discrete data sets) [21,22,23,47,48,49,51,52,54,55,56,57,58,59,61,62,63,67,69,70,74,75,81,86,89,90,91] reported 30-day overall postoperative complications occurring in 678 of 2588 (26.2%) patients in the intervention group and 2059 of 11,004 (18.7%) in the control group. OPC were significantly higher for patients preoperatively treated with anti-TNF-α agents, using RE meta-analysis (OR, 1.23; 95% CI, 1.04–1.45; p = 0.02) (Figure 2). The studies showed a significant heterogeneity (I2 = 44%; p = 0.006) but sensitivity analysis showed a significant reduction in heterogeneity with the exclusion of the study by Brouquet et al., 2018 [49] (OR, 1.17; 95% CI, 1.01–1.36; p = 0.04; I2 = 28%; p = 0.08). No publication bias was observed (Egger’s test: p = 0.204).
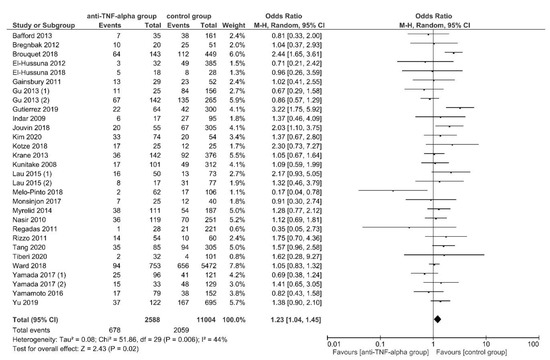
Figure 2.
Random-effects model meta-analysis for 30-day overall postoperative complications in the anti-TNF-α (intervention) and control group. Forest plot of all studies included [21,22,23,47,48,49,51,52,54,55,56,57,58,59,61,62,63,67,69,70,74,75,81,86,89,90,91].
The subgroup analyses found no subgroup difference for subgroups stratified by IBD subtypes, OA, ELEMS, PRI and CSIM (Supplementary Table S4).
Overall Postoperative Infectious Complication
In this case, 20 studies (22 discrete data sets) [21,23,48,53,54,58,59,62,63,64,69,71,74,75,76,79,80,86,89,91] reported 30-day overall postoperative infectious complications in 302 of 2208 (13.7%) patients in the intervention group and 858 of 9333 (9.2%) patients in the control group. No significant differences were found between the examined group, using RE meta-analysis (OR, 1.16; 95% CI, 0.92–1.45; p = 0.21) (Supplementary Figure S1).
The studies showed a significant heterogeneity (I2 = 43%; p = 0.02) which remained stable throughout sensitivity analysis. No publication bias was observed (Egger’s test: p = 0.154) and subgroup analyses found no subgroup difference for subgroups stratified by IBD subtypes, OA, ELEMS, PRI and CSIM (Supplementary Table S5).
Overall Clavien-Dindo Major Complications
A total of 7 studies [23,60,62,66,69,79,81] reported 30-day overall postoperative Clavien-Dindo major complications, manifesting in 101 of 564 (17.9%) patients in the anti-TNF-α group and 176 of 1243 (14.1%) patients in the control group. No difference in OC-DMC was found between the studied group, using FE meta-analysis (OR, 1.13; 95% CI, 0.85–1.50; p = 0.40).
The studies were homogeneous (I2 = 0%; p = 0.91). (Supplementary Figure S2) subgroup analyses found no subgroup difference for subgroups stratified by IBD subtypes; OA; ELEMS; PRI and CSIM (Supplementary Table S6).
Readmission
In this case, 30-day postoperative readmission rates were reported by 15 studies (17 data sets) [21,46,52,55,59,60,61,62,71,75,78,79,87,88,89], with 162 of 1192 (13.6%) patients in the intervention and 259 of 2580 (10.03%) patients in the control group being readmitted. Readmission was presented to be significantly higher for patients in the intervention group, using FE meta-analysis (OR, 1.39; 95% CI, 1.11–1.73; p = 0.004) (Figure 3). The studies were homogeneous (I2 = 0%; p = 0.45) and no publication bias was observed (Egger’s test: p = 0.994).
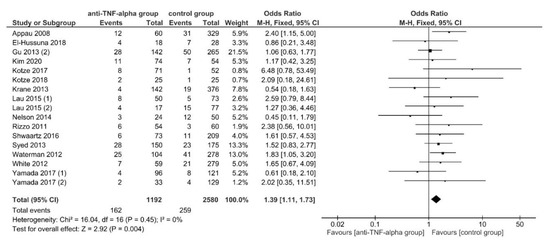
Figure 3.
The fixed-effects model meta-analysis for 30-day postoperative readmission in the anti-TNF-α (intervention) and control group. Forest plot of all studies included. [21,46,52,55,59,60,61,62,71,75,78,79,87,88,89].
After conducting sensitivity analysis, the subgroup analyses found significant subgroup differences for subgroups stratified by IBD subtypes (TSD: p = 0.15; sensitivity analysis: p = 0.05), OA (TSD: p = 0.06; sensitivity analysis: p = 0.02) and PRI (TSD: p = 0.06; sensitivity analysis: p = 0.03). A significantly higher readmission rate was found for patients in the intervention group if the underlying disease was CD (OR, 1.77; 95% CI, 1.23–2.52; p = 0.002), if OA was conducted in >50% of patients (OR, 2.01; 95% CI, 1.32–3.08; p = 0.001) and if PRI was used in <50% (OR, 1.79; 95% CI, 1.32–2.42; p = 0.0002) (Table 3).

Table 3.
The fixed-effect model meta-analysis for 30-day postoperative readmission and intra-abdominal abscesses in the anti-TNF-α (intervention) and control group. Analysis for publication bias and subgroup analysis.
Reoperation
Overall, 14 studies (15 data sets) [46,50,55,60,61,62,69,72,73,74,75,79,87,89] reported 30-day postoperative reoperation rates in 142 of 1493 (9.5%) patients in the intervention and 534 of 5400 (9.9%) patients in the control group. No significant differences were found between the studied groups, using FE meta-analysis (OR, 1.02; 95% CI, 0.82–1.26; p = 0.88) (Supplementary Figure S3). The studies were homogeneous (I2 = 0%; p = 0.80) and no significant publication bias was observed (Egger’s test: p = 0.439).
The subgroup analyses found no subgroup difference for subgroups stratified by IBD subtypes, OA, ELEMS, PRI and CSIM (Supplementary Table S7).
Mortality
In this case, 30-day postoperative mortality was reported by 13 studies [21,46,55,58,60,61,62,63,70,72,73,79,87], occurring in 13 of 1432 (0.9%) patients in the anti-TNF-α group and 95 of 5538 (1.7%) patients in the control group. No significant difference was found between the studied groups, using FE meta-analysis (OR, 0.73; 95% CI, 0.41–1.30; p = 0.28) (Supplementary Figure S4). The studies were homogeneous (I2 = 30%; p = 0.14) and no significant publication bias was observed (Egger’s test: p = 0.361).
The subgroup analyses found a significant subgroup difference for subgroups stratified by CSIM (TSD: p = 0.02; sensitivity analysis: p = 0.006). Mortality was significantly lower for patients in the intervention group if concomitant corticosteroids or immunomodulatory drugs were used in just a minority of patients or not at all (OR, 0.32; 95% CI, 0.12–0.83; p = 0.02). No sensitivity analysis stable subgroup differences were found for subgroups stratified by IBD subtypes, OA, ELEMS and PRI (Supplementary Table S8).
3.3.2. Analysis of Surgical Postoperative Complications
Overall Infectious Surgical-Site Complications
A total of 11 studies [23,53,60,61,65,71,77,82,83,84,85] reported 30-day overall postoperative infectious surgical-site complications, appearing in 112 of 625 (19.9%) patients in the intervention and 324 of 1630 (19.8%) in the control group. No significant differences were found between anti-TNF-α and control group, using RE meta-analysis (OR, 0.81; 95% CI, 0.45–1.45; p = 0.48) (Supplementary Figure S5).
The studies showed a significant substantial heterogeneity (I2 = 76%; p < 0.0001), still results remained stable throughout sensitivity analysis. No significant publication bias was observed (Egger’s test: p = 0.439).
The subgroup analyses found a significant subgroup difference for subgroups stratified by ELEMS (TSD: p < 0.00001; sensitivity analysis: p < 0.00001). Anti-TNF-α treated CD patients who had undergone elective surgery presented with significantly higher OISSC (OR, 2.02; 95% CI, 1.31–3.11; p = 0.002), while CD and UC patients who underwent both elective and emergency surgery developed OISSC significantly less (OR, 0.31; 95% CI, 0.18–0.55; p < 0.0001). No significant sensitivity analysis stable subgroup differences were found for subgroups stratified by IBD subtypes, OA, PRI and CSIM (Supplementary Table S9).
Intra-Abdominal Septic Complications
In this case, 30-day postoperative intra-abdominal septic complications were reported by 9 studies [49,52,64,68,70,79,80,90,92], manifesting in 108 of 830 (13.01%) patients in the intervention and 164 of 2040 (8.04%) patients in the control group. Using FE meta-analysis, patients in the anti-TNF-α group had significantly higher postoperative intra-abdominal septic complications (OR, 1.89; 95% CI, 1.44–2.49; p < 0.00001). The studies were homogeneous (I2 = 47%; p = 0.06) (Figure 4).
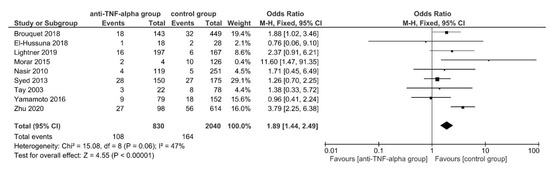
Figure 4.
The fixed-effects model meta-analysis for 30-day postoperative intra-abdominal septic complications in the anti-TNF-α (intervention) and control group. Forest plot of all studies included. [49,52,64,68,70,79,80,90,92].
No subgroup difference was found in subgroup analysis for subgroups stratified by IBD subtypes (TSD: p = 0.47), still 8 [49,64,68,70,79,80,90,92] out of 9 studies [49,52,64,68,70,79,80,90,92] examined CD patient groups. Excluding the only IBD mixed patient group (study by El-Hussuna et al., 2018 [52]) resulted in a significantly higher postoperative intra-abdominal septic complication rate for anti-TNF-α treated CD patients in comparison to the control group (OR, 1.92; 95% CI, 1.45–2.53; p < 0.00001). No sensitivity analysis stable subgroup differences were found for subgroups stratified by OA, ELEMS, PRI and CSIM (Supplementary Table S10).
AL or Abscess
A total of 21 studies (22 data sets) [23,46,50,53,55,60,61,62,63,69,72,73,74,75,78,79,80,87,89,90,93] reported 30-day postoperative AL or abscesses in 93 of 1827 (5.1%) patients in the intervention group and 313 of 7078 (4.4%) patients in the control group. No significant differences were found between the studied groups, using FE model meta-analysis (OR, 1.02; 95% CI, 0.79–1.32; p = 0.86) (Supplementary Figure S6). Studies were homogeneous (I2 = 0%; p = 0.88) and no significant publication bias was observed (Egger’s test: p = 0.216).
The subgroup analysis showed no subgroup differences for subgroups stratified by the IBD subtype, ELEMS and CSIM (Supplementary Table S11).
Intra-Adominal Abscesses
Overall, 18 studies (19 data sets) [23,46,48,50,53,54,55,59,60,61,62,71,74,78,79,80,89,93] reported the presence of intra-abdominal abscesses 30-days postoperatively, occurring in 81 of 1188 (6.8%) patients in the anti-TNF-α group and 229 of 3333 (6.9%) patients in the control group. No significant difference was found between the two groups, using FE model meta-analysis (OR, 1.19; 95% CI, 0.89–1.60; p = 0.24) (Supplementary Figure S7). The studies were homogeneous (I2 = 0%; p = 0.76) and no significant publication bias was observed (Egger’s test: p = 0.85).
After conducting sensitivity analysis, subgroup analysis showed a significant subgroup difference for subgroups stratified by IBD subtypes (TSD: p = 0.15; sensitivity analysis: p = 0.05) and PRI (TSD: p = 0.13; sensitivity analysis: p = 0.05). Intra-abdominal abscess rate was significantly higher for anti-TNF-α treated patients, if the underlying disease was CD (OR, 1.50; 95% CI, 1.0–2.25; p = 0.05) and if only a minority of patients received a protective ileostomy (OR, 1.58; 95% CI, 1.06–2.37; p = 0.02).
No subgroup differences were found for subgroups stratified by OA, ELEMS, and CSIM (Table 3).
3.3.3. Analysis of Other Postoperative Complications
No significant differences were found between the intervention and control group for:
- (1)
- Overall non-infectious postoperative complications [48,54,71,89] (FE: OR, 0.85; 95% CI, 0.56–1.29; p = 0.45; I2 = 23%; p = 0.27) (Supplementary Figure S8).
- (2)
- Overall Clavien-Dindo minor complications [23,60,62,66,69] (FE: OR, 1.30; 95% CI, 0.98–1.72; p = 0.06; I2 = 34%; p = 0.19) (Supplementary Figure S9).
- (3)
- Superficial SSI [23,52,65,71,74,82,83,84,85,89] (RE: OR, 0.67; 95% CI, 0.32–1.40; p = 0.29; I2 = 69%; p = 0.0004–stable throughout sensitivity analysis; Egger’s Test: p = 0.643; no subgroup difference in subgroup analysis for subgroups stratified by IBD subtype: TSD: p = 0.93; sensitivity analysis: p = 0.74) (Supplementary Figure S10; Supplementary Table S12).
- (4)
- Deep or organ space SSI [23,82,83,84,85] (FE: OR, 0.76; 95% CI, 0.48–1.22; p = 0.26; I2 = 0%; p = 0.54) (Supplementary Figure S11).
- (5)
- Fistula formation [74,79,80] (FE: OR, 0.65; 95% CI, 0.17–2.47; p = 0.52; I2 = 0%; p = 0.83) (Supplementary Figure S12).
- (6)
- Ileus [23,48,55,59,62,66,70,74,75,89] (FE: OR, 1.11; 95% CI, 0.81–1.51; p = 0.52; I2 = 0%; p = 0.15; Egger’s Test: p = 0.115; no subgroup difference in subgroup analysis for subgroups stratified by IBD subtype: TSD: p = 0.76; sensitivity analysis: p = 0.5) (Supplementary Figure S13; Supplementary Table S13).
- (7)
- Small bowel obstruction [23,55,60,61,62,74] (FE: OR, 0.76; 95% CI, 0.43–1.32; p = 0.33; I2 = 0%; p = 0.86; no subgroup difference in subgroup analysis for subgroups stratified by IBD subtype: TSD: p = 0.69; sensitivity analysis: p = 0.41) (Supplementary Figure S14; Supplementary Table S14).
- (8)
- Postoperative hemorrhage [23,55,63,75,79,89] (FE: OR, 0.83; 95% CI, 0.49–1.41; p = 0.50; I2 = 0%; p = 0.86; no subgroup difference in subgroup analysis for subgroups stratified by IBD subtype: TSD: p = 0.37; sensitivity analysis: p = 1.0) (Supplementary Figure S15; Supplementary Table S15).
- (9)
- Overall infectious non-surgical-site complications [53,71,78] FE: OR, 1.14; 95% CI, 0.43–3.03; p = 0.79; I2 = 0%; p = 0.53) (Supplementary Figure S16).
- (10)
- Thrombosis [48,62,63,71,75,79,93] (FE: OR, 1.30; 95% CI, 0.75–2.25; p = 0.34; I2 = 14%; p = 0.34; no subgroup difference in subgroup analysis for subgroups stratified by IBD subtype: TSD: p = 0.24; sensitivity analysis: p = 0.14) (Supplementary Figure S17; Supplementary Table S16).
- (11)
- Cardiovascular complications [62,63,70,79] (FE: OR, 0.62; 95% CI, 0.23–1.73; p = 0.36; I2 = 0%; p = 0.96) (Supplementary Figure S18).
- (12)
- Pneumonia [48,50,55,59,60,61,62,79,87,89] (FE: OR, 0.83; 95% CI, 0.48–1.46; p = 0.52; I2 = 0%; p = 0.91; no subgroup difference in subgroup analysis for subgroups stratified by IBD subtype: TSD: p = 0.85; sensitivity analysis: p = 0.58) (Supplementary Figure S19; Supplementary Table S17).
- (13)
- Urinary tract infection [46,48,53,55,60,61,62,87,89] (FE: OR, 1.02; 95% CI, 0.54–1.91; p = 0.96; I2 = 0%; p = 0.65; no subgroup difference in subgroup analysis for subgroups stratified by IBD subtype: TSD: p = 0.68; sensitivity analysis: p = 0.76) (Supplementary Figure S20; Supplementary Table S18).
- (14)
- Sepsis [23,46,48,53,74,79] (FE: OR, 1.24; 95% CI, 0.75–2.04; p = 0.41; I2 = 0%; p = 0.44; no subgroup difference in subgroup analysis for subgroups stratified by IBD subtype: TSD: p = 0.90; sensitivity analysis: p = 0.67) (Supplementary Figure S21; Supplementary Table S19).
4. Discussion
This systematic review and meta-analysis give an extensive overview on the 30-day postoperative complication rates in IBD patients following preoperative anti-TNF-α treatment within the washout period of 12 weeks [18] prior to abdominal surgery.
The results of previously published meta-analyses reported conflicting findings. Some studies reported a significant increase in overall postoperative complications [7,8,9,16] or overall infectious postoperative complications [3,6,7,9,16] for anti-TNF-α treated patients, others did not. [3,5,12,15,17]. This meta-analysis found significantly higher overall postoperative complications, readmission rates and intra-abdominal septic complications for anti-TNF-α treated patients (Figure 2, Figure 3 and Figure 4; Table 3; Supplementary Tables S4 and S10). The analysis for overall postoperative complications showed a significant substantial heterogeneity which was significantly reduced when removing the study by Brouquet et al., 2018 [49] (Figure 2; Supplementary Table S4).
In this specific study, the authors reported an increased risk for overall morbidity and intra-abdominal septic complications for anti-TNF-α treated CD patients after ileocolic resection, regardless of disease severity. Since the studies’ authors did not specify overall postoperative morbidity further, this study might have introduced the observed heterogeneity [49].
In contrast, no significant differences were found between the studied groups for overall infectious complications, Clavien-Dindo major complications, reoperation rates and AL, even after performing thorough subgroup analysis stratified by disease subtypes and potential risk factors (Supplementary Figures S1–S3 and S6; Tables S5–S7 and S11).
The significantly higher postoperative intra-abdominal abscesses and readmission rates were found in the subgroup analyses for patients in the intervention group, when the underlying disease was CD or when only a minority of patients received a protective ileostomy after surgery. In addition, an open surgical approach in >50% of patients were found to be associated with significantly higher readmission rates for patients in the intervention group (Figure 3; Table 3; Supplementary Figure S7). The major surgical procedure for the anti-TNF-α treated group with low protective ileostomy formation (<50% of patients) and significantly higher intra-abdominal abscess rates were ileocolic resections [46,50,60,61,78,79,80] and colectomies [54] with primary anastomosis, mostly conducted in CD patients [46,50,60,61,79,80] (Table 3).
Interestingly, Myrelid et al., 2012 [94] investigated 132 CD patients who underwent ileocecal or ileocolonic resection for CD and found the 30-day postoperative anastomotic complications and reoperation rate to be significantly lower for patients undergoing high-risk resections with split-stoma and delayed anastomosis compared to those with primary anastomosis. Furthermore, the authors reported the risk of anastomotic complications to increase with the number of identified preoperative risk factors [94].
Even though this meta-analysis found no association between preoperative anti-TNF-α treatment and AL (Supplementary Figure S6; Supplementary Table S11), significantly increased intra-abdominal abscesses were found in the anti-TNF-α treated CD group with low protective ileostomy formation (<50% of patients). The benefit of a temporary ileostomy for patients with CD has not been conclusively clarified and is overall rarely used in everyday surgical practice.
In fact, previously conducted meta-analyses [6,7] had reported significantly higher intra-abdominal abscess rates, but significantly increased readmission rates have not been related to preoperative anti-TNF-α therapy previously [15].
Likewise, none of the previously conducted meta-analyses [11,15] reported significant differences in postoperative mortality between the examined groups, which corresponds to the primary finding in this study. However, subgroup analysis found significantly lower mortality rates for the anti-TNF-α-treated patient group when <50% of them were treated concomitantly with corticosteroids and/or immunomodulatory drugs (Supplementary Figure S4; Supplementary Table S8).
This result suggests that the concomitant perioperative immunosuppression in patients preoperatively treated with anti-TNF-α biologics could be detrimental and should therefore be kept to a minimum, in order to increase the chance of overall postoperative survival. On the other hand, a certain protective effect of perioperative immunosuppression can be presumed for IBD patients not treated with anti-TNF-α biologics preoperatively.
No other general (Supplementary Figures S8 and S9), surgical (Supplementary Figures S10–S15; Supplementary Tables S12–S15) or non-surgical complications (Supplementary Figures S16–S21; Supplementary Tables S16–S19) were found to be associated with preoperative anti-TNF-α therapy.
The results of these studies’ analyses have certain limitations that need to be addressed. The majority of included studies were retrospective observational studies [21,22,23,46,47,48,50,51,53,54,55,56,57,58,60,61,62,63,64,65,66,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93] rather than randomized controlled trials, lowering the overall general quality of evidence. Furthermore, several secondary outcomes were quantified, analyzed and reported but not discussed in detail (Supplementary Figures S8–S21; Supplementary Tables S12–S19). The decision was taken to do so, as the main aim of this study was to discuss relevant postoperative complications especially important for surgical practice.
Furthermore, the influence of preoperative timing of anti-TNF-α biologic drug administration on postoperative complications could not be statistically evaluated as the included studies only depict a preoperative time frame, rather than a specific time point at which these biologics were administered [21,22,23,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93].
Finally, potential confounding factors (such as disease severity, comorbidities, concomitant immunosuppression, to name a few) may have influenced or even biased the outcomes of the analyses. This important limitation was addressed by conducting extensive subgroup analysis according to the most commonly reported risk factors. Furthermore, in case of significant or substantial heterogeneity sensitivity analysis was performed to evaluate the effect of excluding one study at a time, on pooled OR.
However, the strength of this study is that it presents the first systematic review and meta-analysis investigating solely patients that have been treated with anti-TNF-α biologics within the washout period of 12 weeks [18] (or detectable serum concentrations [21]) prior to surgery and were followed up exactly 30 days postoperatively. Furthermore, to our knowledge, such extensive subgroup analyses stratified for both the IBD subtype and potential risk factors, were not performed before. [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17].
In addition, we eliminated many sources of bias, still found in other meta-analysis, e.g.,
- (1)
- The overall postoperative complication rate was calculated individually, summation of all individual complications was not performed.
- (2)
- The correct number of patients was included for all studies evaluated in this analysis.
- (3)
- The study offers an insight on how authors assessed for risk of bias in accordance with the NOS scale—authors’ judgment and rationale, underlined by citations, were presented (Supplementary Tables S2.1.–S2.51.).
The outcomes of this systematic review and meta-analysis present some clinical implications, not at least due to the above-mentioned strengths and transparent working processes. The outcomes of this study suggest that the treatment with anti-TNF-α biologic drugs within the washout period of 12 weeks [18] (or detectable serum concentrations [21]) prior to abdominal surgery are associated with important short-term postoperative general and surgical complications for patients with IBD, in particular CD. Still, future prospective studies need to be conducted to assess the magnitude of the influence of preoperative timing of anti-TNF-α treatment and potential confounding factors on postoperative outcomes.
5. Conclusions
In conclusion, current evidence suggests that the treatment with anti-TNF-α agents within the washout period of 12 weeks (3 months [18] or detectable serum concentrations [21]) prior to abdominal surgery is associated with important short-term general and surgical postoperative complications for patients with IBD, in particular with CD. With regard to postoperative mortality, on the one hand, the concomitant treatment with corticosteroids and/or immunomodulatory drugs could be detrimental for patients preoperatively treated with anti-TNF-α biologics, which is why those patients might benefit from a reduction of perioperative immunosuppression, if possible. On the other hand, perioperative immunosuppressive therapy could have a protective effect for IBD patients not treated with anti-TNF-α preoperatively.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11236884/s1, Figure S1: Random-effects model meta-analysis for 30-day overall infectious postoperative complications in the anti-TNF-α (intervention) and control group. Forest plot of all studies included Figure S2: Fixed-effects model meta-analysis for 30-day overall postoperative Clavien-Dindo major complications in the anti-TNF-α (intervention) and control group. Forest plot of all studies included Figure S3: Fixed-effects model meta-analysis for 30-day postoperative reoperation rate in the anti-TNF-α (intervention) and control group. Forest plot of all studies included Figure S4: Fixed-effects model meta-analysis for 30-day postoperative mortality in the anti-TNF-α (intervention) and control group. Forest plot of all studies included Figure S5: Random-effects model meta-analysis for 30-day overall infectious surgical-site postoperative complications in the anti-TNF-α (intervention) and control group. Forest plot of all studies included Figure S6: Fixed-effects model meta-analysis for 30-day postoperative anastomotic leakage in the anti-TNF-α (intervention) and control group. Forest plot of all studies included Figure S7: Fixed-effects model meta-analysis for 30-day postoperative intra-abdominal abscesses in the anti-TNF-α (intervention) and control group. Forest plot of all studies included Figure S8: Fixed-effects model meta-analysis for 30-day overall non-infectious postoperative complications in the anti-TNF-α (intervention) and control group. Forest plot of all studies included Figure S9: Fixed-effects model meta-analysis for 30-day overall postoperative Clavien-Dindo minor complications in the anti-TNF-α (intervention) and control group. Forest plot of all studies included Figure S10: Random-effects model meta-analysis for 30-day postoperative superficial surgical-site infections in the anti-TNF-α (intervention) and control group. Forest plot of all studies included Figure S11: Fixed-effects model meta-analysis for 30-day postoperative deep or organ space surgical-site infections in the anti-TNF-α (intervention) and control group. Forest plot of all studies included Figure S12: Fixed-effects model meta-analysis for 30-day postoperative fistula formations in the anti-TNF-α (intervention) and control group. Forest plot of all studies included Figure S13: Fixed-effects model meta-analysis for 30-day postoperative ileus in the anti-TNF-α (intervention) and control group. Forest plot of all studies included Figure S14: Fixed-effects model meta-analysis for 30-day postoperative small bowel obstructions in the anti-TNF-α (intervention) and control group. Forest plot of all studies included Figure S15: Fixed-effects model meta-analysis for 30-day postoperative hemorrhage in the anti-TNF-α (intervention) and control group. Forest plot of all studies included Figure S16: Fixed-effects model meta-analysis for 30-day overall infectious non-surgical-site postoperative complications in the anti-TNF-α (intervention) and control group. Forest plot of all studies included Figure S17: Fixed-effects model meta-analysis for 30-day postoperative thrombosis in the anti-TNF-α (intervention) and control group. Forest plot of all studies included Figure S18: Fixed-effects model meta-analysis for 30-day postoperative cardiovascular complications in the anti-TNF-α (intervention) and control group. Forest plot of all studies included Figure S19: Fixed-effects model meta-analysis for 30-day postoperative pneumonia in the anti-TNF-α (intervention) and control group. Forest plot of all studies included Figure S20: Fixed-effects model meta-analysis for 30-day postoperative urinary tract infections in the anti-TNF-α (intervention) and control group. Forest plot of all studies included Figure S21: Fixed-effects model meta-analysis for 30-day postoperative sepsis in the anti-TNF-α (intervention) and control group. Forest plot of all studies included Tables S1.1.–S1.7.: Search strategy; Tables S2.1–S2.51: Newcastle Ottawa Quality (NOS) [43] Assessment to assess the quality of included cohort studies; Tables S3.1.–S3.2.: Other 30-day postoperative complications; Table S4: Random-effects model meta-analysis for 30-day overall postoperative complications in the anti-TNF-α (intervention) and control group. Analysis for publication bias; sensitivity analysis and subgroup analysis; Table S5: Random-effects model meta-analysis for 30-day overall infectious postoperative complications in the anti-TNF-α (intervention) and control group. Analysis for publication bias; sensitivity analysis and subgroup analysis; Table S6: Fixed-effects model meta-analysis for 30-day overall postoperative Clavien-Dindo major complications in the anti-TNF-α (intervention) and control group. Subgroup analysis; Table S7: Fixed-effects model meta-analysis for 30-day postoperative reoperation rate in the anti-TNF-α (intervention) and control group. Analysis for publication bias and subgroup analysis; Table S8: Fixed-effects model meta-analysis for 30-day postoperative mortality in the anti-TNF-α (intervention) and control group. Analysis for publication bias and subgroup analysis; Table S9: Random-effects model meta-analysis for 30-day overall postoperative infectious surgical-site complications in the anti-TNF-α (intervention) and control group. Analysis for publication bias; sensitivity analysis and subgroup analysis; Table S10: Fixed-effects model meta-analysis for 30-day postoperative intra-abdominal septic complications in the anti-TNF-α (intervention) and control group. Subgroup analysis; Table S11: Fixed-effects model meta-analysis for 30-day postoperative anastomotic leakage in the anti-TNF-α (intervention) and control group. Analysis for publication bias and subgroup analysis; Table S12: Fixed-effects model meta-analysis for 30-day postoperative superficial surgical-site infections in the anti-TNF-α (intervention) and control group. Analysis for publication bias; sensitivity analysis and subgroup analysis; Table S13: Fixed-effects model meta-analysis for 30-day postoperative ileus in the anti-TNF-α (intervention) and control group. Analysis for publication bias and subgroup analysis; Table S14: Fixed-effects model meta-analysis for 30-day postoperative small bowel obstruction in the anti-TNF-α (intervention) and control group. Analysis for publication bias and subgroup analysis; Table S15: Fixed-effects model meta-analysis for 30-day postoperative hemorrhage in the anti-TNF-α (intervention) and control group. Subgroup analysis; Table S16: Fixed-effects model meta-analysis for 30-day postoperative thrombosis in the anti-TNF-α (intervention) and control group. Subgroup analysis; Table S17: Fixed-effects model meta-analysis for 30-day postoperative pneumonia in the anti-TNF-α (intervention) and control group. Subgroup analysis; Table S18: Fixed-effects model meta-analysis for 30-day postoperative urinary tract infections in the anti-TNF-α (intervention) and control group. Subgroup analysis; Table S19: Fixed-effects model meta-analysis for 30-day postoperative sepsis in the anti-TNF-α (intervention) and control group. Subgroup analysis.
Author Contributions
Conceptualization, K.C.; methodology, K.C.; software, K.C.; validation, K.C., S.R. and P.-A.N.; formal analysis, K.C. and S.R.; investigation K.C. and S.R.; resources, K.C.; data curation, K.C.; writing—original draft preparation, K.C.; writing—review and editing, K.C., S.R., P.-A.N., M.-C.W., D.W. and H.F.; visualization, K.C.; supervision, S.R. and P.-A.N.; project administration, K.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data used to support the findings of this systematic review and meta-analysis are included within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- Wong, D.J.; Roth, E.M.; Feuerstein, J.D.; Poylin, V.Y. Surgery in the age of biologics. Gastroenterol. Rep. 2019, 7, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Ali, U.; Martin, S.T.; Rao, A.D.; Kiran, R.P. Impact of preoperative immunosuppressive agents on postoperative outcomes in Crohn’s disease. Dis. Colon Rectum 2014, 57, 663–674. [Google Scholar] [CrossRef] [PubMed]
- El-Hussuna, A.; Krag, A.; Olaison, G.; Bendtsen, F.; Gluud, L.L. The effect of anti-tumor necrosis factor alpha agents on postoperative anastomotic complications in Crohn’s disease: A systematic review. Dis. Colon Rectum 2013, 56, 1423–1433. [Google Scholar] [CrossRef]
- Hanzel, J.; Almradi, A.; Istl, A.C.; Yang, M.L.; Fleshner, K.A.; Parker, C.E.; Guizzetti, L.; Ma, C.; Singh, S.; Jairath, V. Increased Risk of Infections with Anti-TNF Agents in Patients with Crohn’s Disease after Elective Surgery: Meta-Analysis. Dig. Dis. Sci. 2021, 67, 646–660. [Google Scholar] [CrossRef]
- Law, C.C.Y.; Koh, D.; Bao, Y.; Jairath, V.; Narula, N. Risk of Postoperative Infectious Complications from Medical Therapies in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2020, 26, 1796–1807. [Google Scholar] [CrossRef]
- Lin, Y.S.; Cheng, S.W.; Wang, Y.H.; Chen, K.H.; Fang, C.J.; Chen, C. Systematic review with meta-analysis: Risk of post-operative complications associated with pre-operative exposure to anti-tumour necrosis factor agents for Crohn’s disease. Aliment. Pharmacol. Ther. 2019, 49, 966–977. [Google Scholar] [CrossRef]
- Moosvi, Z.; Duong, J.; Bechtold, M.L.; Nguyen, D.L. Systematic review and meta-analysis: Risks of postoperative complications with preoperative use of anti-tumor necrosis factor-alpha biologics in inflammatory bowel disease patients. Eur. J. Gastroenterol. Hepatol. 2021, 33, 799–816. [Google Scholar] [CrossRef]
- Narula, N.; Charleton, D.; Marshall, J.K. Meta-analysis: Peri-operative anti-TNFα treatment and post-operative complications in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2013, 37, 1057–1064. [Google Scholar] [CrossRef]
- Qiu, Y.; Zheng, Z.; Liu, G.; Zhao, X.; He, A. Effects of preoperative anti-tumour necrosis factor alpha infusion timing on postoperative surgical site infection in inflammatory bowel disease: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2019, 7, 1198–1214. [Google Scholar] [CrossRef]
- Rosenfeld, G.; Qian, H.; Bressler, B. The risks of post-operative complications following pre-operative infliximab therapy for Crohn’s disease in patients undergoing abdominal surgery: A systematic review and meta-analysis. J. Crohns Colitis 2013, 7, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Selvaggi, F.; Pellino, G.; Canonico, S.; Sciaudone, G. Effect of preoperative biologic drugs on complications and function after restorative proctocolectomy with primary ileal pouch formation: Systematic review and meta-analysis. Inflamm. Bowel Dis. 2015, 21, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Uchino, M.; Ikeuchi, H.; Shimizu, J.; Ohge, H.; Haji, S.; Mizuguchi, T.; Mohri, Y.; Yamashita, C.; Kitagawa, Y.; Suzuki, K.; et al. Association between preoperative tumor necrosis factor alpha inhibitor and surgical site infection after surgery for inflammatory bowel disease: A systematic review and meta-analysis. Surg. Today 2021, 51, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xu, Y.; Zheng, T.; He, A.; Liu, G. Meta analysis on association between preoperative infliximab administration and morbidity of postoperative infectious complication in patients with ulcerative colitis. Chin. J. Dig. Dis. 2019, 3, 238–245. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, L.; An, P.; Zhou, B.; Liu, G. Meta-Analysis: The Influence of Preoperative Infliximab Use on Postoperative Complications of Crohn’s Disease. Inflamm. Bowel Dis. 2019, 25, 261–269. [Google Scholar] [CrossRef]
- Yang, Z.P.; Hong, L.; Wu, Q.; Wu, K.C.; Fan, D.M. Preoperative infliximab use and postoperative complications in Crohn’s disease: A systematic review and meta-analysis. Int. J. Surg. 2014, 12, 224–230. [Google Scholar] [CrossRef]
- Zanelli, J.; Chandrapalan, S.; Patel, A.; Arasaradnam, R.P. The impact of pre-operative biologic therapy on post-operative surgical outcomes in ulcerative colitis: A systematic review and meta-analysis. Ther. Adv. Gastroenterol. 2020, 13, 1756284820937089. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Hanauer, S.B. Antitumor necrosis factor therapy for inflammatory bowel disease: A review of agents, pharmacology, clinical results, and safety. Inflamm. Bowel Dis. 1999, 5, 119–133. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021); Cochrane: London, UK, 2021; Available online: www.training.cochrane.org/handbook (accessed on 20 March 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Lau, C.; Dubinsky, M.; Melmed, G.; Vasiliauskas, E.; Berel, D.; McGovern, D.; Ippoliti, A.; Shih, D.; Targan, S.; Fleshner, P. The impact of preoperative serum anti-TNFα therapy levels on early postoperative outcomes in inflammatory bowel disease surgery. Ann. Surg. 2015, 261, 487–496. [Google Scholar] [CrossRef]
- Melo-Pinto, D.; Santos, J.V.; Barbosa, E. Risk factors for postoperative complications in Crohn disease: Analysis of 173 patients. J. Coloproctol. 2018, 38, 214–222. [Google Scholar] [CrossRef]
- Tang, S.; Dong, X.; Liu, W.; Qi, W.; Ye, L.; Yang, X.; Cao, Q.; Ge, X.; Zhou, W. Compare risk factors associated with postoperative infectious complication in Crohn’s disease with and without preoperative infliximab therapy: A cohort study. Int. J. Color. Dis. 2020, 35, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, F.; Ewelukwa, O.; Brar, T.; Forde, J.; Mramba, L.; Glover, S.; Iqbal, A.; Tan, S. Evaluating the impact of vedolizumab on postoperative complications in inflammatory bowel disease patients. Dis. Colon Rectum 2018, 61, E141. [Google Scholar]
- de Buck van Overstraeten, A.; Eshuis, E.J.; Vermeire, S.; Van Assche, G.; Ferrante, M.; D’Haens, G.R.; Ponsioen, C.Y.; Belmans, A.; Buskens, C.J.; Wolthuis, A.M.; et al. Short- and medium-term outcomes following primary ileocaecal resection for Crohn’s disease in two specialist centres. Br. J. Surg. 2017, 104, 1713–1722. [Google Scholar] [CrossRef]
- Guasch, M.; Clos, A.; Manyosa, M.; Lobatón Ortega, T.; Gomez, J.; Pinol, M.; Cabre, E.; Troya, J.; Domenech, E. Prevalence and risk factors for postoperative septic complications in Crohn’s disease. J. Crohns Colitis 2016, 10, S350. [Google Scholar]
- Gudsoorkar, V.; Ibarra, S.; Gonzalez-Almada, A.; Oglat, A.; Koduru, P.; Abraham, B.; Haas, E. P133 an analysis of surgical outcomes in ibd patients treated with and without biologic therapy. Gastroenterology 2018, 154, S68–S69. [Google Scholar] [CrossRef]
- Guo, K.; Ren, J.; Li, G.; Hu, Q.; Wu, X.; Wang, Z.; Wang, G.; Gu, G.; Ren, H.; Hong, Z.; et al. Risk factors of surgical site infections in patients with Crohn’s disease complicated with gastrointestinal fistula. Int. J. Color. Dis. 2017, 32, 635–643. [Google Scholar] [CrossRef]
- Kim, J.; Zaghiyan, K.; Fleshner, P. P640 Risk of post-operative complications among Crohn’s disease patients treated pre-operatively with vedolizumab. A matched case-control study. J. Crohns Colitis 2018, 12, S433–S434. [Google Scholar] [CrossRef][Green Version]
- Oh, S.H.; Hong, S.N.; Kim, M.J.; Kim, E.R.; Chang, D.K.; Kim, Y.-H. P316 The risk of preoperative anti-TNF-α treatment on early postoperative complications in patients with Crohn’s disease. J. Crohns Colitis 2014, 8, S196. [Google Scholar] [CrossRef]
- Rizvi, A.; Kayal, M.; Plietz, M.; Zylberberg, H.; Radcliffe, M.; Yzet, C.; Khaitov, S.; Greenstein, A.; Sylla, P.; Dubinsky, M. 705—Pre-Operative Biologics Significantly Reduce Post-Operative Leaks After Staged Restorative Proctocolectomy. Gastroenterology 2019, 156, S-154. [Google Scholar] [CrossRef]
- Schils, N.; De Buck van Overstraeten, A.; Vermeire, S.; Van Assche, G.; Wolthuis, A.; D’Hoore, A.; Ferrante, M. P445 Perioperative use of vedolizumab seems not associated with short-term postoperative infectious complications in patients with Crohn’s disease undergoing right hemicolectomy with ileocolonic anastomosis. J. Crohns Colitis 2017, 11, S304. [Google Scholar] [CrossRef][Green Version]
- Aaron Brzezinski, L.A.; Del Real, G.A.; Parsi, M.; Lashner, B.; Achkar, J.-P. Infliximab Does Not Increase the Risk of Complications in the Perioperative Period in Patients with Crohn’s Disease. Gastroenterology 2002, 122, A616. [Google Scholar]
- Lau, C.C.; Dubinsky, M.; Melmed, G.Y.; Vasiliauskas, E.A.; McGovern, D.P.; Berel, D.; Ippoliti, A.; Murrell, Z.A.; Shih, D.Q.; Kaur, M.; et al. Higher Preoperative Serum Biologic Levels Are Associated with Postoperative Complications in Crohn’s Disease Patients. Gastroenterology 2013, 144, 190. [Google Scholar] [CrossRef]
- Lau, C.C.; Dubinsky, M.; Melmed, G.Y.; Vasiliauskas, E.A.; McGovern, D.P.; Berel, D.; Murrell, Z.A.; Ippoliti, A.; Shih, D.Q.; Kaur, M.; et al. Preoperative Serum Biologic Levels Do Not Impact Postoperative Outcomes in Ulcerative Colitis. Gastroenterology 2013, 144, 189–190. [Google Scholar] [CrossRef]
- de Silva, S.; Ma, C.; Proulx, M.C.; Crespin, M.; Kaplan, B.S.; Hubbard, J.; Prusinkiewicz, M.; Fong, A.; Panaccione, R.; Ghosh, S.; et al. Postoperative Complications and Mortality Following Colectomy for Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2011, 9, 972–980. [Google Scholar] [CrossRef]
- Desai, P.N.; Sharma, A.; Naik, A.S.; Otterson, M.F.; Zadvornova, Y.; Perera, L.P.; Venu, N.; Stein, D.J. Su1560 Timing of Pre-Operative Anti-Tumor Necrosis Factor Therapy Does Not Affect Early Post-Operative Complication Rates in Inflammatory Bowel Disease Patients Undergoing Intestinal Resection. Gastroenterology 2012, 142, S-1063. [Google Scholar] [CrossRef]
- García, M.J.; Rivero, M.; Miranda-Bautista, J.; Bastón-Rey, I.; Mesonero, F.; Leo-Carnerero, E.; Casas-Deza, D.; Cagigas Fernández, C.; Martin-Cardona, A.; El Hajra, I.; et al. Impact of Biological Agents on Postsurgical Complications in Inflammatory Bowel Disease: A Multicentre Study of Geteccu. J. Clin. Med. 2021, 10, 4402. [Google Scholar] [CrossRef]
- Karjalainen, E.K.; Renkonen-Sinisalo, L.; Mustonen, H.K.; Färkkilä, M.; Lepistö, A.H. Restorative Proctocolectomy in Ulcerative Colitis: Effect of Preoperative Immunomodulatory Therapy on Postoperative Complications and Pouch Failure. Scand. J. Surg. 2021, 110, 51–58. [Google Scholar] [CrossRef]
- Marchal, L.; D’Haens, G.; Van Assche, G.; Vermeire, S.; Noman, M.; Ferrante, M.; Hiele, M.; Bueno De Mesquita, M.; D’Hoore, A.; Penninckx, F.; et al. The risk of post-operative complications associated with infliximab therapy for Cron’s disease: A controlled cohort study. Aliment. Pharmacol. Ther. 2004, 19, 749–754. [Google Scholar] [CrossRef]
- Uchino, M.; Ikeuchi, H.; Matsuoka, H.; Tsuchida, T.; Tomita, N.; Takesue, Y. Risk Factors Associated with Surgical Site Infection After Ileal Pouch-Anal Anastomosis in Ulcerative Colitis. Dis. Colon. Rectum. 2010, 53, 143–149. [Google Scholar] [CrossRef]
- Weber, A.T.; Sack, J.; Ha, C.Y. Anti-TNF Exposure Is Not Associated with Increased Post-Operative Morbidity or Reoperation Among Crohn’s Disease Patients: 744. Am. J. Gastroenterol. 2017, 112, S411, S414–S415. [Google Scholar]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non Randomised Studies in Meta-Analyses. Available online: www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 18 February 2022).
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Appau, K.A.; Fazio, V.W.; Shen, B.; Church, J.M.; Lashner, B.; Remzi, F.; Brzezinski, A.; Strong, S.A.; Hammel, J.; Kiran, R.P. Use of infliximab within 3 months of ileocolonic resection is associated with adverse postoperative outcomes in Crohn’s patients. J. Gastrointest. Surg. 2008, 12, 1738–1744. [Google Scholar] [CrossRef]
- Bafford, A.C.; Powers, S.; Ha, C.; Kruse, D.; Gorfine, S.R.; Chessin, D.B.; Bauer, J.J. Immunosuppressive Therapy Does Not Increase Operative Morbidity in Patients with Crohn’s Disease. J. Clin. Gastroenterol. 2013, 47, 491–495. [Google Scholar] [CrossRef]
- Bregnbak, D.; Mortensen, C.; Bendtsen, F. Infliximab and complications after colectomy in patients with ulcerative colitis. J. Crohns Colitis 2012, 6, 281–286. [Google Scholar] [CrossRef]
- Brouquet, A.; Maggiori, L.; Zerbib, P.; Lefevre, J.H.; Denost, Q.; Germain, A.; Cotte, E.; Beyer-Berjot, L.; Munoz-Bongrand, N.; Desfourneaux, V.; et al. Anti-TNF Therapy Is Associated with an Increased Risk of Postoperative Morbidity after Surgery for Ileocolonic Crohn Disease Results of a Prospective Nationwide Cohort. Ann. Surg. 2018, 267, 221–228. [Google Scholar] [CrossRef]
- Canedo, J.; Lee, S.H.; Pinto, R.; Murad-Regadas, S.; Rosen, L.; Wexner, S.D. Surgical resection in Crohn’s disease: Is immunosuppressive medication associated with higher postoperative infection rates? Color. Dis. 2011, 13, 1294–1298. [Google Scholar] [CrossRef]
- El-Hussuna, A.; Andersen, J.; Bisgaard, T.; Jess, P.; Henriksen, M.; Oehlenschlager, J.; Thorlacius-Ussing, O.; Olaison, G. Biologic treatment or immunomodulation is not associated with postoperative anastomotic complications in abdominal surgery for Crohn’s disease. Scand. J. Gastroenterol. 2012, 47, 662–668. [Google Scholar] [CrossRef]
- El-Hussuna, A.; Qvist, N.; Zangenberg, M.S.; Langkilde, A.; Siersma, V.; Hjort, S.; Gögenur, I. No effect of anti-TNF-α agents on the surgical stress response in patients with inflammatory bowel disease undergoing bowel resections: A prospective multi-center pilot study. BMC Surg. 2018, 18, 91. [Google Scholar] [CrossRef]
- Ferrante, M.; D’Hoore, A.; Vermeire, S.; Declerck, S.; Noman, M.; Van Assche, G.; Hoffman, I.; Rutgeerts, P.; Penninckx, F. Corticosteroids but not infliximab increase short-term postoperative infectious complications in patients with ulcerative colitis. Inflamm. Bowel Dis. 2009, 15, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Gainsbury, M.L.; Chu, D.I.; Howard, L.A.; Coukos, J.A.; Farraye, F.A.; Stucchi, A.F.; Becker, J.M. Preoperative infliximab is not associated with an increased risk of short-term postoperative complications after restorative proctocolectomy and ileal pouch-anal anastomosis. J. Gastrointest. Surg. 2011, 15, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Remzi, F.H.; Shen, B.; Vogel, J.D.; Kiran, R.P. Operative strategy modifies risk of pouch-related outcomes in patients with ulcerative colitis on preoperative anti-tumor necrosis factor-α therapy. Dis. Colon Rectum 2013, 56, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A.; Rivero, M.; Martin-Arranz, M.D.; Sanchez, V.G.; Castro, M.; Barrio, J.; de Francisco, R.; Barreiro-de Acosta, M.; Julia, B.; Cea-Calvo, L.; et al. Perioperative management and early complications after intestinal resection with ileocolonic anastomosis in Crohn’s disease: Analysis from the PRACTICROHN study. Gastroenterol. Rep. 2019, 7, 168–175. [Google Scholar] [CrossRef]
- Indar, A.A.; Young-Fadok, T.M.; Heppell, J.; Efron, J.E. Effect of perioperative immunosuppressive medication on early outcome in Crohn’s disease patients. World J. Surg. 2009, 33, 1049–1052. [Google Scholar] [CrossRef]
- Jouvin, I.; Lefevre, J.H.; Creavin, B.; Pitel, S.; Chafai, N.; Tiret, E.; Beaugerie, L.; Parc, Y. Postoperative Morbidity Risks Following Ileocolic Resection for Crohn’s Disease Treated with Anti-TNF Alpha Therapy: A Retrospective Study of 360 Patients. Inflamm. Bowel Dis. 2018, 24, 422–432. [Google Scholar] [CrossRef]
- Kim, J.Y.; Zaghiyan, K.; Lightner, A.; Fleshner, P. Risk of postoperative complications among ulcerative colitis patients treated preoperatively with vedolizumab: A matched case-control study. BMC Surg. 2020, 20, 46. [Google Scholar] [CrossRef]
- Kotze, P.G.; Magro, D.O.; Martinez, C.A.R.; Saab, B.; Saab, M.P.; Pinheiro, L.V.; Olandoski, M.; Yamamoto, T.; Coy, C.S.R. Adalimumab and postoperative complications of elective intestinal resections in Crohn’s disease: A propensity score case-matched study. Color. Dis. 2018, 20, 211–218. [Google Scholar] [CrossRef]
- Kotze, P.G.; Saab, M.P.; Saab, B.; da Silva Kotze, L.M.; Olandoski, M.; Pinheiro, L.V.; Martinez, C.A.; Ayrizono, M.L.; Magro, D.O.; Coy, C.S. Tumor Necrosis Factor Alpha Inhibitors Did Not Influence Postoperative Morbidity after Elective Surgical Resections in Crohn’s Disease. Dig. Dis. Sci. 2017, 62, 456–464. [Google Scholar] [CrossRef]
- Krane, M.K.; Allaix, M.E.; Zoccali, M.; Umanskiy, K.; Rubin, M.A.; Villa, A.; Hurst, R.D.; Fichera, A. Preoperative infliximab therapy does not increase morbidity and mortality after laparoscopic resection for inflammatory bowel disease. Dis. Colon Rectum 2013, 56, 449–457. [Google Scholar] [CrossRef]
- Kunitake, H.; Hodin, R.; Shellito, P.C.; Sands, B.E.; Korzenik, J.; Bordeianou, L. Perioperative treatment with infliximab in patients with Crohn’s disease and ulcerative colitis is not associated with an increased rate of postoperative complications. J. Gastrointest. Surg. 2008, 12, 1730–1736. [Google Scholar] [CrossRef] [PubMed]
- Lightner, A.L.; McKenna, N.P.; Alsughayer, A.; Harmsen, W.S.; Taparra, K.; Parker, M.E.; Raffals, L.E.; Loftus, E.V., Jr. Biologics and 30-Day Postoperative Complications after Abdominal Operations for Crohn’s Disease: Are There Differences in the Safety Profiles? Dis. Colon Rectum 2019, 62, 1352–1362. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Nagahara, H.; Shibutani, M.; Otani, H.; Sakurai, K.; Toyokawa, T.; Tanaka, H.; Kubo, N.; Muguruma, K.; Kamata, N.; et al. A preoperative low nutritional prognostic index correlates with the incidence of incisional surgical site infections after bowel resection in patients with Crohn’s disease. Surg. Today 2015, 45, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, C.; Nunoo, R.; Asgeirsson, T.; Rivera, R.; Kim, D.; Hoedema, R.; Dujovny, N.; Luchtefeld, M.; Davis, A.T.; Figg, R. Outcomes of ileocolic resection and right hemicolectomies for Crohn’s patients in comparison with non-Crohn’s patients and the impact of perioperative immunosuppressive therapy with biologics and steroids on inpatient complications. Am. J. Surg. 2012, 203, 375–378, discussion 378. [Google Scholar] [CrossRef] [PubMed]
- Monsinjon, M.; Mege, D.; Maggiori, L.; Treton, X.; Bouhnik, Y.; Panis, Y. Postoperative course of laparoscopic subtotal colectomy is affected by prolonged preoperative anti-TNF therapy in patients with acute colitis complicating inflammatory bowel disease. Int. J. Color. Dis. 2017, 32, 1499–1502. [Google Scholar] [CrossRef]
- Morar, P.S.; Hodgkinson, J.D.; Thalayasingam, S.; Koysombat, K.; Purcell, M.; Hart, A.L.; Tarne, J.W.; Faiz, O. Determining Predictors for Intra-abdominal Septic Complications Following Ileocolonic Resection for Crohn’s Disease-Considerations in Pre-operative and Peri-operative Optimisation Techniques to Improve Outcome. J. Crohns Colitis 2015, 9, 483–491. [Google Scholar] [CrossRef]
- Myrelid, P.; Marti-Gallostra, M.; Ashraf, S.; Sunde, M.L.; Tholin, M.; Oresland, T.; Lovegrove, R.E.; Tøttrup, A.; Kjaer, D.W.; George, B.D. Complications in surgery for Crohn’s disease after preoperative antitumour necrosis factor therapy. Br. J. Surg. 2014, 101, 539–545. [Google Scholar] [CrossRef]
- Nasir, B.S.; Dozois, E.J.; Cima, R.R.; Pemberton, J.H.; Wolff, B.G.; Sandborn, W.J.; Loftus, E.V.; Larson, D.W. Perioperative Anti-Tumor Necrosis Factor Therapy Does Not Increase the Rate of Early Postoperative Complications in Crohn’s Disease. J. Gastrointest. Surg. 2010, 14, 1859–1866. [Google Scholar] [CrossRef]
- Nelson, R.; Liao, C.; Fichera, A.; Rubin, D.T.; Pekow, J. Rescue therapy with cyclosporine or infliximab is not associated with an increased risk for postoperative complications in patients hospitalized for severe steroid-refractory ulcerative colitis. Inflamm. Bowel Dis. 2014, 20, 14–20. [Google Scholar] [CrossRef]
- Nørgård, B.M.; Nielsen, J.; Qvist, N.; Gradel, K.O.; de Muckadell, O.B.; Kjeldsen, J. Pre-operative use of anti-TNF-α agents and the risk of post-operative complications in patients with ulcerative colitis—A nationwide cohort study. Aliment. Pharmacol. Ther. 2012, 35, 1301–1309. [Google Scholar] [CrossRef]
- Nørgård, B.M.; Nielsen, J.; Qvist, N.; Gradel, K.O.; de Muckadell, O.B.; Kjeldsen, J. Pre-operative use of anti-TNF-α agents and the risk of post-operative complications in patients with Crohn’s disease—A nationwide cohort study. Aliment. Pharmacol. Ther. 2013, 37, 214–224. [Google Scholar] [CrossRef]
- Regadas, F.S.; Pinto, R.A.; Murad-Regadas, S.M.; Canedo, J.A.; Leal, M.; Nogueras, J.J.; Wexner, S.D. Short-term outcome of infliximab and other medications on patients with inflammatory bowel disease undergoing ileostomy reversal. Color. Dis. 2011, 13, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Armuzzi, A.; Pugliese, D.; Verbo, A.; Papa, A.; Mattana, C.; Rapaccini, G.L.; Guidi, L.; Coco, C. Anti-TNF-alpha therapies do not increase early postoperative complications in patients with inflammatory bowel disease. An Italian single-center experience. Int. J. Color. Dis. 2011, 26, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Selvasekar, C.R.; Cima, R.R.; Larson, D.W.; Dozois, E.J.; Harrington, J.R.; Harmsen, W.S.; Loftus, E.V., Jr.; Sandborn, W.J.; Wolff, B.G.; Pemberton, J.H. Effect of infliximab on short-term complications in patients undergoing operation for chronic ulcerative colitis. J. Am. Coll. Surg. 2007, 204, 956–962, discussion 962–963. [Google Scholar] [CrossRef] [PubMed]
- Serradori, T.; Germain, A.; Scherrer, M.L.; Ayav, C.; Perez, M.; Romain, B.; Palot, J.P.; Rohr, S.; Peyrin-Biroulet, L.; Bresler, L. The effect of immune therapy on surgical site infection following Crohn’s Disease resection. Br. J. Surg. 2013, 100, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Shwaartz, C.; Fields, A.C.; Sobrero, M.; Cohen, B.D.; Divino, C.M. Effect of Anti-TNF Agents on Postoperative Outcomes in Inflammatory Bowel Disease Patients: A Single Institution Experience. J. Gastrointest. Surg. 2016, 20, 1636–1642. [Google Scholar] [CrossRef]
- Syed, A.; Cross, R.K.; Flasar, M.H. Anti-tumor necrosis factor therapy is associated with infections after abdominal surgery in Crohn’s disease patients. Am. J. Gastroenterol. 2013, 108, 583–593. [Google Scholar] [CrossRef]
- Tay, G.S.; Binion, D.G.; Eastwood, D.; Otterson, M.F. Multivariate analysis suggests improved perioperative outcome in Crohn’s disease patients receiving immunomodulator therapy after segmental resection and/or strictureplasty. Surgery 2003, 134, 565–572. [Google Scholar] [CrossRef]
- Tiberi, A.; Pesi, B.; Giudici, F.; Zambonin, D.; Nelli, T.; Cupellini, C.; Ficari, F.; Cianchi, F.; Scaringi, S. Laparoscopic ileo-colic resection and right hemicolectomy for Crohn’s disease and colon cancer: A preliminary comparative study on post-operative outcome. Updates Surg. 2020, 72, 821–826. [Google Scholar] [CrossRef]
- Uchino, M.; Ikeuchi, H.; Bando, T.; Chohno, T.; Sasaki, H.; Horio, Y.; Kuwahara, R.; Minagawa, T.; Goto, Y.; Ichiki, K.; et al. Associations between multiple immunosuppressive treatments before surgery and surgical morbidity in patients with ulcerative colitis during the era of biologics. Int. J. Color. Dis. 2019, 34, 699–710. [Google Scholar] [CrossRef]
- Uchino, M.; Ikeuchi, H.; Bando, T.; Hirose, K.; Hirata, A.; Chohno, T.; Sasaki, H.; Takahashi, Y.; Takesue, Y.; Hida, N.; et al. Does Pre-Operative Multiple Irnmunosuppressive Therapy Associate with Surgical Site Infection in Surgery for Ulcerative Colitis? Digestion 2015, 92, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Uchino, M.; Ikeuchi, H.; Matsuoka, H.; Bando, T.; Ichiki, K.; Nakajima, K.; Tomita, N.; Takesue, Y. Risk factors for surgical site infection and association with infliximab administration during surgery for Crohn’s disease. Dis. Colon Rectum 2013, 56, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Uchino, M.; Ikeuchi, H.; Matsuoka, H.; Bando, T.; Ichiki, K.; Nakajima, K.; Tomita, N.; Takesue, Y. Infliximab administration prior to surgery does not increase surgical site infections in patients with ulcerative colitis. Int. J. Color. Dis. 2013, 28, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.T.; Mytton, J.; Henderson, L.; Amin, V.; Tanner, J.R.; Evison, F.; Radley, S. Anti-TNF therapy is not associated with an increased risk of post-colectomy complications, a population-based study. Color. Dis. 2018, 20, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Waterman, M.; Xu, W.; Dinani, A.; Steinhart, A.H.; Croitoru, K.; Nguyen, G.C.; McLeod, R.S.; Greenberg, G.R.; Cohen, Z.; Silverberg, M.S. Preoperative biological therapy and short-term outcomes of abdominal surgery in patients with inflammatory bowel disease. Gut 2013, 62, 387–394. [Google Scholar] [CrossRef]
- White, E.C.; Melmed, G.Y.; Vasiliauskas, E.; Dubinsky, M.; Ippoliti, A.; McGovern, D.; Targan, S.; Fleshner, P. Does Preoperative Immunosuppression Influence Unplanned Hospital Readmission after Surgery in Patients with Crohn’s Disease? Dis. Colon Rectum 2012, 55, 563–568. [Google Scholar] [CrossRef]
- Yamada, A.; Komaki, Y.; Patel, N.; Komaki, F.; Aelvoet, A.S.; Tran, A.L.; Pekow, J.; Dalal, S.; Cohen, R.D.; Cannon, L.; et al. Risk of Postoperative Complications among Inflammatory Bowel Disease Patients Treated Preoperatively with Vedolizumab. Am. J. Gastroenterol. 2017, 112, 1423–1429. [Google Scholar] [CrossRef]
- Yamamoto, T.; Spinelli, A.; Suzuki, Y.; Saad-Hossne, R.; Teixeira, F.V.; de Albuquerque, I.C.; da Silva, R.N.; de Barcelos, I.F.; Takeuchi, K.; Yamada, A.; et al. Risk factors for complications after ileocolonic resection for Crohn’s disease with a major focus on the impact of preoperative immunosuppressive and biologic therapy: A retrospective international multicentre study. United Eur. Gastroenterol. J. 2016, 4, 784–793. [Google Scholar] [CrossRef]
- Yu, C.S.; Jung, S.W.; Lee, J.L.; Lim, S.B.; Park, I.J.; Yoon, Y.S.; Kim, C.W.; Yang, S.K.; Ye, B.D.; Park, S.H.; et al. The Influence of Preoperative Medications on Postoperative Complications in Patients after Intestinal Surgery for Crohn’s Disease. Inflamm. Bowel Dis. 2019, 25, 1559–1568. [Google Scholar] [CrossRef]
- Zhu, F.; Li, Y.; Guo, Z.; Cao, L.; Feng, D.; Zhang, T.; Zhu, W.; Gong, J. Nomogram to Predict Postoperative Intra-abdominal Septic Complications after Bowel Resection and Primary Anastomosis for Crohn’s Disease. Dis. Colon Rectum 2020, 63, 629–638. [Google Scholar] [CrossRef]
- Zittan, E.; Milgrom, R.; Ma, G.W.; Wong-Chong, N.; O’Connor, B.; McLeod, R.S.; MacRae, H.M.; Greenberg, G.R.; Nguyen, G.C.; Croitoru, K.; et al. Preoperative Anti-tumor Necrosis Factor Therapy in Patients with Ulcerative Colitis Is Not Associated with an Increased Risk of Infectious and Noninfectious Complications after Ileal Pouch-anal Anastomosis. Inflamm. Bowel Dis. 2016, 22, 2442–2447. [Google Scholar] [CrossRef] [PubMed]
- Myrelid, P.; Söderholm, J.D.; Olaison, G.; Sjödahl, R.; Andersson, P. Split stoma in resectional surgery of high-risk patients with ileocolonic Crohn’s disease. Color. Dis. 2012, 14, 188–193. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).