An Overview on the Primary Factors That Contribute to Non-Allergic Asthma in Children
Abstract
1. Introduction
1.1. Exercise-Induced Asthma (EIA)
1.2. Virus-Associated Asthma
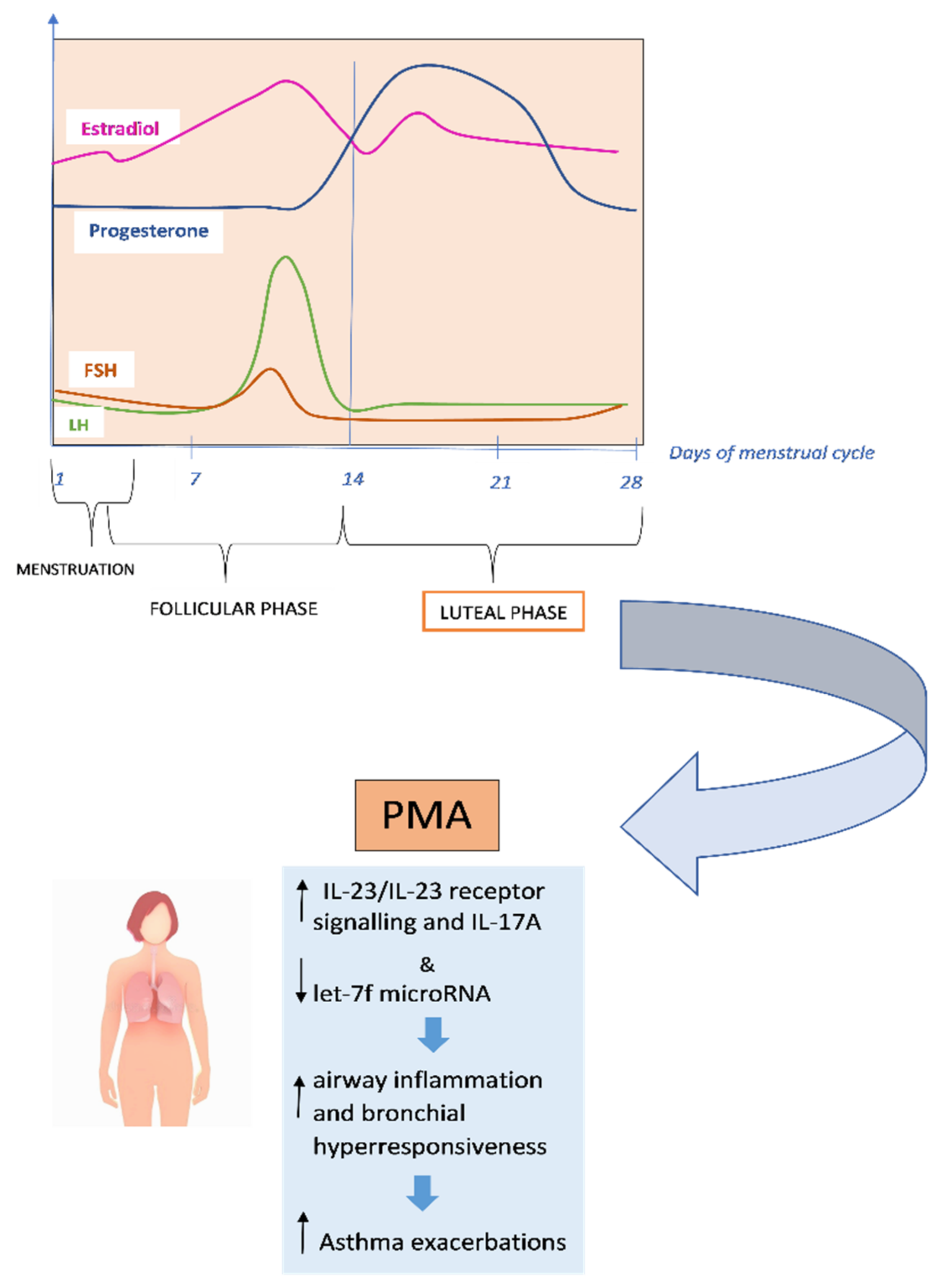
1.3. Premenstrual Asthma
1.4. Air Pollution and Risk of Developing Childhood Asthma
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miraglia del Giudice, M.; Piacentini, G.L.; Capasso, M.; Capristo, C.; Maiello, N.; Boner, A.L.; Capristo, A.F. Formoterol, montelukast, and budesonide in asthmatic children: Effect on lung function and exhaled nitric oxide. Respir. Med. 2007, 101, 1809–1813. [Google Scholar] [CrossRef]
- Di Cicco, M.E.; Leone, M.; Scavone, M.; Del Giudice, M.M.; Licari, A.; Duse, M.; Brambilla, I.; Ciprandi, G.; Caffarelli, C.; Tosca, M. Intermittent and mild persistent asthma: How therapy has changed. Acta Biomed. 2021, 92, e2021523. [Google Scholar]
- Lo Valvo, L.; Leonardi, S.; Marseglia, G.L.; Del Giudice, M.M.; Salpietro, C.; Ciprandi, G.; La Rosa, M. Inhalation therapy in asthmatic and not asthmatic children. Int. J. Immunopathol. Pharmacol. 2011, 24, 61–67. [Google Scholar] [CrossRef]
- Licari, A.; Ciprandi, G.; Marseglia, G.L.; Silvestri, M.; Tosca, M.A.; Anastasio, E.; Brambilla, I.; Caffarelli, C.; Castagnoli, R.; Chini, L.N. Asthma in children and adolescents: The ControL’Asma project. Acta Biomed. 2020, 91, e2020002. [Google Scholar]
- Murphy, V.E.; Gibson, P.G. Premenstrual Asthma: Prevalence, Cycle-to-Cycle Variability and Relationship to Oral Contraceptive Use and Menstrual Symptoms. J. Asthma 2009, 45, 696–704. [Google Scholar] [CrossRef]
- Reddel, H.K.; Bacharier, L.B.; Bateman, E.D.; Brightling, C.E.; Brusselle, G.G.; Buhl, R.; Cruz, A.A.; Duijts, L.; Drazen, J.M.; FitzGerald, J.M.; et al. Global Initiative for Asthma Strategy 2021: Executive summary and rationale for key changes. Eur. Respir. J. 2021, 59, 2102730. [Google Scholar] [CrossRef]
- Jones, T.L.; Neville, D.M.; Chauhan, A.J. Diagnosis and treatment of severe asthma: A phenotype-based approach. Clin. Med. 2018, 18, S36–S40. [Google Scholar] [CrossRef]
- Indinnimeo, L.; Chiappini, E.; Miraglia Del Giudice, M.; Capristo, C.; Cardinale, F.; Cazzato, S.; Chiamenti, G.; Chinellato, I.; Corsello, G.; Cutrera, R.; et al. Guideline on management of the acute asthma attack in children by Italian Society of Pediatrics. Ital. J. Pediatr. 2018, 44, 46. [Google Scholar] [CrossRef]
- Miraglia del Giudice, M.; Matera, M.G.; Capristo, C.; Conte, M.; Santaniello, F.; Chinellato, I.; Leonardi, S.; Miraglia del Giudice, M.C.; Perrone, L. LABAs in asthmatic children: Highlights and new inside. Pulm. Pharmacol. Ther. 2013, 26, 540–543. [Google Scholar] [CrossRef]
- Indinnimeo, L.; Bertuola, F.; Cutrera, R.; De Benedictis, F.M.; Di Pietro, P.; Duse, M.; Gianiorio, P.; Indirli, G.; La Grutta, S.; La Rosa, M.; et al. Clinical evaluation and treatment of acute asthma exacerbations in children. Int. J. Immunopathol. Pharmacol. 2009, 22, 867–878. [Google Scholar] [CrossRef]
- Ciprandi, G.; Cuppari, C.; Salpietro, A.M.; Tosca, M.A.; Rigoli, L.; Grasso, L.; La Rosa, M.; Marseglia, G.L.; Del Giudice, M.M.; Salpietro, C. Serum IL-23 Strongly and Inversely Correlates with FEV1 in Asthmatic Children. Int. Arch. Allergy Immunol. 2012, 159, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Decimo, F.; Capristo, C.; Amelio, R.; Maiello, N.; Capristo, A.F.; Miraglia Del Giudice, M. Evaluation of bronchial hyperreactivity with mannitol dry powder challenge test in a paediatric population with intermittent allergic asthma or allergic rhinitis. Int. J. Immunopathol. Pharmacol. 2011, 24, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Tosca, M.A.; Schiavetti, I.; Medone, E.; del Giudice, M.M.; Ciprandi, G. Role of FEF25–75 in children sent by primary care paediatricians for asthma diagnosis. Acta Paediatr. 2022, 2022, 1–2. [Google Scholar]
- Asero, R.; Tripodi, S.; Dondi, A.; Di Rienzo Businco, A.; Sfika, I.; Bianchi, A.; Candelotti, P.; Caffarelli, C.; Povesi Dascola, C.; Ricci, G.; et al. Prevalence and Clinical Relevance of IgE Sensitization to Profilin in Childhood: A Multicenter Study. Int. Arch. Allergy Immunol. 2015, 168, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Baos, S.; Calzada, D.; Cremades-Jimeno, L.; Sastre, J.; Picado, C.; Quiralte, J.; Florido, F.; Lahoz, C.; Cárdaba, B. Nonallergic Asthma and Its Severity: Biomarkers for Its Discrimination in Peripheral Samples. Front. Immunol. 2018, 9, 1416. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, M.M.; Licari, A.; Brambilla, I.; Tosca, M.A.; Ciprandi, G. Allergen Immunotherapy in Pediatric Asthma: A Pragmatic Point of View. Child 2020, 7, 58. [Google Scholar] [CrossRef]
- Fahy, J.V. Type 2 inflammation in asthma--present in most, absent in many. Nat. Rev. Immunol. 2015, 15, 57–65. [Google Scholar] [CrossRef]
- Peters, M.C.; Mekonnen, Z.K.; Yuan, S.; Bhakta, N.R.; Woodruff, P.G.; Fahy, J.V. Measures of gene expression in sputum cells can identify TH2-high and TH2-low subtypes of asthma. J. Allergy Clin. Immunol. 2014, 133, 388–394. [Google Scholar] [CrossRef]
- Ciprandi, G.; Caimmi, D.; del Giudice, M.M.; La Rosa, M.; Salpietro, C.; Marseglia, G.L. Recent developments in United airways disease. Allergy Asthma Immunol. Res. 2012, 4, 171–177. [Google Scholar] [CrossRef]
- Green, B.J.; Wiriyachaiporn, S.; Grainge, C.; Rogers, G.B.; Kehagia, V.; Lau, R.; Carroll, M.P.; Bruce, K.D.; Howarth, P.H. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS ONE 2014, 9, e100645. [Google Scholar]
- Uddin, M.; Nong, G.; Ward, J.; Seumois, G.; Prince, L.R.; Wilson, S.J.; Cornelius, V.; Dent, G.; Djukanović, R. Prosurvival activity for airway neutrophils in severe asthma. Thorax 2010, 65, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Bullens, D.M.A.; Truyen, E.; Coteur, L.; Dilissen, E.; Hellings, P.W.; Dupont, L.J.; Ceuppens, J.L. IL-17 mRNA in sputum of asthmatic patients: Linking T cell driven inflammation and granulocytic influx? Respir. Res. 2006, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Raedler, D.; Ballenberger, N.; Klucker, E.; Böck, A.; Otto, R.; Prazeres Da Costa, O.; Holst, O.; Illig, T.; Buch, T.; Von Mutius, E.; et al. Identification of novel immune phenotypes for allergic and nonallergic childhood asthma. J. Allergy Clin. Immunol. 2015, 135, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.P. Asthma phenotypes: Nonallergic (intrinsic) asthma. J. Allergy Clin. Immunol. Pract. 2014, 2, 650–652. [Google Scholar] [CrossRef] [PubMed]
- Panettieri, R.A. Neutrophilic and Pauci-immune Phenotypes in Severe Asthma. Immunol. Allergy Clin. N. Am. 2016, 36, 569–579. [Google Scholar] [CrossRef]
- Kostakou, E.; Kaniaris, E.; Filiou, E.; Vasileiadis, I.; Katsaounou, P.; Tzortzaki, E.; Koulouris, N.; Koutsoukou, A.; Rovina, N. Acute Severe Asthma in Adolescent and Adult Patients: Current Perspectives on Assessment and Management. J. Clin. Med. 2019, 8, 1283. [Google Scholar] [CrossRef]
- Novak, N.; Bieber, T. Allergic and nonallergic forms of atopic diseases. J. Allergy Clin. Immunol. 2003, 112, 252–262. [Google Scholar] [CrossRef]
- Klain, A.; Indolfi, C.; Dinardo, G.; Contieri, M.; Decimo, F.; Miraglia Del Giudice, M. Exercise-Induced Bronchoconstriction in Children. Front. Med. 2021, 8, 814976. [Google Scholar] [CrossRef]
- Lin, L.L.; Huang, S.J.; Ou, L.S.; Yao, T.C.; Tsao, K.C.; Yeh, K.W.; Huang, J.L. Exercise-induced bronchoconstriction in children with asthma: An observational cohort study. J. Microbiol. Immunol. Infect. 2019, 52, 471–479. [Google Scholar] [CrossRef]
- Brooks, E.G.; Hayden, M.L. Exercise-induced asthma. Nurs. Clin. North Am. 2003, 38, 689–696. [Google Scholar] [CrossRef]
- Minic, P.B.; Sovtic, A.D. Exercise intolerance and exercise-induced bronchoconstriction in children. Front. Biosci. 2017, 9, 21–32. [Google Scholar] [CrossRef][Green Version]
- Leonardi, S.; Vitaliti, G.; Marseglia, G.L.; Caimmi, D.; Lionetti, E.; Miraglia Del Giudice, M.; Salpietro, C.; Spicuzza, L.; Ciprandi, G.; La Rosa, M. Function of the airway epithelium in asthma. J. Biol. Regul. Homeost. Agents 2012, 26, S41–S48. [Google Scholar]
- Parsons, J.P.; Hallstrand, T.S.; Mastronarde, J.G.; Kaminsky, D.A.; Rundell, K.W.; Hull, J.H.; Storms, W.W.; Weiler, J.M.; Cheek, F.M.; Wilson, K.C.; et al. An official American Thoracic Society clinical practice guideline: Exercise-induced bronchoconstriction. Am. J. Respir. Crit. Care Med. 2013, 187, 1016–1027. [Google Scholar] [CrossRef]
- Weiler, J.M.; Brannan, J.D.; Randolph, C.C.; Hallstrand, T.S.; Parsons, J.; Silvers, W.; Storms, W.; Zeiger, J.; Bernstein, D.I.; Blessing-Moore, J. Exercise-induced bronchoconstriction update-2016. J. Allergy Clin. Immunol. 2016, 138, 1292–1295.e36. [Google Scholar] [CrossRef]
- Duong, M.L.; Subbarao, P.; Adelroth, E.; Obminski, G.; Strinich, T.; Inman, M.; Pedersen, S.; O’Byrne, P.M. Sputum eosinophils and the response of exercise-induced bronchoconstriction to corticosteroid in asthma. Chest 2008, 133, 404–411. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Goto, H.; Kobayashi, H.; Chow, W.C.; Peng, W.H.; Tang, R.B. Changes in Serum Eosinophil Cationic Protein Levels after Exercise Challenge in Asthmatic Children. J. Asthma 2009, 44, 569–573. [Google Scholar] [CrossRef]
- Tahan, F.; Saraymen, R.; Gumus, H. The role of lipoxin A4 in exercise-induced bronchoconstriction in asthma. J. Asthma 2008, 45, 161–164. [Google Scholar] [CrossRef]
- Hallstrand, T.S.; Chi, E.Y.; Singer, A.G.; Gelb, M.H.; Henderson, W.R. Secreted phospholipase A2 group X overexpression in asthma and bronchial hyperresponsiveness. Am. J. Respir. Crit. Care Med. 2007, 176, 1072–1078. [Google Scholar] [CrossRef]
- Zietkowski, Z.; Skiepko, R.; Tomasiak, M.M.; Bodzenta-Lukaszyk, A. Endothelin-1 in exhaled breath condensate of allergic asthma patients with exercise-induced bronchoconstriction. Respir. Res. 2007, 8, 76. [Google Scholar] [CrossRef]
- Parsons, J.P.; Mastronarde, J.G. Exercise-induced asthma. Curr. Opin. Pulm. Med. 2009, 15, 25–28. [Google Scholar] [CrossRef]
- Del Giacco, S.R.; Firinu, D.; Bjermer, L.; Carlsen, K.-H. Exercise and asthma: An overview. Eur. Clin. Respir. J. 2015, 2, 27984. [Google Scholar] [CrossRef]
- Randolph, C. Pediatric exercise-induced bronchoconstriction: Contemporary developments in epidemiology, pathogenesis, presentation, diagnosis, and therapy. Curr. Allergy Asthma Rep. 2013, 13, 662–671. [Google Scholar] [CrossRef]
- Dryden, D.M.; Spooner, C.H.; Stickland, M.K.; Vandermeer, B.; Tjosvold, L.; Bialy, L.; Wong, K.; Rowe, B.H. Exercise-Induced Bronchoconstriction and Asthma. Evid. Rep. Technol. Assess. 2010, 189, 1–154. [Google Scholar]
- Godfrey, S.; Springer, C.; Bar-Yishay, E.; Avital, A. Cut-off points defining normal and asthmatic bronchial reactivity to exercise and inhalation challenges in children and young adults. Eur. Respir. J. 1999, 14, 659–668. [Google Scholar] [CrossRef]
- Del Giudice, M.M.; Pezzulo, A.; Capristo, C.; Alterio, E.; Caggiano, S.; de Benedictis, D.; Capristo, A.F. Leukotriene modifiers in the treatment of asthma in children. Ther. Adv. Respir. Dis. 2009, 3, 245–251. [Google Scholar] [CrossRef]
- Duse, M.; Santamaria, F.; Verga, M.C.; Bergamini, M.; Simeone, G.; Leonardi, L.; Tezza, G.; Bianchi, A.; Capuano, A.; Cardinale, F. Correction: Inter-society consensus for the use of inhaled corticosteroids in infants, children and adolescents with airway diseases. Ital. J. Pediatr. 2022, 48, 35. [Google Scholar] [CrossRef]
- Piacentini, G.L.; Peroni, D.G.; Miraglia Del Giudice, M.; Bodini, A.; Costella, S.; Vicentini, L.; Boner, A.L. Effect of montelukast on exhaled NO in asthmatic children exposed to relevant allergens. Pediatr. Allergy Immunol. 2002, 13, 137–139. [Google Scholar] [CrossRef]
- Wanrooij, V.H.M.; Willeboordse, M.; Dompeling, E.; Van De Kant, K.D.G. Exercise training in children with asthma: A systematic review. Br. J. Sports Med. 2014, 48, 1024–1031. [Google Scholar] [CrossRef]
- Tancredi, G.; Quattrucci, S.; Scalercio, F.; De Castro, G.; Zicari, A.M.; Bonci, E.; Cingolani, S.; Indinnimeo, L.; Midulla, F. 3-min step test and treadmill exercise for evaluating exercise-induced asthma. Eur. Respir. J. 2004, 23, 569–574. [Google Scholar] [CrossRef]
- Milanese, M.; Miraglia del Giudice, E.; Peroni, D.G. Asthma, exercise and metabolic dysregulation in paediatrics. Allergol. Immunopathol. 2019, 47, 289–294. [Google Scholar] [CrossRef]
- Beuther, D.A.; Martin, R.J. Efficacy of a heat exchanger mask in cold exercise-induced asthma. Chest 2006, 129, 1188–1193. [Google Scholar] [CrossRef]
- Brenner, A.M.; Loren, M.L.; Weiser, P.C.; Krogh, L.A. Effectiveness of a Portable Face Mask in Attenuating Exercise-Induced Asthma. JAMA 1980, 244, 2196–2198. [Google Scholar] [CrossRef]
- Lorente, F.; Laffond, E.; Moreno, E.; Dávila, I. Viral infection and asthma: Immunologic mechanisms. Allergol. Immunopathol. 2001, 29, 126–133. [Google Scholar] [CrossRef]
- Hansbro, N.G.; Horvat, J.C.; Wark, P.A.; Hansbro, P.M. Understanding the mechanisms of viral induced asthma: New therapeutic directions. Pharmacol. Ther. 2008, 117, 313–353. [Google Scholar] [CrossRef]
- Johnston, S.L.; Pattemore, P.K.; Sanderson, G.; Smith, S.; Lampe, F.; Josephs, L.; Symington, P.; Toole, S.; Myint, S.H.; Tyrrell, D.A.J.; et al. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ 1995, 310, 1225. [Google Scholar] [CrossRef]
- Yoo, J.K.; Kim, T.S.; Hufford, M.M.; Braciale, T.J. Viral infection of the lung: Host response and sequelae. J. Allergy Clin. Immunol. 2013, 132, 1263–1276. [Google Scholar] [CrossRef]
- Wu, P.; Hartert, T.V. Evidence for a causal relationship between respiratory syncytial virus infection and asthma. Expert Rev Anti Infect. Ther. 2011, 9, 731–745. [Google Scholar] [CrossRef]
- Busse, W.W.; Lemanske, R.F.; Gern, J.E. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet 2010, 376, 826–834. [Google Scholar] [CrossRef]
- Jackson, D.J.; Johnston, S.L. The role of viruses in acute exacerbations of asthma. J. Allergy Clin. Immunol. 2010, 125, 1178–1187. [Google Scholar] [CrossRef]
- Stewart, C.J.; Hasegawa, K.; Wong, M.C.; Ajami, N.J.; Petrosino, J.F.; Piedra, P.A.; Espinola, J.A.; Tierney, C.N.; Camargo, C.A.; Mansbach, J.M. Respiratory Syncytial Virus and Rhinovirus Bronchiolitis Are Associated With Distinct Metabolic Pathways. J. Infect. Dis. 2018, 217, 1160–1169. [Google Scholar] [CrossRef]
- Corne, J.M.; Marshall, C.; Smith, S.; Schreiber, J.; Sanderson, G.; Holgate, S.T.; Johnston, S.L. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: A longitudinal cohort study. Lancet 2002, 359, 831–834. [Google Scholar] [CrossRef]
- Zhu, Z.; Tang, W.; Gwaltney, J.M.; Wu, Y.; Elias, J.A. Rhinovirus stimulation of interleukin-8 in vivo and in vitro: Role of NF-kappaB. Am. J. Physiol. 1997, 273, L814–L824. [Google Scholar] [CrossRef] [PubMed]
- Terajima, M.; Yamaya, M.; Sekizawa, K.; Okinaga, S.; Suzuki, T.; Yamada, N.; Nakayama, K.; Ohrui, T.; Oshima, T.; Numazaki, Y.; et al. Rhinovirus infection of primary cultures of human tracheal epithelium: Role of ICAM-1 and IL-1beta. Am. J. Physiol. 1997, 273, L749–L759. [Google Scholar] [CrossRef] [PubMed]
- Message, S.D.; Laza-Stanca, V.; Mallia, P.; Parker, H.L.; Zhu, J.; Kebadze, T.; Contoli, M.; Sanderson, G.; Kon, O.M.; Papi, A.; et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc. Natl. Acad. Sci. USA 2008, 105, 13562–13567. [Google Scholar] [CrossRef]
- Legg, J.P.; Hussain, I.R.; Warner, J.A.; Johnston, S.L.; Warner, J.O. Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am. J. Respir. Crit. Care Med. 2003, 168, 633–639. [Google Scholar] [CrossRef]
- Román, M.; Calhoun, W.J.; Hinton, K.L.; Avendaño, L.F.; Simon, V.; Escobar, A.M.; Gaggero, A.; Díaz, P.V. Respiratory syncytial virus infection in infants is associated with predominant Th-2-like response. Am. J. Respir. Crit. Care Med. 1997, 156, 190–195. [Google Scholar] [CrossRef]
- Mejías, A.; Chávez-Bueno, S.; Jafri, H.S.; Ramilo, O. Respiratory syncytial virus infections: Old challenges and new opportunities. Pediatr. Infect. Dis. J. 2005, 24, S189–S197. [Google Scholar] [CrossRef]
- Al-nakib, W.; Tyrrell, D.A.J. Drugs against rhinoviruses. J. Antimicrob. Chemother. 1992, 30, 115–117. [Google Scholar] [CrossRef]
- Edwards, M.R.; Johnson, M.W.; Johnston, S.L. Combination therapy: Synergistic suppression of virus-induced chemokines in airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2006, 34, 616–624. [Google Scholar] [CrossRef]
- Skevaki, C.L.; Christodoulou, I.; Spyridaki, I.S.; Tiniakou, I.; Georgiou, V.; Xepapadaki, P.; Kafetzis, D.A.; Papadopoulos, N.G. Budesonide and formoterol inhibit inflammatory mediator production by bronchial epithelial cells infected with rhinovirus. Clin. Exp. Allergy 2009, 39, 1700–1710. [Google Scholar] [CrossRef]
- Darveaux, J.I.; Lemanske, R.F. Infection-related asthma. J. Allergy Clin. Immunol. Pract. 2014, 2, 658–663. [Google Scholar] [CrossRef]
- Kumar, S.; Roy, R.D.; Sethi, G.R.; Saigal, S.R. Mycoplasma pneumoniae infection and asthma in children. Trop. Doct. 2019, 49, 117–119. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Chen, C.; Liu, K. Mycoplasma pneumoniae infection and risk of childhood asthma: A systematic review and meta-analysis. Microb. Pathog. 2021, 155, 104893. [Google Scholar] [CrossRef]
- Shah, R.; Newcomb, D.C. Sex Bias in Asthma Prevalence and Pathogenesis. Front. Immunol. 2018, 9, 2997. [Google Scholar] [CrossRef]
- Fuseini, H.; Newcomb, D.C. Mechanisms Driving Gender Differences in Asthma. Curr. Allergy Asthma Rep. 2017, 17, 19. [Google Scholar] [CrossRef]
- Matteis, M.; Polverino, F.; Spaziano, G.; Roviezzo, F.; Santoriello, C.; Sullo, N.; Bucci, M.R.; Rossi, F.; Polverino, M.; Owen, C.A.; et al. Effects of sex hormones on bronchial reactivity during the menstrual cycle. BMC Pulm. Med. 2014, 14, 108. [Google Scholar] [CrossRef]
- Yung, J.A.; Fuseini, H.; Newcomb, D.C. Hormones, sex, and asthma. Ann. Allergy Asthma Immunol. 2018, 120, 488–494. [Google Scholar] [CrossRef]
- Bernstein, J.A. Progestogen Sensitization: A Unique Female Presentation of Anaphylaxis. Curr. Allergy Asthma Reports 2020, 20, 4. [Google Scholar] [CrossRef]
- Tan, K.S. Premenstrual asthma: Epidemiology, pathogenesis and treatment. Drugs 2001, 61, 2079–2086. [Google Scholar] [CrossRef]
- Fuseini, H.; Yung, J.A.; Cephus, J.Y.; Zhang, J.; Goleniewska, K.; Polosukhin, V.V.; Peebles, R.S.; Newcomb, D.C. Testosterone Decreases House Dust Mite-Induced Type 2 and IL-17A-Mediated Airway Inflammation. J. Immunol. 2018, 201, 1843–1854. [Google Scholar] [CrossRef]
- Newcomb, D.C.; Cephus, J.Y.; Boswell, M.G.; Fahrenholz, J.M.; Langley, E.W.; Feldman, A.S.; Zhou, W.; Dulek, D.E.; Goleniewska, K.; Woodward, K.B.; et al. Estrogen and progesterone decrease let-7f microRNA expression and increase IL-23/IL-23 receptor signaling and IL-17A production in patients with severe asthma. J. Allergy Clin. Immunol. 2015, 136, 1025–1034.e11. [Google Scholar] [CrossRef]
- Semik-Orzech, A.; Skoczyński, S.; Pierzchała, W. Serum estradiol concentration, estradiol-to-progesterone ratio and sputum IL-5 and IL-8 concentrations are increased in luteal phase of the menstrual cycle in perimenstrual asthma patients. Eur. Ann. Allergy Clin. Immunol. 2017, 49, 161–170. [Google Scholar] [CrossRef]
- Sánchez-Ramos, J.L.; Pereira-Vega, A.R.; Alvarado-Gómez, F.; Maldonado-Pérez, J.A.; Svanes, C.; Gómez-Real, F. Risk factors for premenstrual asthma: A systematic review and meta-analysis. Expert Rev. Respir. Med. 2017, 11, 57–72. [Google Scholar] [CrossRef]
- Thornton, J.; Lewis, J.; Lebrun, C.M.; Licskai, C.J. Clinical characteristics of women with menstrual-linked asthma. Respir. Med. 2012, 106, 1236–1243. [Google Scholar] [CrossRef][Green Version]
- 2022 GINA Main Report—Global Initiative for Asthma—GINA. Available online: https://ginasthma.org/gina-reports/ (accessed on 28 June 2022).
- Tan, K.S.; McFarlane, L.C.; Lipworth, B.J. Modulation of airway reactivity and peak flow variability in asthmatics receiving the oral contraceptive pill. Am. J. Respir. Crit. Care Med. 1997, 155, 1273–1277. [Google Scholar] [CrossRef]
- Vélez-Ortega, A.C.; Temprano, J.; Reneer, M.C.; Ellis, G.I.; McCool, A.; Gardner, T.; Khosravi, M.; Marti, F. Enhanced generation of suppressor T cells in patients with asthma taking oral contraceptives. J. Asthma 2013, 50, 223. [Google Scholar] [CrossRef]
- Calcaterra, V.; Nappi, R.E.; Farolfi, A.; Tiranini, L.; Rossi, V.; Regalbuto, C.; Zuccotti, G. Perimenstrual Asthma in Adolescents: A Shared Condition in Pediatric and Gynecological Endocrinology. Child 2022, 9, 233. [Google Scholar] [CrossRef]
- Pereira-Vega, A.; Sánchez Ramos, J.L.; Maldonado Pérez, J.A.; Vázquez Oliva, R.; Bravo Nieto, J.M.; Vázquez Rico, I.; Ignacio García, J.M.; Romero Palacios, P.; Alwakil Olbah, M.; Medina Gallardo, J.F. Premenstrual asthma and leukotriene variations in the menstrual cycle. Allergol. Immunopathol. 2012, 40, 368–373. [Google Scholar] [CrossRef]
- Tiotiu, A.I.; Novakova, P.; Nedeva, D.; Chong-Neto, H.J.; Novakova, S.; Steiropoulos, P.; Kowal, K. Impact of Air Pollution on Asthma Outcomes. Int. J. Environ. Res. Public. Health 2020, 17, 6212. [Google Scholar] [CrossRef]
- Gowers, A.M.; Cullinan, P.; Ayres, J.G.; Anderson, H.R.; Strachan, D.P.; Holgate, S.T.; Mills, I.C.; Maynard, R.L. Does outdoor air pollution induce new cases of asthma? Biological plausibility and evidence; a review. Respirology 2012, 17, 887–898. [Google Scholar] [CrossRef]
- Glencross, D.A.; Ho, T.R.; Camiña, N.; Hawrylowicz, C.M.; Pfeffer, P.E. Air pollution and its effects on the immune system. Free Radic. Biol Med. 2020, 151, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Bowatte, G.; Lodge, C.; Lowe, A.J.; Erbas, B.; Perret, J.; Abramson, M.J.; Matheson, M.; Dharmage, S.C. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: A systematic review and a meta-analysis of birth cohort studies. Allergy 2015, 70, 245–256. [Google Scholar] [PubMed]
- Khreis, H.; Kelly, C.; Tate, J.; Parslow, R.; Lucas, K.; Nieuwenhuijsen, M. Exposure to traffic-related air pollution and risk of development of childhood asthma: A systematic review and meta-analysis. Environ Int. 2017, 100, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Gehring, U.; Wijga, A.H.; Koppelman, G.H.; Vonk, J.M.; Smit, H.A.; Brunekreef, B. Air pollution and the development of asthma from birth until young adulthood. Eur. Respir. J. 2020, 56, 2000147. [Google Scholar] [CrossRef] [PubMed]
- Eguiluz-Gracia, I.; Mathioudakis, A.G.; Bartel, S.; Vijverberg, S.J.H.; Fuertes, E.; Comberiati, P.; Cai, Y.S.; Tomazic, P.V.; Diamant, Z.; Vestbo, J.; et al. The need for clean air: The way air pollution and climate change affect allergic rhinitis and asthma. Allergy 2020, 75, 2170–2184. [Google Scholar] [CrossRef] [PubMed]
- Di Cicco, M.; Sepich, M.; Ragazzo, V.; Peroni, D.G.; Comberiati, P. Potential effects of E-cigarettes and vaping on pediatric asthma. Minerva Pediatr. 2020, 72, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Xian, S.; Chen, Y. E-cigarette users are associated with asthma disease: A meta-analysis. Clin. Respir. J. 2021, 15, 457–466. [Google Scholar] [CrossRef]
- Drago, G.; Perrino, C.; Canepari, S.; Ruggieri, S.; L’Abbate, L.; Longo, V.; Colombo, P.; Frasca, D.; Balzan, M.; Cuttitta, G.; et al. Relationship between domestic smoking and metals and rare earth elements concentration in indoor PM 2.5. Environ. Res. 2018, 165, 71–80. [Google Scholar] [CrossRef]
- Gillespie-Bennett, J.; Pierse, N.; Wickens, K.; Crane, J.; Howden-Chapman, P.; Shields, H.; Viggers, H.; Free, S.; Phipps, R.; Fjallstrom, P.; et al. The respiratory health effects of nitrogen dioxide in children with asthma. Eur. Respir. J. 2011, 38, 303–309. [Google Scholar] [CrossRef]
- Nurmatov, U.B.; Tagiyeva, N.; Semple, S.; Devereux, G.; Sheikh, A. Volatile organic compounds and risk of asthma and allergy: A systematic review. Eur. Respir. Rev. 2015, 24, 92–101. [Google Scholar] [CrossRef]
- Maung, T.Z.; Bishop, J.E.; Holt, E.; Turner, A.M.; Pfrang, C. Indoor Air Pollution and the Health of Vulnerable Groups: A Systematic Review Focused on Particulate Matter (PM), Volatile Organic Compounds (VOCs) and Their Effects on Children and People with Pre-Existing Lung Disease. Int. J. Environ. Res. Public Health 2022, 19, 8752. [Google Scholar] [CrossRef]
- Annesi-Maesano, I. The air of Europe: Where are we going? Eur. Respir. Rev. 2017, 26, 170024. [Google Scholar] [CrossRef]
- EUR-Lex-32016L2284-EN. EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_2016.344.01.0001.01.ENG&toc=OJ:L:2016:344:TOC (accessed on 25 September 2022).
- Yoshihara, S.; Kanno, N.; Yamada, Y.; Ono, M.; Fukuda, N.; Numata, M.; Abe, T.; Arisaka, O. Effects of early intervention with inhaled sodium cromoglycate in childhood asthma. Lung 2006, 184, 63–72. [Google Scholar] [CrossRef]
- Fitzgerald, D.A.; Mellis, C.M. Leukotriene receptor antagonists in virus-induced wheezing: Evidence to date. Treat. Respir. Med. 2006, 5, 407–417. [Google Scholar] [CrossRef]
- Debelleix, S.; Siao-Him Fa, V.; Begueret, H.; Berger, P.; Kamaev, A.; Ousova, O.; Marthan, R.; Fayon, M. Montelukast reverses airway remodeling in actively sensitized young mice. Pediatr. Pulmonol. 2018, 53, 701–709. [Google Scholar] [CrossRef]
- Guan, R.; Liu, Y.; Ren, D.; Li, J.; Xu, T.; Hu, H. The efficacy and safety of fluticasone propionate/formoterol compared with fluticasone propionate/salmeterol in treating pediatric asthma: A systematic review and meta-analysis. J. Int. Med. Res. 2020, 48, 300060519889442. [Google Scholar] [CrossRef]
- Kahlon, G.K.; Pooni, P.A.; Bhat, D.; Dhooria, G.S.; Bhargava, S.; Arora, K.; Gill, K.S. Role of montelukast in multitrigger wheezers attending chest clinic in Punjab, India. Pediatr. Pulmonol. 2021, 56, 2530–2536. [Google Scholar] [CrossRef]
- Hilty, M.; Burke, C.; Pedro, H.; Cardenas, P.; Bush, A.; Bossley, C.; Davies, J.; Ervine, A.; Poulter, L.; Pachter, L.; et al. Disordered microbial communities in asthmatic airways. PLoS ONE 2010, 5, e8578. [Google Scholar] [CrossRef]
- García-Rivero, J.L. The Microbiome and Asthma. Arch. Bronconeumol. 2020, 56, 1–2. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220. [Google Scholar] [CrossRef]
- Di Cicco, M.; Pistello, M.; Jacinto, T.; Ragazzo, V.; Piras, M.; Freer, G.; Pifferi, M.; Perpni, D. Does lung microbiome play a causal or casual role in asthma? Pediatr Pulmonol. 2018, 53, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J. Asthma microbiome studies and the potential for new therapeutic strategies. Curr. Allergy Asthma Rep. 2013, 13, 453–461. [Google Scholar] [CrossRef] [PubMed][Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klain, A.; Dinardo, G.; Salvatori, A.; Indolfi, C.; Contieri, M.; Brindisi, G.; Decimo, F.; Zicari, A.M.; Miraglia del Giudice, M. An Overview on the Primary Factors That Contribute to Non-Allergic Asthma in Children. J. Clin. Med. 2022, 11, 6567. https://doi.org/10.3390/jcm11216567
Klain A, Dinardo G, Salvatori A, Indolfi C, Contieri M, Brindisi G, Decimo F, Zicari AM, Miraglia del Giudice M. An Overview on the Primary Factors That Contribute to Non-Allergic Asthma in Children. Journal of Clinical Medicine. 2022; 11(21):6567. https://doi.org/10.3390/jcm11216567
Chicago/Turabian StyleKlain, Angela, Giulio Dinardo, Alessandra Salvatori, Cristiana Indolfi, Marcella Contieri, Giulia Brindisi, Fabio Decimo, Anna Maria Zicari, and Michele Miraglia del Giudice. 2022. "An Overview on the Primary Factors That Contribute to Non-Allergic Asthma in Children" Journal of Clinical Medicine 11, no. 21: 6567. https://doi.org/10.3390/jcm11216567
APA StyleKlain, A., Dinardo, G., Salvatori, A., Indolfi, C., Contieri, M., Brindisi, G., Decimo, F., Zicari, A. M., & Miraglia del Giudice, M. (2022). An Overview on the Primary Factors That Contribute to Non-Allergic Asthma in Children. Journal of Clinical Medicine, 11(21), 6567. https://doi.org/10.3390/jcm11216567







