Efficacy of Serotonin Type 3 Receptor Antagonist Ramosetron on Diarrhea-Predominant Irritable Bowel Syndrome (IBS-D)-Like Symptoms in Patients with Quiescent Inflammatory Bowel Disease: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
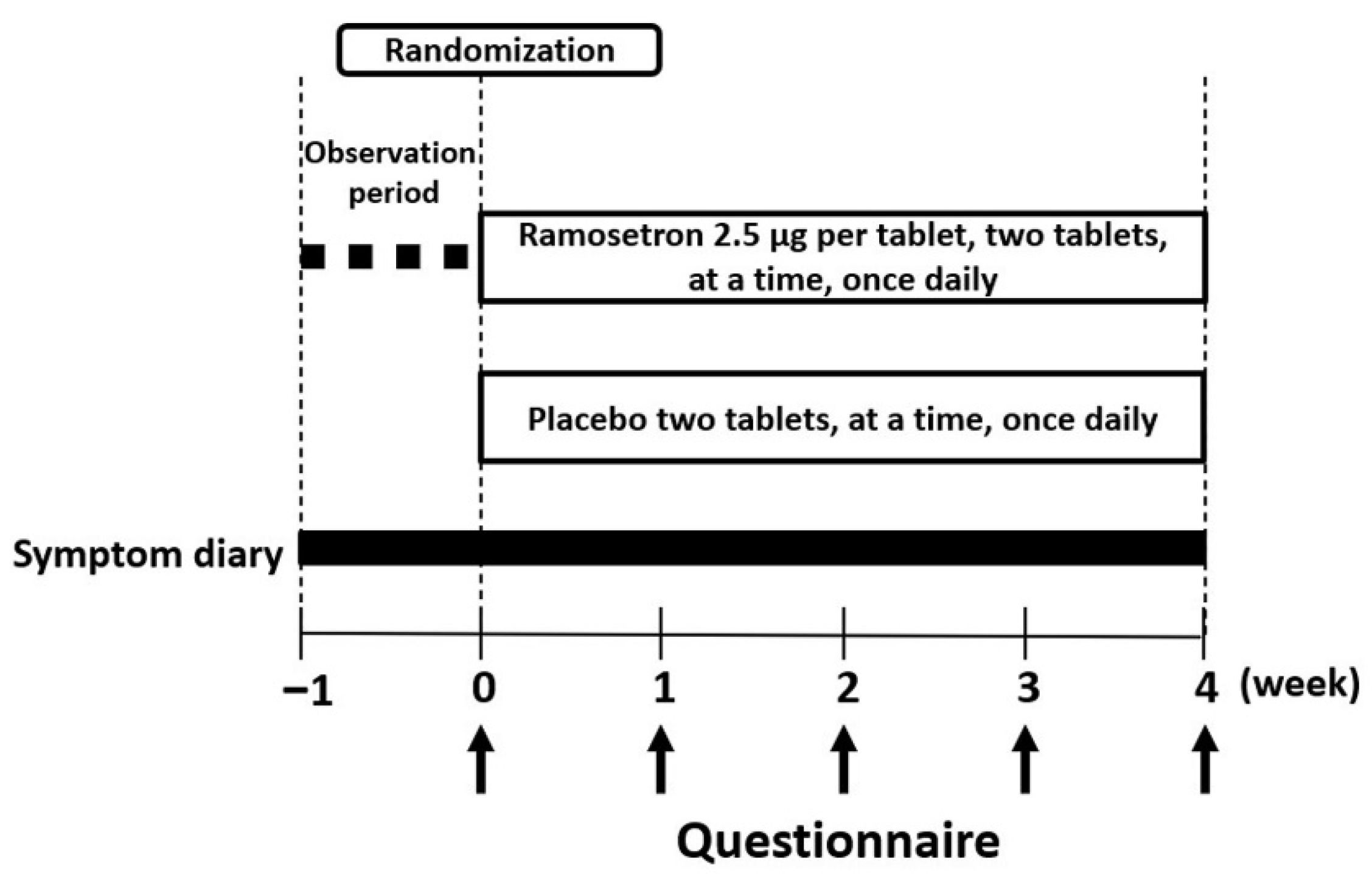
2.2. Study Design
2.3. Data Collection, Efficacy and End Points
2.4. Statistical Analysis
3. Results
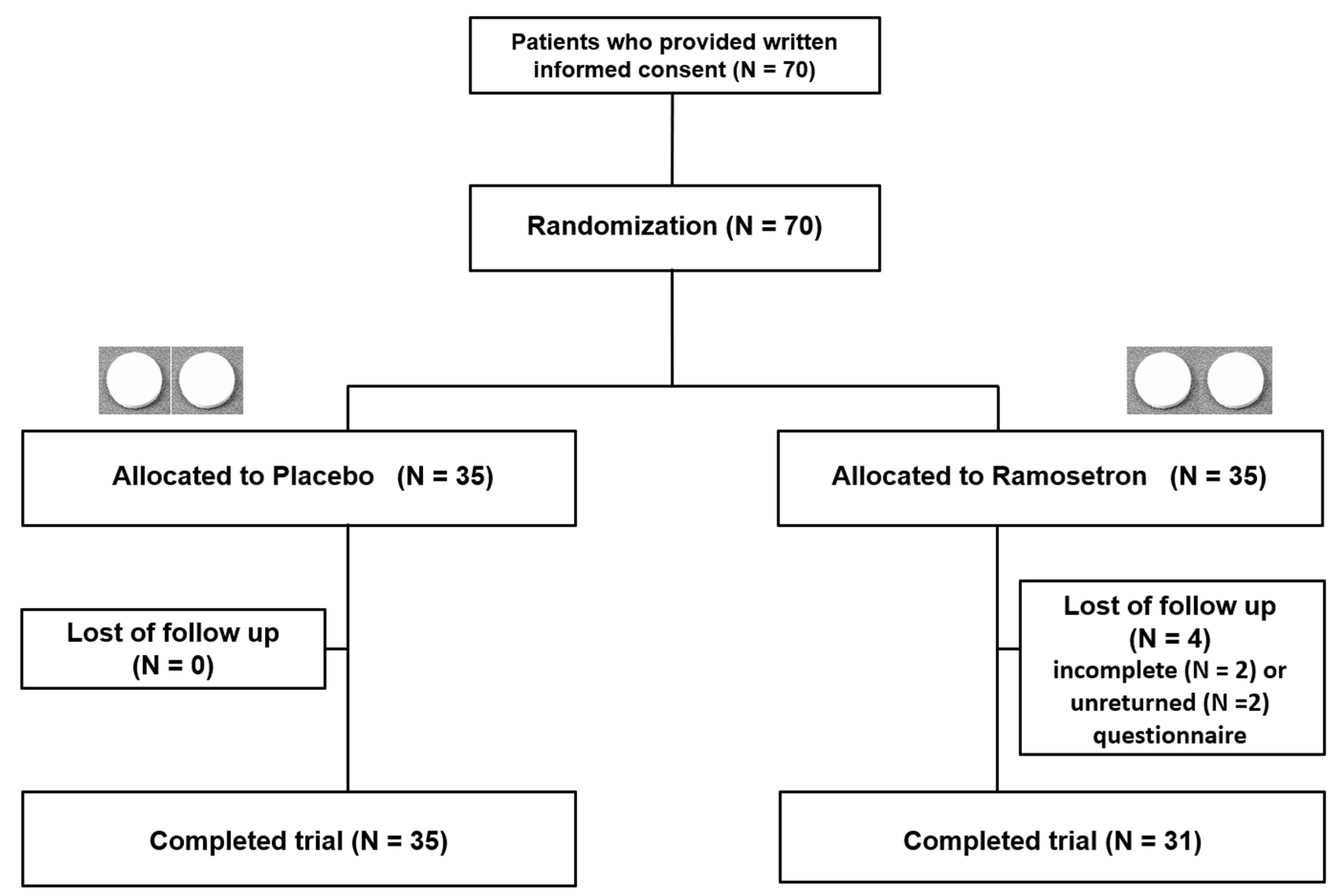
3.1. Enrolment and Baseline Characteristics of the Patients
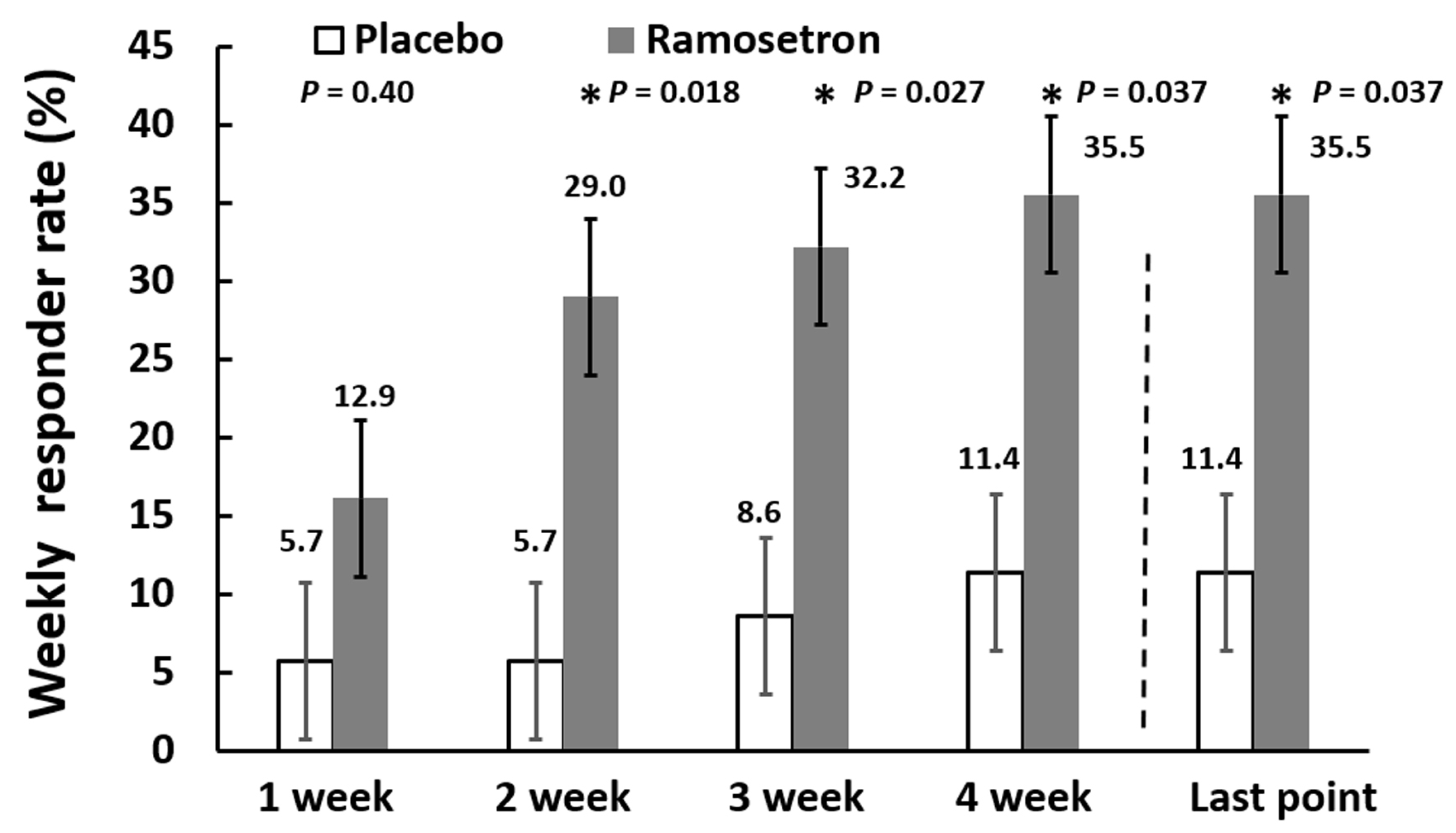
3.2. Effect of Ramosetron on Overall IBS-D-Like Symptoms in Patients with Quiescent IBD
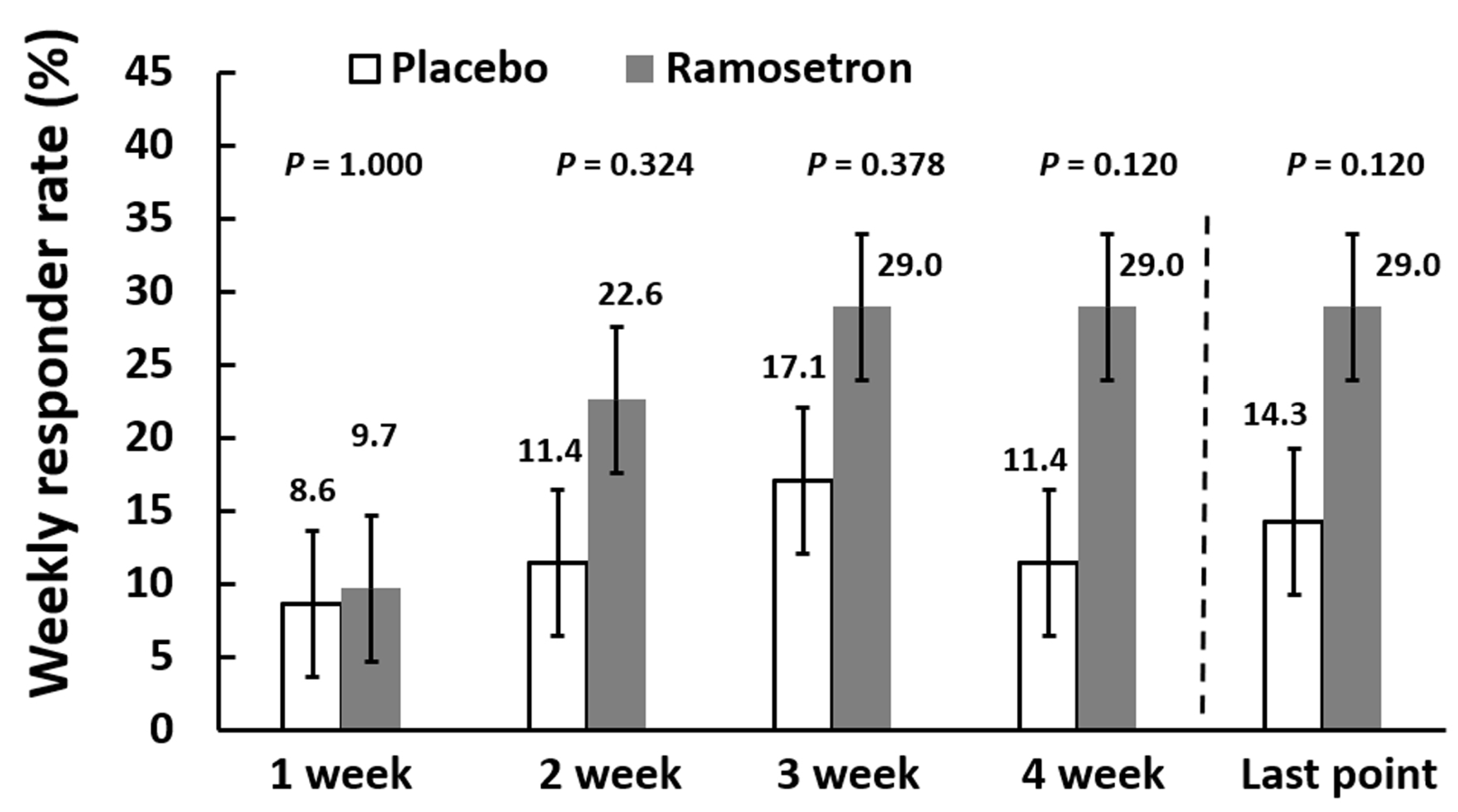
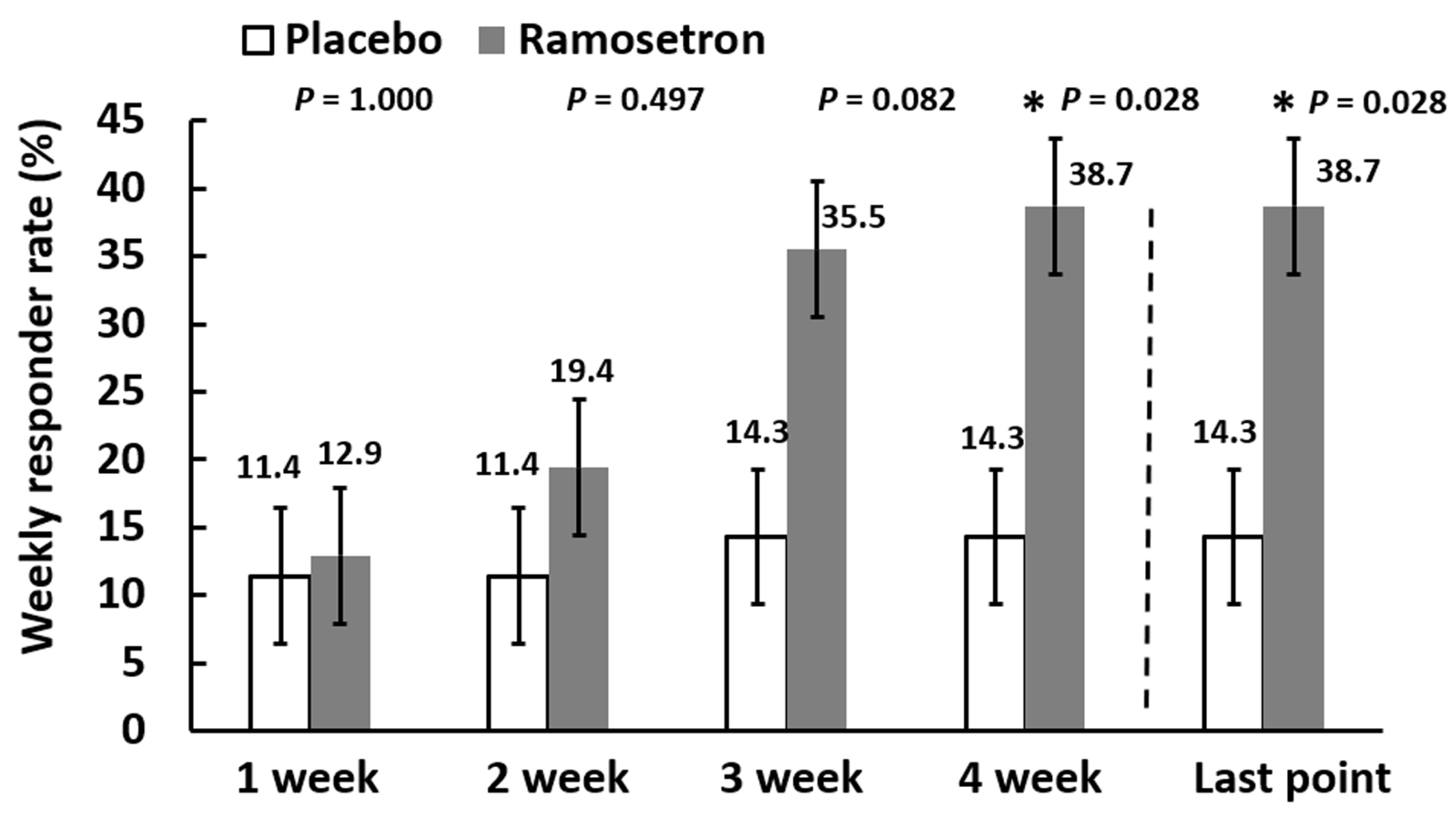
3.3. Effect of Ramosetron on Abdominal Discomfort/Pain and Abnormal Bowel Habits in Patients with Quiescent IBD
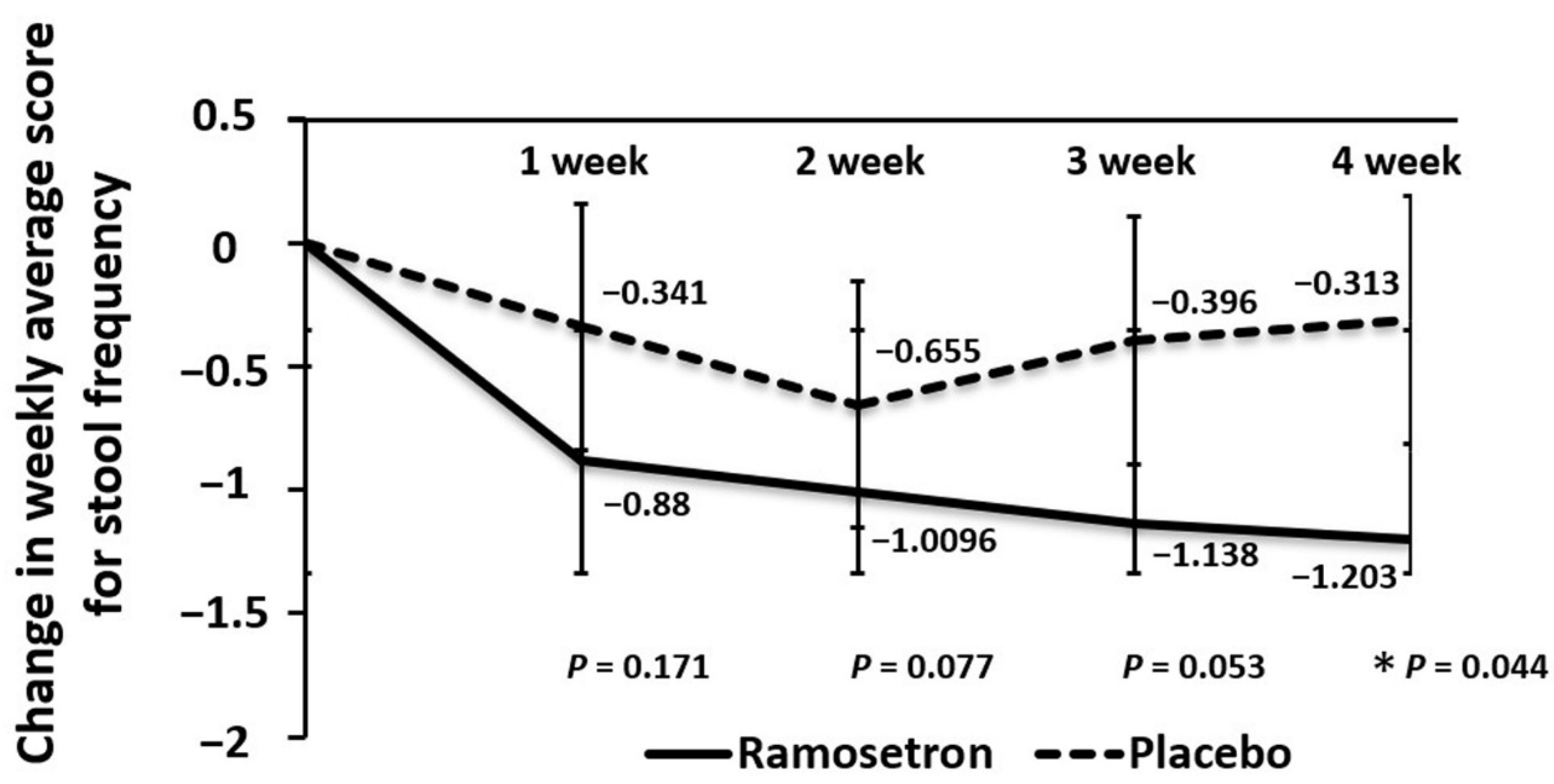
3.4. Effect of Ramosetron on Weekly Changes in Stool Frequency
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, D.H.; Cheon, J.H. Pathogenesis of inflammatory bowel disease and recent advances in biologic therapies. Immune Netw. 2017, 17, 25–40. [Google Scholar] [CrossRef]
- Simrén, M.; Axelsson, J.; Gillberg, R.; Abrahamsson, H.; Svedlund, J.; Björnsson, E.S. Quality of life in inflammatory bowel disease in remission: The impact of IBS-like symptoms and associated psychological factors. Am. J. Gastroenterol. 2002, 97, 389–396. [Google Scholar] [CrossRef]
- Tomita, T.; Kato, Y.; Takimoto, M.; Yamasaki, T.; Kondo, T.; Kono, T.; Tozawa, K.; Yokoyama, Y.; Ikehara, H.; Ohda, Y.; et al. Prevalence of irritable bowel syndrome-like symptoms in Japanese patients with inactive inflammatory bowel disease. J. Neurogastroenterol. Motil. 2016, 22, 661–669. [Google Scholar] [CrossRef][Green Version]
- Vivinus-Nébot, M.; Frin-Mathy, G.; Bzioueche, H.; Dainese, R.; Bernard, G.; Anty, R.; Filippi, J.; Saint-Paul, M.C.; Tulic, M.K.; Verhasselt, V.; et al. Functional bowel symptoms in quiescent inflammatory bowel diseases: Role of epithelial barrier disruption and low-grade inflammation. Gut 2014, 63, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Gracie, D.J.; Williams, C.J.; Sood, R.; Mumtaz, S.; Bholah, M.H.; Hamlin, P.J.; Ford, A.C. Negative effects on psychological health and quality of life of genuine irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2017, 15, 376–384.e5. [Google Scholar] [CrossRef] [PubMed]
- Keohane, J.; O’Mahony, C.; O’Mahony, L.; O’Mahony, S.; Quigley, E.M.; Shanahan, F. Irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease: A real association or reflection of occult inflammation? Am. J. Gastroenterol. 2010, 105, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Fukudo, S.; Ida, M.; Akiho, H.; Nakashima, Y.; Matsueda, K. Effect of ramosetron on stool consistency in male patients with irritable bowel syndrome with diarrhea. Clin. Gastroenterol. Hepatol. 2014, 12, 953–959.e4. [Google Scholar] [CrossRef]
- Fukudo, S.; Kinoshita, Y.; Okumura, T.; Ida, M.; Akiho, H.; Nakashima, Y.; Nishida, A.; Haruma, K. Ramosetron reduces symptoms of irritable bowel syndrome with Diarrhea and improves quality of life in women. Gastroenterology 2016, 150, 358–366.e8. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Kim, N.Y.; Kwon, J.K.; Huh, K.C.; Lee, O.Y.; Lee, J.S.; Choi, S.C.; Sohn, C.I.; Myung, S.J.; Park, H.J.; et al. Efficacy of ramosetron in the treatment of male patients with irritable bowel syndrome with diarrhea: A multicenter, randomized clinical trial, compared with mebeverine. Neurogastroenterol. Motil. 2011, 23, 1098–1104. [Google Scholar] [CrossRef]
- Matsueda, K.; Harasawa, S.; Hongo, M.; Hiwatashi, N.; Sasaki, D. A randomized, double-blind, placebo-controlled clinical trial of the effectiveness of the novel serotonin type 3 receptor antagonist ramosetron in both male and female Japanese patients with diarrhea-predominant irritable bowel syndrome. Scand. J. Gastroenterol. 2008, 43, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Matsueda, K.; Harasawa, S.; Hongo, M.; Hiwatashi, N.; Sasaki, D. A phase II trial of the novel serotonin type 3 receptor antagonist ramosetron in Japanese male and female patients with diarrhea-predominant irritable bowel syndrome. Digestion 2008, 77, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yu, T.; Tang, Y.; Xiong, W.; Shen, X.; Jiang, L.; Lin, L. Efficacy and safety of 5-hydroxytryptamine 3 receptor antagonists in irritable bowel syndrome: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2017, 12, e0172846. [Google Scholar]
- Qi, Q.; Zhang, Y.; Chen, F.; Zuo, X.; Li, Y. Ramosetron for the treatment of irritable bowel syndrome with diarrhea: A systematic review and meta-analysis of randomized controlled trials. BMC Gastroenterol. 2018, 18, 5. [Google Scholar] [CrossRef]
- Walmsley, R.S.; Ayres, R.C.; Pounder, R.E.; Allan, R.N. A simple clinical colitis activity index. Gut 1998, 43, 29–32. [Google Scholar] [CrossRef]
- Rachmilewitz, D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: A randomized trial. BMJ 1989, 298, 82–86. [Google Scholar] [CrossRef]
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional bowel disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef]
- Kanazawa, M.; Nakajima, S.; Oshima, T.; Whitehead, W.E.; Sperber, A.D.; Palsson, O.S.; Drossman, D.A.; Miwa, H.; Fukudo, S. Validity and reliability of the Japanese version of the Rome III diagnostic questionnaire for irritable bowel syndrome and functional dyspepsia. J. Neurogastroenterol. Motil. 2015, 21, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Tomita, T.; Oshima, T.; Fukui, H.; Watari, J.; Miwa, H. Efficacy of serotonin type 3 receptor antagonist ramosetron in the treatment of diarrhea-predominant irritable bowel syndrome with inactive Crohn’s disease, a pilot trial. Neurogastroenterol. Motil. 2014, 26 (Suppl. S1), 55. [Google Scholar]
- Fujii, Y.; Saitoh, Y.; Tanaka, H.; Toyooka, H. Ramosetron vs. granisetron for the prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy. Can. J. Anaesth. 1999, 46, 991–993. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; He, X.; Yang, S.; Ai, B.; Zhang, C.; Huang, D.; Dong, M.; Liu, P.; Zhou, S.; Han, X. Ramosetron versus ondansetron in the prevention of chemotherapy-induced gastrointestinal side effects: A prospective randomized controlled study. Chemotherapy 2007, 53, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Shin, S.W.; Song, E.K.; Lee, N.R.; Kim, J.S.; Ahn, J.S.; Yun, H.J.; Cho, Y.H.; Park, K.U.; Kim, S.Y.; et al. Ramosetron versus Ondansetron in combination with aprepitant and dexamethasone for the prevention of highly Emetogenic chemotherapy-induced nausea and vomiting: A multicenter, randomized phase III trial, KCSG PC10-21. Oncologist 2015, 20, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Miyata, K.; Yamano, M.; Kamato, T.; Akuzawa, S. Effect of serotonin (5-HT)3-receptor antagonists YM060, YM114 (KAE-393), ondansetron and granisetron on 5-HT4 receptors and gastric emptying in rodents. Jpn. J. Pharmacol. 1995, 69, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, L.A.; Labus, J.S.; Coveleskie, K.; Hammer, C.; Rappold, G.; Tillisch, K.; Bueller, J.A.; Suyenobu, B.; Jarcho, J.M.; McRoberts, J.A.; et al. The HTR3A polymorphism c. -42C>T is associated with amygdala responsiveness in patients with irritable bowel syndrome. Gastroenterology 2011, 140, 1943–1951. [Google Scholar] [CrossRef]
- Miyata, K.; Ito, H.; Fukudo, S. Involvement of the 5-HT3 receptor in CRH-induced defecation in rats. Am. J. Physiol. 1998, 274, G827–G831. [Google Scholar] [CrossRef]
- Fukui, H.; Oshima, T.; Tanaka, Y.; Oikawa, Y.; Makizaki, Y.; Ohno, H.; Tomita, T.; Watari, J.; Miwa, H. Effect of probiotic Bifidobacterium bifidum G9-1 on the relationship between gut microbiota profile and stress sensitivity in maternally separated rats. Sci. Rep. 2018, 8, 12384. [Google Scholar] [CrossRef]
- Enck, P.; Aziz, Q.; Barbara, G.; Farmer, A.D.; Fukudo, S.; Mayer, E.A.; Niesler, B.; Quigley, E.M.; Rajilić-Stojanović, M.; Schemann, M.; et al. Irritable bowel syndrome. Nat. Rev. Dis. Primers 2016, 2, 16014. [Google Scholar] [CrossRef]
- Oliver, B.; van Wijngaarden, I.; Soudijn, W. 5-HT(3) receptor antagonists and anxiety; a preclinical and clinical review. Eur. Neuropsychopharmacol. 2000, 10, 77–95. [Google Scholar] [CrossRef]
- Qiu, X.; Zhao, X.; Cui, X.; Mao, X.; Tang, N.; Jiao, C.; Wang, D.; Zhang, Y.; Ye, Z.; Zhang, H. Characterization of fungal and bacterial dysbiosis in young adult Chinese patients with Crohn’s disease. Ther. Adv. Gastroenterol. 2020, 13, 1756284820971202. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Loening-Baucke, V.; Vaneechoutte, M.; Doerffel, Y. Active Crohn’s disease and ulcerative colitis can be specifically diagnosed and monitored based on the biostructure of the fecal flora. Inflamm. Bowel. Dis. 2008, 14, 147–161. [Google Scholar] [CrossRef]
- DuPont, A.W. Postinfectious irritable bowel syndrome. Clin. Infect. Dis. 2008, 46, 594–599. [Google Scholar] [PubMed]
- Balemans, D.; Mondelaers, S.U.; Cibert-Goton, V.; Stakenborg, N.; Aguilera-Lizarraga, J.; Dooley, J.; Liston, A.; Bulmer, D.C.; Vanden Berghe, P.; Boeckxstaens, G.E.; et al. Evidence for long-term sensitization of the bowel in patients with post-infectious-IBS. Sci. Rep. 2017, 7, 13606. [Google Scholar] [CrossRef] [PubMed]
| Placebo | Ramosetron | p Value | |
|---|---|---|---|
| Number | 35 (CD: 31, UC: 4) | 31 (CD: 25, UC: 6) | |
| Age (year) | 44.5 ± 9.9 | 46.6 ± 12.4 | 0.589 |
| Gender, male (n) | 29 (82.8%) | 26 (83.9%) | 1.00 |
| BMI (kg/m2) | 20.9 ± 3.0 | 21.6 ± 3.5 | 0.550 |
| Type of CD | |||
| L1 (ileal) | 9 | 6 | |
| L2 (colonic) | 5 | 5 | |
| L3 (ileocolonic) | 17 | 14 | |
| Type of UC | |||
| Proctitis | 0 | 4 | |
| Left-sided | 2 | 1 | |
| Total colon | 2 | 1 | |
| Duration of disease (year) | 17.2 ± 7.6 | 19.9 ± 9.9 | 0.291 |
| Smoking (n) | 13 (37.1%) | 14 (45.2%) | 0.618 |
| Medication | |||
| 5-ASA/sulfasalazine | 20 | 21 | |
| Prednisolone | 0 | 0 | |
| Azathioprine/6-MP | 1 | 2 | |
| Anti-TNF-α antibody | 18 | 14 | |
| CRP | 0.2 ± 0. 3 | 0.2 ± 0. 3 | 0.810 |
| CDAI | 68.2 ± 40.9 | 73.4 ± 3.3 | 0.639 |
| CAI | 0.25 ± 0.5 | 0.83 ± 0.7 | 0.239 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomita, T.; Fukui, H.; Morishita, D.; Mori, S.; Oshima, T.; Shinzaki, S.; Miwa, H. Efficacy of Serotonin Type 3 Receptor Antagonist Ramosetron on Diarrhea-Predominant Irritable Bowel Syndrome (IBS-D)-Like Symptoms in Patients with Quiescent Inflammatory Bowel Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Clin. Med. 2022, 11, 6882. https://doi.org/10.3390/jcm11236882
Tomita T, Fukui H, Morishita D, Mori S, Oshima T, Shinzaki S, Miwa H. Efficacy of Serotonin Type 3 Receptor Antagonist Ramosetron on Diarrhea-Predominant Irritable Bowel Syndrome (IBS-D)-Like Symptoms in Patients with Quiescent Inflammatory Bowel Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Journal of Clinical Medicine. 2022; 11(23):6882. https://doi.org/10.3390/jcm11236882
Chicago/Turabian StyleTomita, Toshihiko, Hirokazu Fukui, Daisuke Morishita, Sumire Mori, Tadayuki Oshima, Shinichiro Shinzaki, and Hiroto Miwa. 2022. "Efficacy of Serotonin Type 3 Receptor Antagonist Ramosetron on Diarrhea-Predominant Irritable Bowel Syndrome (IBS-D)-Like Symptoms in Patients with Quiescent Inflammatory Bowel Disease: A Randomized, Double-Blind, Placebo-Controlled Trial" Journal of Clinical Medicine 11, no. 23: 6882. https://doi.org/10.3390/jcm11236882
APA StyleTomita, T., Fukui, H., Morishita, D., Mori, S., Oshima, T., Shinzaki, S., & Miwa, H. (2022). Efficacy of Serotonin Type 3 Receptor Antagonist Ramosetron on Diarrhea-Predominant Irritable Bowel Syndrome (IBS-D)-Like Symptoms in Patients with Quiescent Inflammatory Bowel Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Journal of Clinical Medicine, 11(23), 6882. https://doi.org/10.3390/jcm11236882










