PSMA Expression in Solid Tumors beyond the Prostate Gland: Ready for Theranostic Applications?
Abstract
1. Introduction
2. Salivary Glands Cancer
3. Thyroid Cancer
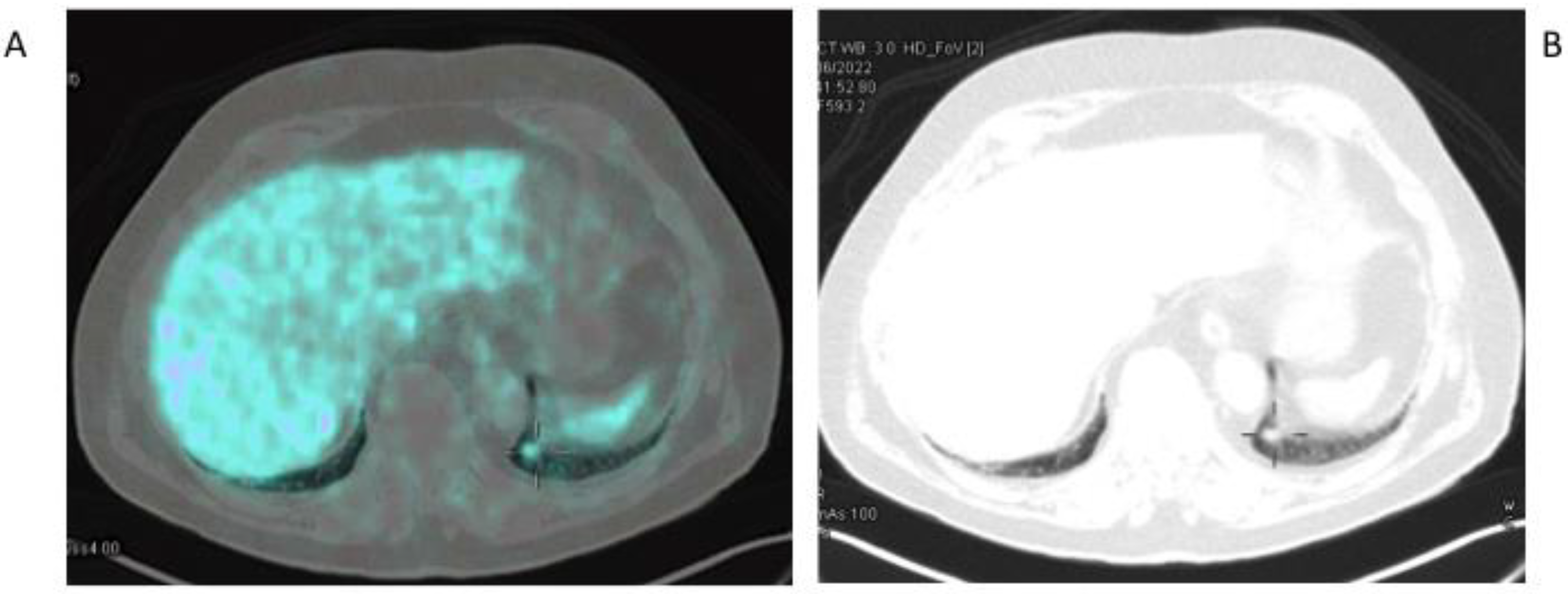
4. Hepatocellular Carcinoma
5. Renal Cell Carcinoma
6. Glioblastoma
7. Breast Cancer
8. Lung Cancer
9. Other Cancers
10. Is There an Added Value of PSMA-Based Imaging over 18F-FDG PET/CT for Extra-Prostatic Tumors?
11. Conclusions and Future Perspectives
| Cancer Type | Histotype (n. of pts) | PSMA PET Findings (n. of pts) | Criteria for Eligibility to RLT | Dose × n. Cycles (n. of pts) | Clinical/Biochemical Response (n. of pts) | Radiological Response (n. of pts) | Reference |
|---|---|---|---|---|---|---|---|
| Salivary gland cancer | AdCC (4) | Lung metastases (2) Liver metastases (1) Bone metastases (1) Locoregional recurrence (1) | PSMA uptake > normal liver on PET imaging | 6–7.4 GBq × 2 (2) 6–7.4GBq × 4 (2) | Symptom relief (3) PD (1) | Stable lung lesions, minimal progression of liver metastases (1) SD (1) PD (2) | [15] |
| Adenocarcinoma NOS (1) | Bone metastases | PSMA uptake > normal liver on PET imaging | 6–7.4 GBq × 1 | Treatment was discontinued due to side-effect development (fatigue, xerostomia) | N.A. | [15] | |
| Acinar cell carcinoma (1) | Inguinal lymph nodes Lung metastases Bone metastases | PSMA uptake > normal liver on PET imaging | 6–7.4 GBq × 2 | Significant pain relief but treatment was discontinued due to side-effect development (fatigue, xerostomia) | PD | [15] | |
| Thyroid cancer | RrDTC (1) | Neck and lung metastases | PSMA+ lesions (no other criteria for eligibility were specified) | 7.4 GBq × 1 | N.A. Patient died after 2 weeks due to cardiac arrest | N.A. | [27] |
| RrDTC (2) | Neck metastases (2) Lung metastases (2) Liver metastases (1) | PSMA+ lesions (no other criteria for eligibility were specified) | 6 GBq × 2 | Slightly temporary (7 months) decrease in Tg levels (1) Increase in Tg levels (1) | PR (1) PD (1) | [21] | |
| PDTC (1) | Bone metastases | PSMA+ lesions (no other criteria for eligibility were specified) | 6.3 Gbq × 1 and 7.4 Gbq × 1 | Temporary (7 months) stable Tg levels | Temporary SD followed by appearance of FDG positive lesions | [20] | |
| Liver cancer | HCC (2) | Liver lesions (2) | PSMA+ lesions (no other criteria for eligibility were specified) | 5.9–6.2 GBq × 1 | N.A. Treatment was discontinued due to poor dosimetry at SPECT/CT | N.A. | [35] |
| Brain cancer | GBM (1) | Right parietal mass | PSMA uptake > normal liver on PET imaging in patients not eligible for surgery or who refused RT/CHT | 8.4 GBq × 1 | N.A. | Increased uptake on SPECT imaging after therapy but no radiological FU was reported | [56] |
| GBM (1) | Left temporal nodular lesion extending to left gangliocapsular region | PSMA uptake > normal liver on PET imaging in patients not eligible for surgery or who refused RT/CHT | 3.7 GBq × 3 | Improvements in QoL and pain relief | Significant reduction in lesion size at restaging MRI | [57] | |
| Breast cancer | Triple negative breast cancer (1) | Local recurrence and metastatic lymph nodes | PSMA+ lesions (no other criteria for eligibility were specified) | 7.5 GBq × 2 | PD | PD after the second cycle | [58] |
| Testicular Cancer | Testicular mixed germ cell tumor (1) | Bone, liver, lymph nodes, lung metastases | PSMA+ lesions (no other criteria for eligibility were specified) | 7.5 GBq × 1 | Increased α-fetoprotein levels | PD | [108] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Denise, S.; O’Keefe, D.S.; Bacich, D.J.; Huang, S.S.; Heston, W.D.W. A perspective on the evolving story of PSMA biology, PSMA-based imaging and endoradiotherapeutic strategies. J. Nucl. Med. 2018, 59, 1007–1013. [Google Scholar]
- Eiber, M.; Fendler, W.P.; Rowe, S.P.; Calais, J.; Hofman, M.S.; Maurer, T.; Schwarzenboeck, S.M.; Kratowchil, C.; Herrmann, K.; Giesel, F.L. Prostate-specific membrane antigen ligands for imaging and therapy. J. Nucl. Med. 2017, 58, 67S–76S. [Google Scholar] [CrossRef] [PubMed]
- Fragomeni, R.A.S.; Amir, T.; Sheikhbahaei, S.; Harvey, S.C.; Javadi, M.S.; Solnes, L.B.; Kiess, A.P.; Allaf, M.E.; Pomper, M.G.; Gorin, M.A.; et al. Imaging of nonprostate cancers using PSMA-targeted radiotracers: Rationale, current state of the field, and a call to arms. J. Nucl. Med. 2018, 59, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Siva, S.; Udovicich, C.; Tran, B.; Zargar, H.; Murphy, D.G.; Hofman, M.S. Expanding the role of small-molecule PSMA ligands beyond PET staging of prostate cancer. Nat. Rev. Urol. 2020, 17, 107–118. [Google Scholar] [CrossRef]
- Lutje, S.; Heskamp, S.; Cornelissen, A.S.; Poeppel, T.D.; van den Broek, S.A.; Rosenbaum-Krumme, S.; Bockisch, A.; Gotthardt, M.; Rijpkema, M.; Boerman, O.C. PSMA ligands for radionuclide imaging and therapy of prostate cancer: Clinical status. Theranostics 2015, 5, 1388–1401. [Google Scholar] [CrossRef]
- Richter, S.; Wuest, F. 18F-Labeled peptides: The future is bright. Molecules 2014, 19, 20536–20556. [Google Scholar] [CrossRef]
- Ducharme, J.; Goertzen, A.L.; Patterson, J.; Demeter, S. Practical aspects of 18F-FDG PET when receiving 18F-FDG from a distant supplier. J. Nucl. Med. Technol. 2009, 37, 164–169. [Google Scholar] [CrossRef]
- De Galiza Barbosa, F.; Queiroz, M.A.; Nunes, R.F.; Costa, L.B.; Zaniboni, E.C.; Marin, J.F.G.; Cerri, G.G.; Buchpiguel, C.A. Nonprostatic diseases on PSMA PET imaging: A spectrum of benign and malignant findings. Cancer Imaging 2020, 20, 23. [Google Scholar] [CrossRef]
- Rupp, N.J.; Umbricht, C.A.; Pizzuto, D.A.; Lenggenhager, D.; Töpfer, A.; Müller, J.; Muehlematter, U.J.; Ferraro, D.A.; Messerli, M.; Morand, G.B.; et al. First clinicopathologic evidence of a Non-PSMA-Related uptake mechanism for (68)Ga-PSMA-11 in salivary glands. J. Nucl. Med. 2019, 60, 1270–1276. [Google Scholar] [CrossRef]
- Nishida, H.; Kondo, Y.; Kusaba, T.; Kadowaki, H.; Daa, T. Immunohistochemical reactivity of prostate-specific membrane antigen in salivary gland Tumors. Head Neck Pathol. 2022, 16, 427–433. [Google Scholar] [CrossRef]
- Van Boxtel, W.; Lütje, S.; van Engen-van Grunsven, I.C.H.; Verhaegh, G.W.; Schalken, J.A.; Jonker, M.A.; Nagarajah, J.; Gotthardt, M.; van Herpen, C.M.L. 68Ga-PSMA-HBED-CC PET/CT imaging for adenoid cystic carcinoma and salivary duct carcinoma: A phase 2 imaging study. Theranostics 2020, 10, 2273–2283. [Google Scholar] [CrossRef] [PubMed]
- Klein Nulent, T.J.W.; van Es, R.J.J.; Krijger, G.C.; de Bree, R.; Willems, S.M.; de Keizer, B. Prostate-specific membrane antigen PET imaging and immunohistochemistry in adenoid cystic carcinoma-a preliminary analysis. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Konig, L.; Hauswald, H.; Flechtenmacher, C.; Heller, M.; Debus, J.; Haberkorn, U.; Kratochwil, l.; Giesel, F. Uptake of prostate-specifc membrane antigen (PSMA) in adenoid cystic carcinoma—Is PSMA-PET-CT a helpful tool in radiation oncology? Clin. Transl. Radiat. Oncol. 2017, 7, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Isgoren, S.; Hekimsoy, T.; Koroglu, E.; Demir, H. PET/CT with 68Ga-PSMA and 18F-FDG in Metastatic Adenoid Cystic Carcinoma: Report of 2 Cases. Clin. Nucl. Med. 2022, 47, e423–e424. [Google Scholar] [CrossRef]
- Klein Nulent, T.J.W.; van Es, R.J.J.; Willems, S.M.; Braat, A.J.A.T.; Devriese, L.A.; de Bree, R.; de Keizer, B. First experiences with 177Lu-PSMA-617 therapy for recurrent or metastatic salivary gland cancer. EJNMMI Res. 2021, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Roman, B.R.; Morris, L.G.; Davies, L. The thyroid cancer epidemic, 2017 perspective. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 332. [Google Scholar] [CrossRef]
- Luster, M.; Clarke, S.E.; Dietlein, M.; Lassmann, M.; Lind, P.; Oyen, W.J.; Tennvall, J.; Bombardieri, E. Guidelines for radioiodine therapy of differentiated thyroid cancer. EJNMMI 2008, 35, 1941–1959. [Google Scholar] [CrossRef]
- Bertagna, F.; Albano, D.; Giovanella, L.; Bonacina, M.; Durmo, R.; Giubbini, R.; Treglia, G. 68Ga-PSMA PET thyroid incidentalomas. Hormones 2019, 18, 145–149. [Google Scholar] [CrossRef]
- Bychkov, A.; Vutrapongwatana, U.; Tepmongkol, S.; Keelawat, S. PSMA expression by microvasculature of thyroid tumors–Potential implications for PSMA theranostics. Sci. Rep. 2017, 7, 5202. [Google Scholar] [CrossRef]
- Wächter, S.; Di Fazio, P.; Maurer, E.; Manoharan, J.; Keber, C.; Pfestroff, A.; Librizzi, D.; Bartsch, D.K.; Luster, M.; Eilsberger, F. Prostate-specific membrane antigen in anaplastic and poorly differentiated thyroid cancer—A new diagnostic and therapeutic target? Cancers 2021, 13, 5688. [Google Scholar] [CrossRef]
- De Vries, L.H.; Lodewijk, L.; Braat, A.J.; Krijger, G.C.; Valk, G.D.; Lam, M.G.; Rinkes, I.H.; Vriens, M.R.; de Keizer, B. 68 Ga-PSMA PET/CT in radioactive iodine-refractory differentiated thyroid cancer and first treatment results with 177 Lu-PSMA-617. EJNMMI Res. 2020, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Lawhn-Heath, C.; Yom, S.S.; Liu, C.; Villanueva-Meyer, J.E.; Aslam, M.; Smith, R.; Narwal, M.; Juarez, R.; Behr, S.C.; Pampaloni, M.H.; et al. Gallium-68 prostate-specific membrane antigen ([68 Ga] Ga-PSMA-11) PET for imaging of thyroid cancer: A feasibility study. EJNMMI Res. 2020, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Malhotra, G.; Agrawal, R.; Sonavane, S.; Meshram, V.; Asopa, R.V. Evidence of prostate-specific membrane antigen expression in metastatic differentiated thyroid cancer using 68Ga-PSMA-HBED-CC PET/CT. Clin. Nucl. Med. 2018, 43, e265–e268. [Google Scholar] [CrossRef] [PubMed]
- Pitalua-Cortes, Q.; García-Perez, F.O.; Vargas-Ahumada, J.; Gonzalez-Rueda, S.; Gomez-Argumosa, E.; Ignacio-Alvarez, E.; Soldevilla-Gallardo, I.; Torres-Agredo, L. Head-to-head comparison of 68Ga-PSMA-11 and 131I in the follow-up of well-differentiated metastatic thyroid cancer: A new potential theragnostic agent. Front. Endocrinol. 2021, 12, 794759. [Google Scholar] [CrossRef]
- Aashiq, M.; Silverman, D.A.; Na’ara, S.; Takahashi, H.; Amit, M. Radioiodine-refractory thyroid cancer: Molecular basis of redifferentiation therapies, management, and novel therapies. Cancers 2019, 11, 1382. [Google Scholar] [CrossRef]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Baum, R.; Bozkurt, M.F.; Czernin, J.; Delgado Bolton, R.C.; Ezziddin, S.; Forrer, F.; Hicks, R.J.; et al. EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands (177Lu-PSMA-RLT). EJNMMI 2019, 46, 2536–2544. [Google Scholar] [CrossRef]
- Assadi, M.; Ahmadzadehfar, H. 177Lu-DOTATATE and 177Lu-prostate-specific membrane antigen therapy in a patient with advanced metastatic radioiodine-refractory differentiated thyroid cancer after failure of tyrosine kinase inhibitors treatment. World J. Nucl. Med. 2019, 18, 406–408. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Izuishi, K.; Yamamoto, Y.; Mori, H.; Kameyama, R.; Fujihara, S.; Masaki, T.; Suzuki, Y. Molecular mechanisms of [18F]fluorodeoxyglucose accumulation in liver cancer. Oncol. Rep. 2014, 31, 701–706. [Google Scholar] [CrossRef]
- Sacks, A.; Peller, P.J.; Surasi, D.S.; Chatburn, L.; Mercier, G.; Subramaniam, R.M. Value of PET/CT in the management of primary hepatobiliary tumors, part 2. Am. J. Roentgenol. 2011, 197, W260–W265. [Google Scholar] [CrossRef]
- Sheikhbahaei, S.; Werner, R.A.; Solnes, L.B.; Pienta, K.J.; Pomper, M.G.; Gorin, M.A.; Rowe, S.P. Prostate-Specific Membrane Antigen (PSMA)-Targeted PET imaging of prostate cancer: An update on important pitfalls. Semin. Nucl. Med. 2019, 49, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Kesler, M.; Levine, C.; Hershkovitz, D.; Mishani, E.; Menachem, Y.; Lerman, H.; Zohar, Y.; Shibolet, O.; Even-Sapir, E. 68Ga-PSMA is a novel PET-CT tracer for imaging of hepatocellular carcinoma: A prospective pilot study. J. Nucl. Med. 2019, 60, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Kuyumcu, S.; Has-Simsek, D.; Iliaz, R.; Sanli, Y.; Buyukkaya, F.; Akyuz, F.; Turkmen, C. Evidence of prostate-specific membrane antigen expression in hepatocellular carcinoma using 68Ga-PSMA PET/CT. Clin. Nucl. Med. 2019, 44, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Gündoğan, C.; Ergül, N.; Çakır, M.S.; Kılıçkesmez, Ö.; Gürsu, R.U.; Aksoy, T.; Çermik, T.F. 68Ga-PSMA PET/CT versus 18F-FDG PET/CT for imaging of hepatocellular carcinoma. Mol. Imaging Radionucl. Ther. 2021, 30, 79. [Google Scholar] [CrossRef] [PubMed]
- Hirmas, N.; Leyh, C.; Sraieb, M.; Barbato, F.; Schaarschmidt, B.M.; Umutlu, L.; Nader, M.; Wedemeyer, H.; Ferdinandus, J.; Rischpler, C.; et al. 68Ga-PSMA-11 PET/CT improves tumor detection and impacts management in patients with hepatocellular carcinoma. J. Nucl. Med. 2021, 62, 1235–1241. [Google Scholar] [CrossRef]
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Gore, J.L.; Sun, M.; Wood, C.; Russo, P. Epidemiology of renal cell carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef]
- Uijen, M.J.M.; Derks, Y.H.W.; Merkx, R.I.J.; Schilham, M.G.M.; Roosen, J.; Privé, B.M.; van Lith, S.A.M.; van Herpen, C.M.L.; Gotthardt, M.; Heskamp, S.; et al. PSMA radioligand therapy for solid tumors other than prostate cancer: Background, opportunities, challenges, and first clinical reports. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4350–4368. [Google Scholar] [CrossRef]
- Gorin, M.A.; Rowe, S.P.; Hooperc, J.E.; Kates, M.; Hammers HJSzabo, Z.; Pomper, M.G.; Allaf, M.E. PSMA-targeted 18F-DCFPyL PET/CT imaging of clear renal cell carcinoma: Results from a rapid autopsy. Eur. Urol. 2017, 71, 145–146. [Google Scholar] [CrossRef]
- Spatz, S.; Tolkach, Y.; Jung, K.; Stephan, C.; Busch, J.; Ralla, B.; Rabien, A.; Feldmann, G.; Brossart, P.; Bundschuh, R.A.; et al. Comprehensive evaluation of prostate specific membrane antigen expression in the vasculature of renal tumors: Implications for imaging studies and prognostic role. J. Urol. 2018, 199, 370–377. [Google Scholar] [CrossRef]
- Sawicki, L.M.; Buchbender, C.; Boos, J.; Giessing, M.; Ermert, J.; Antke, C.; Antoch, G.; Hautzel, H. Diagnostic potential of PET/CT using a 68Ga-labelled prostate-specific membrane antigen ligand in whole-body staging of renal cell carcinoma: Initial experience. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 102–107. [Google Scholar] [CrossRef]
- Rhee, H.; Blazak, J.; Tham, C.M.; Ng, K.L.; Shepherd, B.; Lawson, M.; Preston, J.; Vela, I.; Thomas, P.; Wood, S. Pilot study: Use of gallium-68 PSMA PET for detection of metastatic lesions in patients with renal tumour. EJNMMI Res. 2016, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, L.; Basso, U.; Maruzzo, M.; Novara, G. The role of radiolabeled prostate-specific membrane antigen positron emission tomography/computed tomography for the evaluation of renal cancer. Eur. Urol. Focus 2020, 6, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.P.; Gorin, M.A.; Hammers, H.J.; Som Javadi, M.; Hawasli, H.; Szabo, Z.; Cho, S.Y.; Pomper, M.G.; Allaf, M.E. Imaging of metastatic clear cell renal cell carcinoma with PSMA-targeted ¹⁸F-DCFPyL PET/CT. Ann. Nucl. Med. 2015, 29, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, A.; Joy, A.; Nanabala, R.; Unni, M.; Tk, P. Complimentary pattern of uptake in 18F-FDG PET/CT and 68Ga-prostate-specific membrane antigen PET/CT in a case of metastatic clear cell renal carcinoma. Clin. Nucl. Med. 2016, 41, e517–e519. [Google Scholar] [CrossRef] [PubMed]
- Saadat, S.; Tie, B.; Wood, S.; Vela, I.; Rhee, H. Imaging tumour thrombus of clear cell renal cell carcinoma: FDG PET or PSMA PET? Direct in vivo comparison of two technologies. Urol. Case Rep. 2018, 16, 4–5. [Google Scholar] [CrossRef]
- Rowe, S.P.; Gorin, M.A.; Hammers, H.J.; Pomper, M.G.; Allaf, M.E.; Javadi, M.S. Detection of 18F-FDG PET/CT occult lesions with 18F-DCFPyL PET/CT in a patient with metastatic renal cell carcinoma. Clin. Nucl. Med. 2016, 41, 83–85. [Google Scholar] [CrossRef]
- Siva, S.; Callahan, J.; Pryor, D.; Martin, J.; Lawrentschuk, N.; Hofman, M.S. Utility of 68 Ga prostate specific membrane antigen-positron emission tomography in diagnosis and response assessment of recurrent renal cell carcinoma. J. Med. Imaging Radiat. Oncol. 2017, 61, 372–378. [Google Scholar] [CrossRef]
- Nadebaum, D.P.; Hofman, M.S.; Mitchell, C.A.; Siva, S.; Hicks, R.J. Oligometastatic renal cell carcinoma with sarcomatoid differentiation demonstrating variable imaging phenotypes on 68Ga-PSMA and 18F-FDG PET/CT: A case report and review of the literature. Clin. Genitourin. Cancer 2017, 16, 1–5. [Google Scholar] [CrossRef]
- Demirci, E.; Ocak, M.; Kabasakal, L.; Decristoforo, C.; Talat, Z.; Halaç, M.; Kanmaz, B. 68Ga-PSMA PET/CT imaging of metastatic clear cell renal cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1461–1462. [Google Scholar] [CrossRef]
- Yin, Y.; Campbell, S.P.; Markowski, M.C.; Pierorazio, P.M.; Pomper, M.G.; Allaf, M.E.; Rowe, S.P.; Gorin, M.A. Inconsistent detection of sites of metastatic non-clear cell renal cell carcinoma with PSMA-Targeted [18F]DCFPyL PET/CT. Mol. Imaging Biol. 2019, 21, 567–573. [Google Scholar] [CrossRef]
- Omuro, A.; De Angelis, L.M. Glioblastoma and other malignant gliomas: A clinical review. JAMA 2013, 310, 1842–1850. [Google Scholar] [PubMed]
- Wernicke, A.G.; Edgar, M.A.; Lavi, E.; Liu, H.; Salerno, P.; Bander, N.H.; Gutin, P.H. Prostate-specific membrane antigen as a potential novel vascular target for treatment of glioblastoma multiforme. Arch. Pathol. Lab. Med. 2011, 135, 1486–1489. [Google Scholar] [CrossRef] [PubMed]
- Saffar, H.; Noohi, M.; Tavangar, S.M.; Saffar, H.; Azimi, S. Expression of Prostate-Specific Membrane Antigen (PSMA) in brain glioma and its correlation with tumor grade. Iran J. Pathol. 2018, 13, 45–53. [Google Scholar] [PubMed]
- Bertagna, F.; Albano, D.; Cerudelli, E.; Gazzilli, M.; Giubbini, R.; Treglia, G. Potential of radiolabeled PSMA PET/CT or PET/MRI diagnostic procedures in gliomas/glioblastomas. Curr. Radiopharm. 2020, 13, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Kunikowska, J.; Kuliński, R.; Muylle, K.; Koziara, H.; Królicki, L. 68Ga-Prostate-specific membrane antigen-11 PET/CT: A new imaging option for recurrent glioblastoma multiforme? Clin. Nucl. Med. 2020, 45, 11–18. [Google Scholar] [CrossRef]
- Kunikowska, J.; Charzyńska, I.; Kuliński, R.; Pawlak, D.; Maurin, M.; Królicki, L. Tumor uptake in glioblastoma multiforme after IV injection of [177Lu] Lu-PSMA-617. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1605–1606. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ballal, S.; Yadav, M.P.; ArunRaj, S.T.; Haresh, K.P.; Gupta, S.; Damle, N.A.; Garg, A.; Tripathi, M.; Bal, C. 177Lu-/68Ga-PSMA theranostics in recurrent glioblastoma multiforme: Proof of concept. Clin. Nucl. Med. 2020, 45, e512–e513. [Google Scholar] [CrossRef]
- Tolkach, Y.; Gevensleben, H.; Bundschuh, R.; Koyun, A.; Huber, D.; Kehrer, C.; Hecking, T.; Keyver-Paik, M.D.; Kaiser, C.; Ahmadzadehfar, H.; et al. Prostate-specific membrane antigen in breast cancer: A comprehensive evaluation of expression and a case report of radionuclide therapy. Breast Cancer Res. Treat. 2018, 169, 447–455. [Google Scholar] [CrossRef]
- Wernicke, A.G.; Varma, S.; Greenwood, E.A.; Christos, P.J.; Chao, K.S.C.; Liu, H.; Bander, N.H.; Shin, S.J. Prostate-specific membrane antigen expression in tumor-associated vasculature of breast cancers. Apmis 2014, 122, 482–489. [Google Scholar] [CrossRef]
- Kasoha, M.; Unger, C.; Solomayer, E.F.; Bohle, R.M.; Zaharia, C.; Khreich, F.; Wagenpfeil, S.; Juhasz-Böss, I. Prostate-specific membrane antigen (PSMA) expression in breast cancer and its metastases. Clin. Exp. Metastasis 2017, 34, 479–490. [Google Scholar] [CrossRef]
- Sathekge, M.; Lengana, T.; Modiselle, M.; Vorster, M.; Zeevaart, J.; Maes, A.; Ebenhan, T.; Van de Wiele, C. 68Ga-PSMA-HBED-CC PET imaging in breast carcinoma patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Medina-Ornelas, S.; García-Perez, F.; Estrada-Lobato, E.; Ochoa-Carrillo, F. 68Ga-PSMA PET/CT in the evaluation of locally advanced and metastatic breast cancer, a single center experience. Am. J. Nucl. Med. Mol. Imaging 2020, 10, 135–142. [Google Scholar] [PubMed]
- Morgenroth, A.; Tinkir, E.; Vogg, A.T.J.; Sankaranarayanan, R.A.; Baazaoui, F.; Mottaghy, F.M. Targeting of prostate-specific membrane antigen for radio-ligand therapy of triple-negative breast cancer. Breast Cancer Res. 2019, 21, 116. [Google Scholar] [CrossRef] [PubMed]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of lung cancer. Contemp. Oncol. Współczesna Onkol. 2021, 25, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J., Jr.; Wu, Y.L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef]
- Zheng, M. Classification and pathology of lung cancer. Surg. Oncol. Clin. 2016, 25, 447–468. [Google Scholar] [CrossRef] [PubMed]
- Chia, J.Y.; Loi, H.Y.; Khor, L.K.; Lee, K.C.; Seow, Y.H. Primary lung adenocarcinoma with 68gallium prostate-specific membrane antigen-PET/CT scan avidity in a patient on surveillance after prostatectomy. Clin. Genitourin. Cancer 2018, 16, e525–e527. [Google Scholar] [CrossRef]
- Sharma, P. 68Ga-PSMA-Avid small cell lung cancer on PET/CT: Incidental second malignancy in treated prostate cancer. Clin. Nucl. Med. 2020, 45, 1016–1017. [Google Scholar] [CrossRef]
- Karyağar, S.S. PSMA-positive secondary tumors in 68Ga-PSMA PET/CT imaging in patients with prostate cancer. Eur. Arch. Med. Res. 2020, 36, 246–250. [Google Scholar] [CrossRef]
- Osman, M.M.; Iravani, A.; Hicks, R.J.; Hofman, M.S. Detection of synchronous primary malignancies with 68Ga-labeled prostate-specific membrane antigen PET/CT in patients with prostate cancer: Frequency in 764 patients. J. Nucl. Med. 2017, 58, 1938–1942. [Google Scholar] [CrossRef]
- Pyka, T.; Weirich, G.; Einspieler, I.; Maurer, T.; Theisen, J.; Hatzichristodoulou, G.; Schwamborn, K.; Schwaiger, M.; Eiber, M. 68Ga-PSMA-HBED-CC PET for differential diagnosis of suggestive lung lesions in patients with prostate cancer. J. Nucl. Med. 2016, 57, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Jochumsen, M.R.; Gormsen, L.C.; Nielsen, G.L. 68Ga-PSMA avid primary adenocarcinoma of the lung with complementary low 18F-FDG uptake. Clin. Nucl. Med. 2018, 43, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Wang, S.S.; Song, W.H.; Pan, Y.; Yu, H.P.; Si, T.G.; Liu, Y.; Cui, X.N.; Guo, Z. Expression of prostate-specific membrane antigen in lung cancer cells and tumor neovasculature endothelial cells and its clinical significance. PLoS ONE 2015, 10, e0125924. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.H.; Heitkötter, B.; Schulze, A.B.; Schliemann, C.; Steinestel, K.; Trautmann, M.; Marra, A.; Hillejan, L.; Mohr, M.; Evers, G.; et al. Prostate specific membrane antigen (PSMA) expression in non-small cell lung cancer. PLoS ONE 2017, 12, e0186280. [Google Scholar] [CrossRef]
- Malik, D.; Kumar, R.; Mittal, B.R.; Singh, H.; Bhattacharya, A.; Sood, A.; Sharma, V.; Singh, H. 68 Ga-labelled PSMA (prostate specific membrane antigen) expression in signet-ring cell gastric carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1276–1277. [Google Scholar] [CrossRef]
- Laurens, S.T.; Witjes, F.; Janssen, M.; Flucke, U.; Gottardt, M. 68Ga-prostate-specific membrane antigen uptake in gastrointestinal stromal tumor. Clin. Nucl. Med. 2018, 43, 60–61. [Google Scholar] [CrossRef]
- Sasikumar, A.; Joy, A.; Pillai, M.R. 68Ga-PSMA uptake in an incidentally detected gastrointestinal stromal tumor in a case of suspected carcinoma prostate. Clin. Nucl. Med. 2017, 42, e447–e448. [Google Scholar] [CrossRef]
- Huang, Y.T.; Fong, W.; Thomas, P. Rectal Carcinoma on 68Ga-PSMA PET/CT. Clin. Nucl. Med. 2016, 41, e167–e168. [Google Scholar] [CrossRef]
- Stoykow, C.; Huber-Schumacher, S.; Almanasreh, N.; Jilg, C.; Ruf, J. Strong PSMA radioligand uptake by rectal carcinoma: Who put the “S” in PSMA? Clin. Nucl. Med. 2017, 42, 225–226. [Google Scholar] [CrossRef]
- Hangaard, L.; Jochumsen, M.R.; Vendelbo, M.H.; Bouchelouche, K. Metastases from colorectal cancer avid on 68Ga-PSMA PET/CT. Clin. Nucl. Med. 2017, 42, 532–533. [Google Scholar] [CrossRef]
- Sonni, I.; Caron, J.; Kishan, A.U.; Muthusamy, V.R.; Calais, J. PSMA expression in the neovasculature associated with rectal adenocarcinoma: A potential stromal target for nuclear theranostics. Clin. Nucl. Med. 2020, 45, e309–e310. [Google Scholar] [CrossRef] [PubMed]
- Haffner, M.C.; Kronberger, I.E.; Ross, J.S.; Sheehan, C.E.; Zitt, M.; Mühlmann, G.; Öfner, D.; Zelger, B.; Ensinger, C.; Yang, X.J.; et al. Prostate-specific membrane antigen expression in the neovasculature of gastric and colorectal cancers. Hum. Pathol. 2009, 40, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hadi, M.; Ismail, Y.; Younis, L. Prostate-specific membrane antigen (PSMA) immunoexpression in the neovasculature of colorectal carcinoma in Egyptian patients. Pathol.-Res. Pract. 2014, 210, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Cuda, T.J.; Riddell, A.D.; Liu, C.; Whitehall, V.L.; Borowsky, J.; Wyld, D.K.; Burge, M.E.; Ahern, E.; Griffin, A.; Lyons, N.J.; et al. PET imaging quantifying 68Ga-PSMA-11 uptake in metastatic colorectal cancer. J. Nucl. Med. 2020, 61, 1576–1579. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Emmett, L.; Violet, J.Y.; Zhang, A.; Lawrence, N.J.; Stockler, M.; Francis, R.J.; Iravani, A.; Williams, S.; Azad, A.; et al. TheraP: A randomized phase 2 trial of 177Lu-PSMA-617 theranostic treatment vs cabazitaxel in progressive metastatic castration-resistant prostate cancer (Clinical Trial Protocol ANZUP 1603). BJU Int. 2019, 124, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Ke, Z.F.; Yang, Z.; Wang, Z.; Yang, S.C.; Luo, C.Q.; Wang, L.T. Prostate-specific membrane antigen: A new potential prognostic marker of osteosarcoma. Med. Oncol. 2012, 29, 2234–2239. [Google Scholar] [CrossRef]
- Sasikumar, A.; Joy, A.; Pillai, M.R.; Alex, T.M.; Narayanan, G. 68Ga-PSMA PET/CT in osteosarcoma in fibrous dysplasia. Clin. Nucl. Med. 2017, 42, 446–447. [Google Scholar] [CrossRef]
- Heitkötter, B.; Trautmann, M.; Grünewald, I.; Bögemann, M.; Rahbar, K.; Gevensleben, H.; Wardelmann, E.; Hartmann, W.; Steinestel, K.; Huss, S. Expression of PSMA in tumor neovasculature of high grade sarcomas including synovial sarcoma, rhabdomyosarcoma, undifferentiated sarcoma and MPNST. Oncotarget 2017, 8, 4268. [Google Scholar] [CrossRef]
- Vaz, S.; Oliveira, C.; Castanheira, J.C.; Silva, Â.F.; Costa, D.C. Gastric GIST incidentally detected on 68Ga-PSMA-PET/CT: Correlation between functional imaging and histology. Clin. Nucl. Med. 2018, 43, e488–e491. [Google Scholar] [CrossRef]
- Sirtl, S.; Todica, A.; Ilhan, H.; Zorniak, M.; Bartenstein, P.; Mayerle, J. Incidental finding of a PSMA-positive pancreatic cancer in a patient suffering from a metastasized PSMA-positive prostate cancer. Diagnostics 2021, 11, 129. [Google Scholar] [CrossRef]
- Lu, Y.; Li, C. Incidental findings of coexisting metastatic pancreatic cancer in patients with prostate cancer on 18F–Prostate-specific membrane antigen PET/CT. Clin. Nucl. Med. 2022, 11, 10–97. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.; Schembri, G.P.; Hsiao, E. Serous cystadenoma of the pancreas showing uptake on 68Ga PSMA PET/CT. Clin. Nucl. Med. 2017, 42, 56–57. [Google Scholar] [CrossRef] [PubMed]
- Mhawech-Fauceglia, P.; Zhang, S.; Terracciano, L.; Sauter, G.; Chadhuri, A.; Herrmann, F.R.; Penetrante, R. Prostate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: An immunohistochemical study using mutiple tumour tissue microarray technique. Histopathology 2007, 50, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Zhang, H.; Wang, X.; Liu, J.; Yuan, Z.; Hao, J. Prostate-specific membrane antigen as a marker of pancreatic cancer cells. Med. Oncol. 2014, 31, 857. [Google Scholar] [CrossRef] [PubMed]
- Stock, K.; Steinestel, K.; Wiesch, R.; Mikesch, J.H.; Hansmeier, A.; Trautmann, M.; Beller, N.; Rehkämper, J.; Wardelmann, E.; Heitkötter, B.; et al. Neovascular prostate-specific membrane antigen expression is associated with improved overall survival under palliative chemotherapy in patients with pancreatic ductal adenocarcinoma. BioMed Res. Int. 2017, 2017, 25. [Google Scholar] [CrossRef]
- Krishnaraju, V.S.; Kumar, R.; Mittal, B.R.; Sharma, V.; Singh, H.; Nada, R.; Bal, A.; Rohilla, M.; Singh, H.; Rana, S.S. Differentiating benign and malignant pancreatic masses: Ga-68 PSMA PET/CT as a new diagnostic avenue. Eur. Radiol. 2021, 31, 2199–2208. [Google Scholar] [CrossRef]
- Gala, J.L.; Loric, S.; Guiot, Y.; Denmeade, S.R.; Gady, A.; Brasseur, F.; Heusterspreute, M.; Eschwege, P.; Nayer, P.D.; Van Cangh, P.; et al. Expression of prostate-specific membrane antigen in transitional cell carcinoma of the bladder: Prognostic value? Clin. Cancer Res. 2000, 6, 4049–4054. [Google Scholar]
- Samplaski, M.K.; Heston, W.; Elson, P.; Magi-Galluzzi, C.; Hansel, D.E. Folate hydrolase (prostate-specific antigen) 1 expression in bladder cancer subtypes and associated tumor neovasculature. Mod. Pathol. 2011, 24, 1521–1529. [Google Scholar] [CrossRef]
- Gupta, M.; Choudhury, P.S.; Gupta, G.; Gandhi, J. Metastasis in urothelial carcinoma mimicking prostate cancer metastasis in Ga-68 prostate-specific membrane antigen positron emission tomography-computed tomography in a case of synchronous malignancy. Indian J. Nucl. 2016, 31, 222. [Google Scholar] [CrossRef]
- Campbell, S.P.; Baras, A.S.; Ball, M.W.; Kates, M.; Hahn, N.M.; Bivalacqua, T.J.; Johnson, M.H.; Pomper, M.G.; Allaf, M.E.; Rowe, S.P.; et al. Low levels of PSMA expression limit the utility of 18F-DCFPyL PET/CT for imaging urothelial carcinoma. Ann. Nucl. Med. 2018, 32, 69–74. [Google Scholar] [CrossRef]
- Wernicke, A.G.; Kim, S.; Liu, H.; Bander, N.H.; Pirog, E.C. Prostate-specific membrane antigen (PSMA) expression in the neovasculature of gynecologic malignancies: Implications for PSMA-targeted therapy. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, G.; Grech, C.; Pils, D.; Pammer, J.; Neudert, B.; Pötsch, N.; Baltzer, P.; Traub-Weidinger, T.; Seebacher, V.; Aust, S. Prostate-Specific Membrane Antigen (PSMA) Expression in Tumor-Associated Neovasculature Is an Independent Prognostic Marker in Patients with Ovarian Cancer. J. Pers. Med. 2022, 12, 551. [Google Scholar] [CrossRef] [PubMed]
- Aide, N.; Poulain, L.; Elie, N.; Briand, M.; Giffard, F.; Blanc-Fournier, C.; Joly, F.; Lasnon, C. A PSMA-targeted theranostic approach is unlikely to be efficient in serous ovarian cancers. EJNMMI Res. 2021, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S.; Reuter, V.E.; Heston, W.D.; Bander, N.H.; Grauer, L.S.; Gaudin, P.B. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999, 59, 3192–3198. [Google Scholar] [PubMed]
- Haffner, M.C.; Laimer, J.; Chaux, A.; Schäfer, G.; Obrist, P.; Brunner, A.; Kronberger, I.E.; Laimer, K.; Gurel, B.; Koller, J.B.; et al. High expression of prostate-specific membrane antigen in the tumor-associated neo-vasculature is associated with worse prognosis in squamous cell carcinoma of the oral cavity. Mod. Pathol. 2012, 25, 1079–1085. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Snow, H.; Hazell, S.; Francis, N.; Mohammed, K.; O’Neill, S.; Davies, E.; Mansfield, D.; Messiou, C.; Hujairi, N.; Nicol, D.; et al. Prostate-specific membrane antigen expression in melanoma metastases. J. Cutan. Pathol. 2020, 47, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Brada, M.D.; Rushing, E.J.; Bächinger, D.; Zoller, L.; Burger, I.A.; Hüllner, M.W.; Moch, H.; Huber, A.; Eckhard, A.H.; Rupp, N.J. Immunohistochemical expression pattern of theragnostic targets SSTR2 and PSMA in endolymphatic sac tumors: A single institution case series. Head Neck Pathol. 2022, 11, 1–7. [Google Scholar] [CrossRef]
- Simsek, D.H.; Civan, C.; Ekenel, M.; Kuyumcu, S.; Sanli, Y. 177Lu-PSMA therapy for metastatic testicular mixed germ cell tumor. Clin. Nucl. Med. 2021, 46, 415–418. [Google Scholar] [CrossRef]
- Milowsky, M.I.; Nanus, D.M.; Kostakoglu, L.; Sheehan, C.E.; Vallabhajosula, S.; Goldsmith, S.J.; Ross, J.S.; Bander, N.H. Vascular targeted therapy with anti–prostate-specific membrane antigen monoclonal antibody J591 in advanced solid tumors. J. Clin. Oncol. 2007, 25, 540–547. [Google Scholar] [CrossRef]
- Abramova, O.B.; Kaplan, M.A.; Grin, M.A.; Yuzhakov, V.V.; Suvorov, N.V.; Mironov, A.F.; Drozhzhina, V.V.; Churikova, T.P.; Kozlovtseva, E.A.; Bandurko, L.N.; et al. Photodynamic therapy of melanoma b16 with chlorin E6 conjugated with a PSMA-ligand. Bull. Exp. Biol. Med. 2021, 171, 468–471. [Google Scholar] [CrossRef]
- Civan, C.; Isik, E.G.; Simsek, D.H. Metastatic poorly differentiated thyroid cancer with heterogeneous distribution of 18F-FDG, 68Ga-DOTATATE, and 68Ga-PSMA on PET/CT. Clin. Nucl. Med. 2021, 46, e212–e213. [Google Scholar] [CrossRef] [PubMed]
- Lengana, T.; Lawal, I.O.; Mokoala, K.; Vorster, M.; Sathekge, M.M. 68Ga-PSMA: A one-stop shop in radioactive iodine refractory thyroid cancer? Nucl. Med. Mol. Imaging 2019, 53, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Lütje, S.; Gomez, B.; Cohnen, J.; Umutlu, L.; Gotthardt, M.; Poeppel, T.D.; Bockisch, A.; Rosenbaum-Krumme, S. Imaging of prostate-specific membrane antigen expression in metastatic differentiated thyroid cancer using 68Ga-HBED-CC-PSMA PET/CT. Clin. Nucl. Med. 2017, 42, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, P.; Russell, J.; Rooper, L.M.; Ladenson, P.W.; Pomper, M.G.; Rowe, S.P. The prostate-specific membrane antigen (PSMA)-targeted radiotracer 18F-DCFPyL detects tumor neovasculature in metastatic, advanced, radioiodine-refractory, differentiated thyroid cancer. Med. Oncol. 2020, 37, 98. [Google Scholar] [CrossRef]
- Taywade, S.K.; Damle, N.A.; Bal, C. PSMA Expression in papillary thyroid carcinoma: Opening a new horizon in management of thyroid cancer? Clin. Nucl. Med 2016, 41, e263–e265. [Google Scholar] [CrossRef]
- Sasikumar, A.; Joy, A.; Pillai, M.R.A.; Oommen, K.E.; Jayakumar, R. Rare case of intratracheal metastasis detected on 68Ga-Prostate-specific membrane antigen PET/CT scan in a case of thyroglobulin elevated negative iodine scan syndrome. Clin. Nucl. Med. 2018, 43, 282–283. [Google Scholar] [CrossRef]
- Zhao, B.; Dong, A.; Zuo, C. Prostate-specific membrane antigen-avid bone metastases from urothelial carcinoma of the bladder. Clin. Nucl. Med. 2022, 47, 892–894. [Google Scholar] [CrossRef]
- Parihar, A.S.; Sood, A.; Mittal, B.R.; Kumar, R.; Singh, H.; Dhatt, S.S. 68Ga-PSMA-HBED-CC PET/CT and 18F-FDG PET/CT in ewing sarcoma. Clin. Nucl. Med. 2020, 45, e57–e58. [Google Scholar] [CrossRef]
- Can, C.; Gündoğan, C.; Kömek, H. Is 68Ga-Prostate-specific membrane antigen PET/CT superior than 18F-FDG PET/CT for evaluation of metastatic osteosarcoma? Clin. Nucl. Med. 2021, 46, e233–e235. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauri, C.; Chiurchioni, L.; Russo, V.M.; Zannini, L.; Signore, A. PSMA Expression in Solid Tumors beyond the Prostate Gland: Ready for Theranostic Applications? J. Clin. Med. 2022, 11, 6590. https://doi.org/10.3390/jcm11216590
Lauri C, Chiurchioni L, Russo VM, Zannini L, Signore A. PSMA Expression in Solid Tumors beyond the Prostate Gland: Ready for Theranostic Applications? Journal of Clinical Medicine. 2022; 11(21):6590. https://doi.org/10.3390/jcm11216590
Chicago/Turabian StyleLauri, Chiara, Lorenzo Chiurchioni, Vincenzo Marcello Russo, Luca Zannini, and Alberto Signore. 2022. "PSMA Expression in Solid Tumors beyond the Prostate Gland: Ready for Theranostic Applications?" Journal of Clinical Medicine 11, no. 21: 6590. https://doi.org/10.3390/jcm11216590
APA StyleLauri, C., Chiurchioni, L., Russo, V. M., Zannini, L., & Signore, A. (2022). PSMA Expression in Solid Tumors beyond the Prostate Gland: Ready for Theranostic Applications? Journal of Clinical Medicine, 11(21), 6590. https://doi.org/10.3390/jcm11216590








