Abstract
Background: Limb–girdle muscular dystrophy R2 (LGMD R2) is most frequently misdiagnosed as immune-mediated necrotizing myopathy (IMNM). This study aimed to compare the clinicopathological data of IMNM and LGMD R2 to find distinguishing features. Methods: We retrospectively reassessed the medical data of patients with IMNM (n = 41) and LGMD R2 (n = 8) treated at Tongji Hospital from January 2017 to December 2021. Results: In our cohort, patients with LGMD R2 had a longer interval of onset to first visit, mild muscle weakness with late upper limb involvement, less myalgia, no cervical muscle weakness or dysphagia, no extramuscular organs affected except cardiac involvement, and lack of various autoantibodies, such as antinuclear antibodies. These features were completely reversed in IMNM. Moreover, thigh MRIs showed that muscle edema prominently affecting the adductor magnus was a characteristic of IMNM, while extensive fatty replacement was more common in LGMD R2 (p = 0.0086). Necrotic myofibers presented in both entities (p = 0.1693), while features such as ring/whorled and splitting myofibers were more often found in LGMD R2 (p = 0.0112 and p < 0.0001, respectively). Conversely, sarcoplasmic p62 expression was more pronounced in IMNM (p < 0.05). There were 4 of 8 (50%) patients with LGMD R2 initially considered as seronegative IMNM, and therefore unnecessarily treated with immunosuppressive drugs. Insufficient recognition of the early clinical, imaging, and histopathological features of LGMD R2 is the main reason for misdiagnosis. Conclusions: These findings may help clinicians differentiate seronegative IMNM and LGMD R2, reducing early misdiagnosis and mismanagement. Particularly, prominent adductor magnus edema on MRI and abundant p62 staining seem to be good markers for IMNM, while the presence of splitting myofibers is a crucial clue to early hereditary myopathy, including LGMD R2.
1. Introduction
Immune-mediated necrotizing myopathy (IMNM) is a recently described subtype of idiopathic inflammatory myopathies (IIMs), which are mainly defined by three subtypes according to different myositis-specific autoantibodies (MSAs), including anti-signal recognition particle (SRP) positive IMNM, anti-3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) positive IMNM, and IMNM with no known autoantibodies (seronegative IMNM) [1]. Patients with IMNM present with rapidly progressive muscle weakness, often requiring early aggressive immunotherapies [1]. Several adult-onset muscular dystrophies can mimic IMNM, leading to clinical misdiagnosis [2,3]. Dysferlinopathy is an autosomal recessively inherited muscular dystrophy caused by mutations in the DYSF gene, which encodes the muscle-specific protein dysferlin, and its deficiency leads to sarcolemma repair abnormalities and secondary inflammatory activation [4]. Dysferlinopathy presents with a distal Miyoshi myopathy (MM) phenotype, or more often, a proximal phenotype known as limb–girdle muscular dystrophy R2 (LGMD R2) (formerly LGMD 2B) [5].
With the in-depth understanding of IMNM in recent years, we noted that LGMD R2 is most frequently misdiagnosed as IMNM, especially seronegative IMNM. They have much more similar clinicopathological features, including proximal muscle weakness, remarkably elevated serum creatine kinase (CK), scattered myonecrosis on a muscle biopsy, a relative paucity of inflammatory infiltrates with macrophage predominance, and MAC deposition on the sarcolemma. Genetic testing is useful for distinguishing the two entities; however, most patients with early LGMD R2 lack the typical clinicopathological features. In this scenario, genetic testing is usually considered only in the case of no response to immunotherapies. Antibody testing for HMGCR or SRP is now readily available; however, it plays limited roles in distinguishing seronegative IMNM from LGMD R2. Therefore, the clinicopathological distinction between the two entities is important for early diagnosis and clinical decision making.
Although the features of LGMDR2 and IMNM have been described in previous studies [1,3,6,7], data fully describing the differences between the two entities are still lacking. In this study, we compared the clinicopathological features of IMNM and LGMD R2, investigated the causes of early misdiagnosis of LGMD R2 as seronegative IMNM, and identified some features contributing to the early diagnosis of seronegative IMNM and LGMD R2.
2. Materials and Methods
2.1. Patients
Muscle biopsies and medical data were obtained from the Department of Neurology at Tongji Hospital from January 2017 to December 2021. The diagnosis for IMNM met the clinico-sero-pathological criteria formulated by the European Neuromuscular Centre International Workshop [8]. The exclusion criteria included drug-, infection-, toxic-, and other MSA-associated myopathies, as well as endocrine myopathy [2,3]. Patients with insufficient medical data were also excluded. A total of 41 patients with IMNM were enrolled, including 23 patients with the anti-SRP autoantibody, 6 patients with the anti-HMGCR autoantibody, and 12 patients with seronegative IMNM. The diagnosis criteria for LGMD R2 are as follows: (1) progressive proximal muscle weakness; (2) pathogenic DYSF gene mutation confirmed by genetic analyses; (3) complete or nearly complete dysferlin protein loss (≤ 20%) in muscle biopsies demonstrated by Western blot (WB). A total of 8 patients with LGMD R2 were enrolled. Their pathogenic mutations and WB bands are shown in Supplementary Table S1 and Supplementary Figure S1, respectively.
2.2. Data Collection
All participants except 2 patients with LGMD R2 were tested for MSAs and myositis-associated autoantibodies (MAAs), including anti-SRP, -HMGCR, -Mi2, -TIF1-γ, -NXP-2, -SAE, -MDA5, -Jo1, -PL7, -PL12, -EJ, -OJ, -Ku, -PMScl100, -PMScl75, and -Ro52 autoantibodies by a commercial laboratory. A line blot immunoassay was used for detection of all MSAs and MAAs except HMGCR, which was performed by a cell-based assay. Connective tissue disease (CTD)-associated autoantibodies, such as antinuclear antibodies, anti-neutrophil cytoplasmic antibodies (ANCA) and rheumatoid factors, serum CK, and lactate dehydrogenase (LDH) levels were routinely performed.
Muscle magnetic resonance imaging (MRI) was performed to assess muscle edema, atrophy, fatty replacement, and myofascial edema. The degree of muscle edema and fatty replacement were graded by the area of muscle involvement as previously described [9]: normal muscle (score 1); <30% of the muscle (score 2); 30–60% of the muscle (score 3); and > 60% of the muscle (score 4). A lung CT scan was performed to detect interstitial lung disease (ILD). Cardiac abnormalities were evaluated by cardiac MRI (myocardial fibrosis, or ventricular/atrial dilatation or dysfunction) and echocardiogram (Echo) (systolic dysfunction or chamber dilatation). Muscle strength was assessed using the Medical Research Council (MRC) grading systems.
2.3. Genetic Analysis
Genetic analysis was performed at the genetic diagnostic center of Tongji Hospital. A whole exome sequencing was tested to identify the pathogenic variants, which was validated using sanger sequencing.
2.4. Western Blot
An anti-dysferlin antibody (NCL-hamlet, 1:1000, Leica, Wetzlar, Germany) was used for WB analysis as previously described [10].
2.5. Skeletal Muscle Biopsies, and Histological and Immunohistochemical Staining
A muscle biopsy was performed on all patients. Muscle specimens were frozen in isopentane cooled in liquid nitrogen and then stored in a refrigerator at −80 °C. Frozen muscle specimens were sliced into 7 μm sections for histological and immunohistochemical (IHC) staining. Histological staining, such as hematoxylin and eosin (H&E), NADH-tetrazolium reductase (NADH-TR), and acid phosphatase (AcP), was routinely performed. The following primary antibodies were used to recognize: CD4 (1:100, ABclonal, Wuhan, China), CD8 (1:2000, Proteintech, Wuhan, China), CD20, (1:50, ABclonal, Wuhan, China), CD68 (1:100, Santa Cruz Biotechnology, CA, USA), major histocompatibility complex class I (MHC-I, 1:400, Abcam, Cambridge, UK), C5b9 (1:50, DAKO, Copenhagen, Denmark), sequestosome 1 (p62, 1:400, Proteintech, Wuhan, China), and dysferlin (NLC-Hamlet, 1:40, Leica, Wetzlar, Germany).
2.6. Histological and IHC Analysis
Five high-power fields (HPFs) for each muscle specimen at 200× magnification were selected for further analysis. Average cell counts (positive staining/HPF) were used for semiquantitative analysis of CD68+ macrophages, CD4+ T cells, CD8+ T cells, and CD20+ B cells. For inflammatory cells, 0 = almost no staining (<5 cells/HPF), 1 = little staining (5–20 cells/HPF), 2 = moderate staining (21–50 cells/HPF), and 3 = abundant staining (> 50 cells/HPF). The comparison of myofibers positive for MAC, MHC-I, and p62 between IMNM and LGMD R2 was determined by the percentage of positive myofibers. For myofibers, 0 = no staining (0% of myofibers), 1 = little staining (< 3% of myofibers), 2 = moderate staining (3–10% of myofibers), 3 = abundant staining (11–30% of myofibers), and 4 = very high staining (> 30% of myofibers). Cell counts were performed using Image J software.
2.7. Statistical Analysis
Categorical variables were expressed as frequencies and percentages, while continuous variables were expressed as medians and ranges. Fisher’s exact two-tailed test and the Mann–Whitney U test were used for comparison of categorical variables and continuous variables, respectively. Statistical analysis was performed using GraphPad Prism software. The p values < 0.05 were considered statistically significant.
3. Results
The clinical and histopathological comparisons between IMNM and LGMD R2 are shown in Table 1 and Table 2, respectively. Patients with IMNM had an older age of onset (p = 0.0167) and a shorter interval of onset to first visit (p = 0.0175) when compared with patients with LGMD R2. There was no difference in gender between the two conditions.

Table 1.
Comparison of the clinical features between IMNM and LGMD R2.

Table 2.
Comparison of the histopathological features between IMNM and LGMD R2.
3.1. Initial Presentation
Limb weakness as initial presentation was common in both IMNM and LGMD R2, with proximal muscles especially affected. Early upper and lower limb weakness was seen in most patients with IMNM; however, no upper limb weakness was noted in any of the patients with LGMD R2. One patient (12.5%) with LGMD R2 reported pelvic zone muscle weakness, while some patients with IMNM reported myalgia (19.5%).
3.2. Clinical Assessment
At the time of diagnosis, IMNM had more severe muscle weakness (p = 0.0046) compared with LGMD R2. Muscle weakness of the upper limbs was more common in IMNM (p = 0.0003). Cervical muscle weakness, dysphagia, extramuscular symptoms (skin rash, arthritis, and ILD), and complications (malignancy and CTD) were noted only in IMNM. The frequency of myalgia was markedly increased in IMNM (36.6%), especially in seronegative IMNM (66.67%). Cardiac abnormalities occurred in the two entities. Similar to MSAs, the serum antinuclear antibody was especially common in IMNM (75%), but absent in LGMD R2 (p = 0.0003).
3.3. Muscle MRI
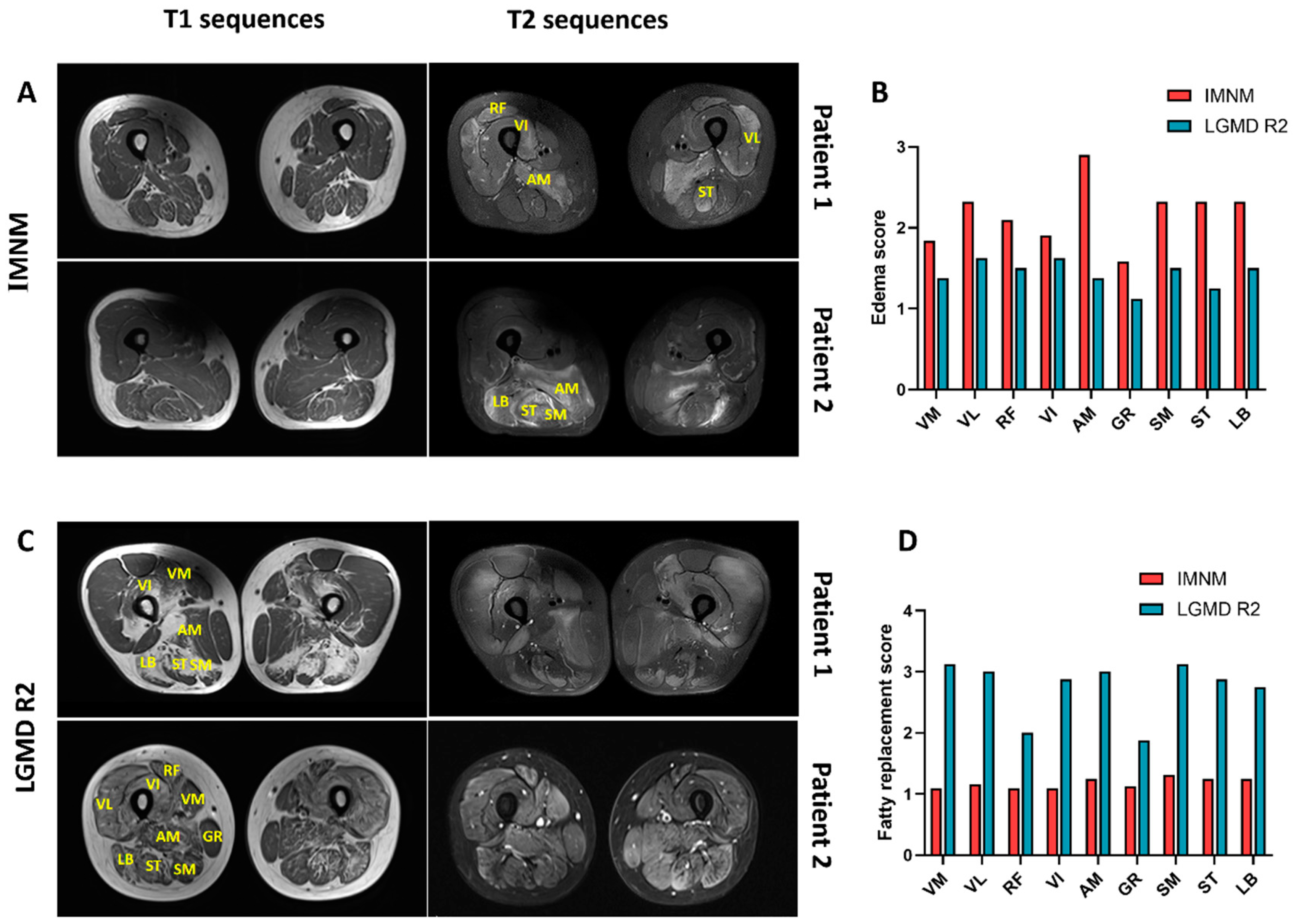
Representative thigh MRI images of patients with IMNM and LGMD R2 are shown in Figure 1. Muscle edema was more pronounced in IMNM and mainly affected the adductor magnus (AM), followed by posterior thigh muscle groups (long biceps femoris (LB), semitendinosus (ST), and semimembranosus (SM)), the vastus lateralis (VL), and rectus femoris (RF) (Figure 1A,B). Fatty replacement and muscle atrophy were more common in LGMD R2 (p = 0.0086, p = 0.0277, respectively), and extensive fatty replacement was frequently involved in all three compartments of the thigh (Figure 1C,D).
Figure 1.
Representative thigh muscle MRI of IMNM and LGMD R2. (A) Slight or no fatty replacement on T1 images and a marked T2 hyperintensity in patients with IMNM. (B) Muscle edema with adductor magnus (AM) prominently affected. (C) Extensive fatty replacement on T1 images and slight T2 hyperintensity in patients with LGMD R2. (D) Fatty replacement occurred in all three compartments of the thigh. Note: VM: vastus medialis; VL: vastus lateralis; RF: rectus femoris; VI: vastus internus; GR: gracilis; SM: semimembranosus; ST: semitendinosus; LB: long biceps femoris. IMNM: n = 31; LGMD R2: n = 8.
3.4. Histopathology
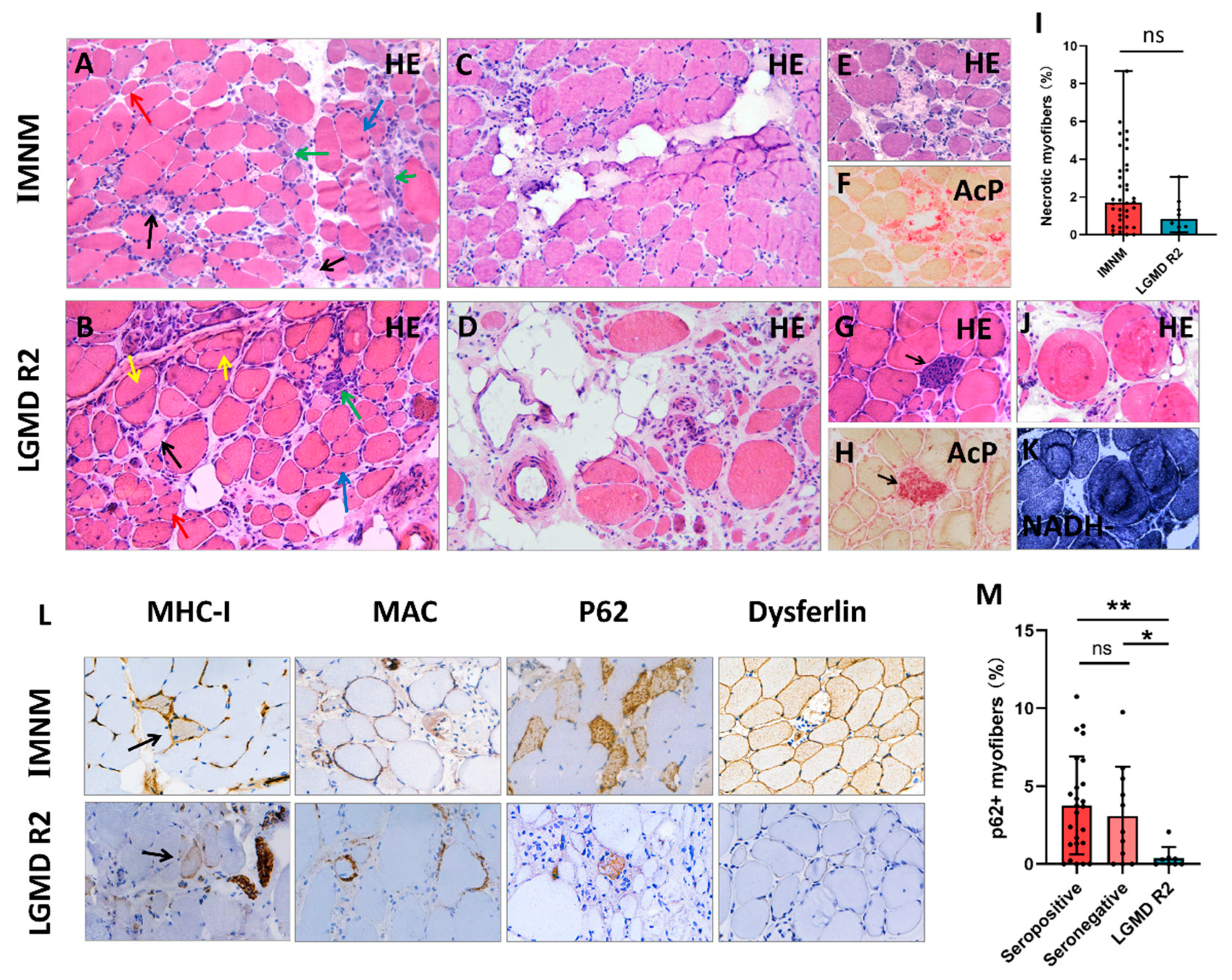
Histopathological features of IMNM and LGMD R2 are summarized in Table 2, and representative images are shown in Figure 2. Histological analysis showed that myofiber necrosis, regeneration, atrophy, and myophagocytosis presented in both entities (Figure 2A,B,E–H). IMNM trended towards more necrotic myofibers compared to LGMD R2, but was not statistically significant (Figure 2I). Chronic myopathic features, such as internalized nuclei, splitting myofibers, and ring/whorled myofibers were more common in LGMD R2 (p = 0.0001, p < 0.0001, p = 0.0112, respectively). Fiber size variability and fatty replacement were more obvious in LGMD R2, especially in patients with a long course of disease (Figure 2C,D).
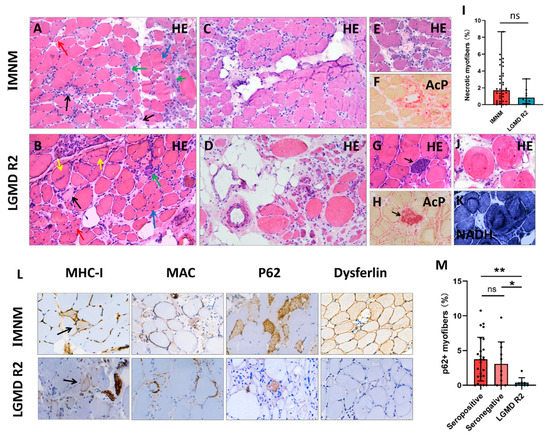
Figure 2.
Representative histopathological images of IMNM and LGMD R2. (A,B) Hematoxylin-eosin (H&E) staining showing profound necrotic myofibers (black arrows), regenerating myofibers (basophilic cells, green arrows), degenerating myofibers (myofibers with internalized nuclei, blue arrows), atrophic myofibers (red arrows), and splitting myofibers (yellow arrows). (C) Slight fat replacement in an IMNM biopsy. (D) Severe fat replacement with fiber size variability (dramatically atrophic or enlarged myofibers) in an LGMD R2 patient with a long course of disease. (E–H) H&E and acid phosphatase (AcP) staining showing necrotic myofibers undergoing myophagocytosis. (I) More necrotic myofibers in IMNM biopsies. (J,K) H&E and NADH staining showing ring/whorled myofibers in a LGMD R2 biopsy. (L) Immunohistochemical staining for major histocompatibility complex class I (MHC-I, black arrows), membrane attack complex (MAC), sequestosome 1 (p62), and dysferlin. (M) More myofibers positive for p62 in seropositive and seronegative IMNM biopsies. Images (A–D): original magnification 200 ×; images (E–H,J–L): original magnification 400×. ns, no significance; * p < 0.05, ** p < 0.01.
A few inflammatory infiltrates were noted in both IMNM and LGMD R2 biopsies with CD68+ macrophage predominance. There were no significant differences in the numbers of CD68+ macrophages, CD4+ T cells, CD8+ T cells, and CD20+ B cells (Supplementary Figure S2), as well as MHC-I expression and MAC deposition on the sarcolemma between IMNM and LGMD R2. Positive p62 staining was observed in the two entities, whereas the percentage of p62 positive fibers in IMNM biopsies was remarkably higher than in LGMD R2 biopsies (p < 0.05, Figure 2M). Dysferlin deficiency only presented in LGMD R2 (Figure 2L); however, dysferlin staining was susceptible to false negative staining (normal IHC labeling) or faint staining (Supplementary Figure S1).
3.5. Diagnosis and Outcome
There were four patients with LGMD R2 (50%) who were initially diagnosed as seronegative IMNM and received immunotherapies. No reliable clues indicative of hereditary myopathy was the leading cause of early misdiagnosis, including no family history (n = 4), lack of clinical sign of muscle atrophy (n = 3), and no severe fat replacement observed on the muscle biopsy (n = 4) or muscle MRI (n = 3) (Supplementary Figures S1 and S3). These patients showed no improvement (n = 3) or worsened (n = 1) after immunotherapies. Then, genetic analysis was performed and confirmed the mutations of the DYSF gene.
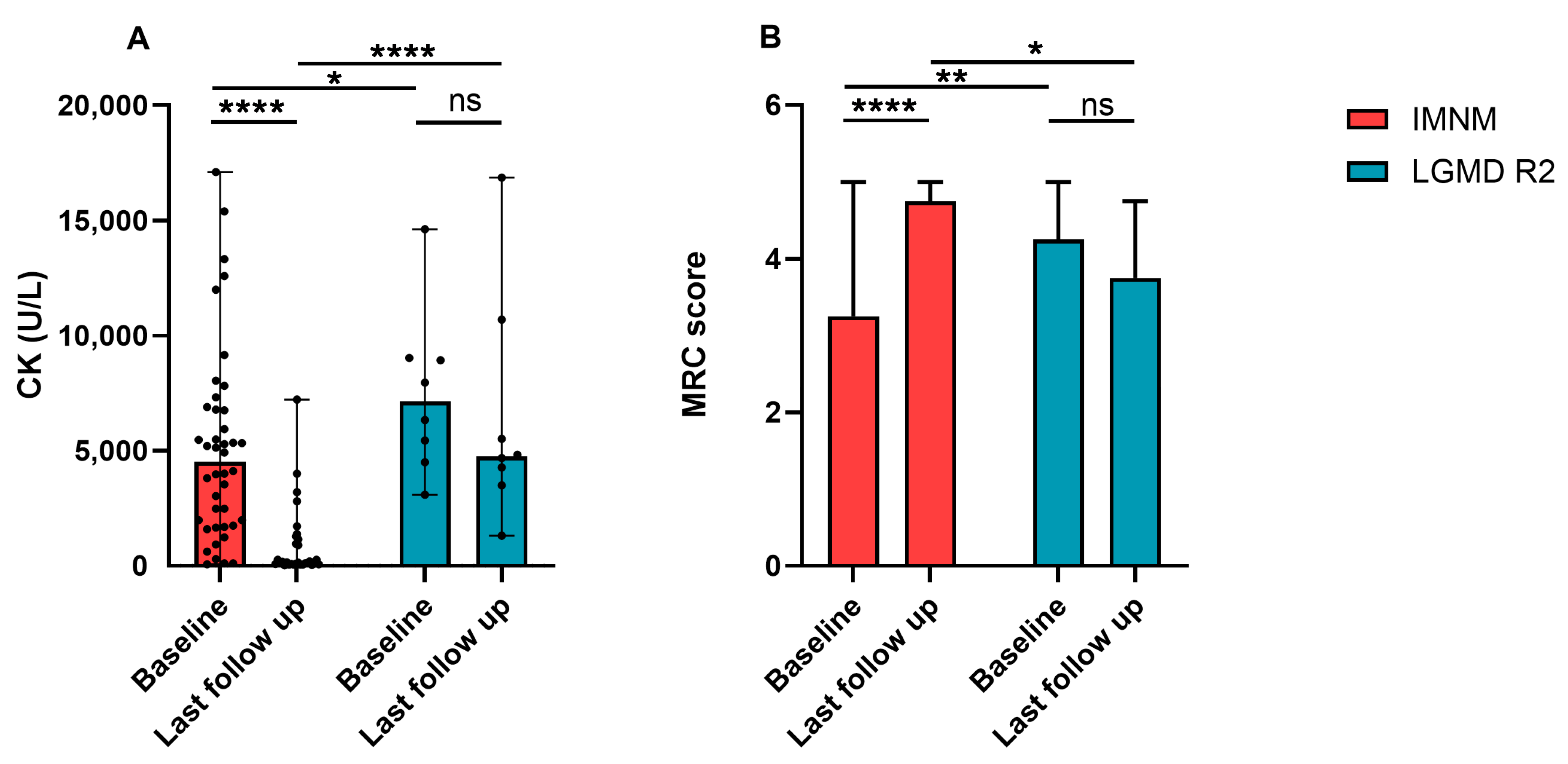
Most patients with IMNM improved after immunotherapies. Their serum CK dramatically decreased and MRC scores significantly improved (Figure 3A,B). Patients with LGMD R2 progressed slowly, MRC scores decreased with the course of the disease, and serum CK maintained a high level, but had a descending trend (Figure 3A,B).
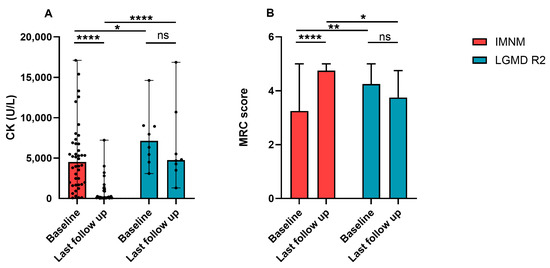
Figure 3.
Changes of serum CK levels and MRC scores of patients with IMNM and LGMD R2. (A) CK levels of patients with IMNM and LGMD R2 at baseline and the last follow-up. (B) MRC scores of patients with IMNM and LGMD R2 at baseline and the last follow-up. Data was shown by medians and range. CK: creatine kinase; MRC: Medical Research Council; ns: no significance; * p < 0.05, ** p < 0.01, **** p < 0.0001.
3.6. Seropositive IMNM vs. LGMD R2, and Seronegative IMNM vs. LGMD R2
The clinical and histopathological comparisons between seropositive/seronegative IMNM and LGMD R2 are shown in Table 1 and Table 2, respectively. Overall, the differences were consistent with these findings observed in IMNM and LGMD R2. Of note, myalgia was more common in seronegative patients compared to LGMD R2 patients (p = 0.0281).
4. Discussion
In our center, up to 50% of patients with LGMD R2 were initially considered as seronegative IMNM, and therefore unnecessarily treated with corticosteroids or immunosuppressors with several possible side effects. We compared detailed clinical, imaging, and histopathological features between IMNM and LGMD R2, and investigated the causes of early misdiagnosis of LGMD R2. Our study highlighted that LGMD R2 can mimic IMNM; however, there were a number of differences between the two entities. Patients with LGMD R2 had a longer interval of onset to first visit, mild muscle weakness with late upper limb involvement, less myalgia, no cervical muscle weakness or dysphagia, no extramuscular organs affected except cardiac involvement, and lack of various autoantibodies including the antinuclear antibody. These clinical features were completely reversed in IMNM. Thigh MRIs showed muscle edema with the adductor magnus prominently affected as a feature of IMNM, while extensive fatty replacement was more common in LGMD R2. Ring/whorled and splitting myofibers were more often found in LGMD R2, while sarcoplasmic p62 expression was more pronounced in IMNM. Insufficient recognition of the early clinical, imaging, and histopathological features of LGMD R2 was the leading cause of misdiagnosis.
IMNM and LGMD R2 frequently presented with proximal weakness; however, the patterns of weakness are remarkably different. Patients with IMNM usually had a proximal weakness with nearly equal involvement of the upper and lower limbs at onset. It then became increasingly apparent that proximal muscle weakness predominantly affected the lower limbs. Patients with LGMD R2 usually started with a proximal muscle weakness of the lower limbs or the pelvic girdle. The upper limbs and shoulder girdle muscles were usually involved later and more mildly during the course of the disease [11].
IMNM is a severe myopathy with an acute or subacute course [12], whereas muscle weakness in patients with LGMD R2 was usually mild at first visit, manifested as difficulty in climbing stairs/mountains, difficulty in rising from the floor, and tiring easily. Mild weakness with significantly elevated CK levels may be a feature of LGMD R2, as there is no relationship between the severity of LGMD R2 and serum CK [10]. Overall, muscle weakness in LGMD R2 progresses slowly with the disease duration, whereas serum CK tends to decrease [10,13].
Myalgia appears to be a clinical feature helping differentiate the two entities. A previous review by Fanin et al. highlighted myalgia as a frequently observed feature of LGMD R2 [5]. However, the largest international multicenter study on dysferlinopathy (including 193 patients) described less common muscle pain (13%) [11]. This is consistent with our result (12.5%) and another multicenter study (5/40) [10]. Muscle atrophy plays a limited role in the early diagnosis of LGMD R2, as mild muscle wasting is difficult to find clinically.
Myocardial abnormalities were frequently detected in both IMNM and LGMD R2, but no patient reported any cardiac symptoms. Cardiac involvement is a relatively common extramuscular manifestation of IMNM [14], associated with a poor prognosis of IMNM [15,16]. The dysferlin protein also presents in the myocardium, and its deficiency leads to myocardial injury [17,18,19]. In a previous study, myocardial damage was detected by cardiac MRI in four of nine patients with LGMD 2B [18]. Symptomatic cardiomyopathy seems to be limited, whereas subclinical cardiac damage does occur in the two entities. Long-term studies are still needed to determine the prognostic significance of subclinical cardiac damage in these patients.
Muscle edema is closely associated with inflammatory activity, suggesting the diagnosis of IIMs, such as IMNM, while extensive fatty replacement is more likely to be an inherited myopathy, such as LGMD R2 [20,21]. However, only one of four patients with LGMD R2 initially considered as IMNM had prominent fat replacement on the thigh MRI (Supplementary Figure S3). Adductor magnus edema was evident in most IMNM, but usually absent in LGMD R2, suggesting it could be a potentially useful tool to differentiate IMNM from early LGMD R2 or hereditary myopathies.
LGMD featured with variable degrees of dystrophic pathology, including remarkable fiber size variability, increased internalized nuclei, splitting and ring/whorled myofibers, and severe fat replacement, especially in those with a long course of disease [22]. All four patients initially suspected as IMNM lacked severe fat replacement or fiber size variability, whereas splitting myofibers were usually noted (Supplementary Figure S1), strongly suggesting that splitting myofibers may be a key clue to differentiating early LGMD from IMNM.
Dystrophic pathology was found on the muscle biopsy of one patient with the anti-SRP antibody in our study and several patients with the anti-HMGCR antibody in previous studies [23,24,25]. These patients had a chronic course, resembling LGMD, but responded favorably to immunotherapies [23]. The chronic LGMD-like phenotype appears to be a feature of seropositive IMNM, as there have been no reports of seronegative patients presenting with a slowly progressive weakness. Therefore, serum MSAs should be routinely assessed when patients with suspected LGMD have no family history, and no definitive genetic abnormality or unavailable genetic sequencing. Other serum antibodies, such as the antinuclear antibody, can also be detected in most IMNM, supporting the view that the immune mechanism plays a key role in the pathogenesis of IMNM, but is a secondary, non-specific change in LGMD R2 [4,26].
Immunostaining for p62 (an autophagy marker) may be helpful for distinguishing the two entities. Autophagy is required for successful differentiation of myoblasts and functional regeneration of skeletal muscle [27,28]. Dysferlin deficiency in LGMD R2 leads to damaged muscle regeneration [29,30], which may be responsible for its rarely p62 staining in LGMD R2. Inversely, LC3 (another autophagy marker) and p62 accumulated in regenerating myofibers in IMNM [31], suggesting a better tissue repair ability. Although p62 staining has been considered an interesting diagnostic hallmark of IMNM [1], precise mechanisms of autophagy in IMNM are yet to be elucidated, and much more studies are still needed.
Immunostaining for dysferlin is necessary, but not always reliable in distinguishing the two entities. Almost normal IHC staining was noted in the muscle biopsy from an LGMD R2 patient who had a family history and a pathogenic DYSF mutation. Prior studies have demonstrated that partial dysferlin defects were difficult to identify using IHC staining, leading to false negative staining (normal IHC labeling) or faint staining [5,32]. Harris et al. also observed patients with DYSF mutations presenting with normal dysferlin protein levels [11]. Therefore, gene sequencing is ultimately required to confirm the diagnosis of LGMD R2.
One patient with LGMD R2 progressed gradually during corticosteroid treatment. Her weakness of the lower limbs worsened, followed by upper limb involvement and myalgia. Subsequent genetic analysis confirmed pathogenic mutations in the DYSF gene. Then, she was weaned off immunosuppressants and her weakness improved slightly, and myalgia disappeared. Indeed, patients with LGMD R2 seem to worsen and develop a series of side effects after corticosteroid or immunosuppressive treatment [5,33,34]. Our study provides a toolkit for clinicians to timely screen possible LGMD R2 from IMNM, especially seronegative IMNM, avoiding the use of potentially harmful immunosuppressive drugs.
However, there are still some limitations. First, some muscular dystrophy patients may be hidden in seronegative cases. Considering the possibility of a mistaken diagnosis of seronegative IMNM, we made a comparison between seropositive IMNM and LGMD R2 at the same time. Second, other recessive LGMDs and distal MM phenotypes were not included in our study. We focused on the differences between IMNM and LGMD R2 rather than all LGMDs, as LGMD R2 is the most common subtype in our country, accounting for 49.5% of all LGMDs [35]. Moreover, as shown, the clinicopathological features of LGMD R2 are more similar to IMNM. Particularly, sarcolemmal MAC deposition can be observed in LGMD R2, but is rarely seen in other muscular dystrophies [8,36]. Distal MM features initial distal weakness and prominent calf muscle atrophy, particularly the gastrocnemius involvement, which is not often misdiagnosed as IMNM [37]. One patient with distal MM was excluded from our study due to incomplete medical data. Even so, most findings are suitable for distal MM, as there is no striking difference in clinical presentation (except weakness pattern) and examination between distal MM and LGMD R2 [11]. Finally, this study was limited by a small number of patients with LGMD R2, leading to potential information scarcity and selection bias, such as the lack of patients with asymptomatic hyperCKemia at onset.
5. Conclusions
Our study provided some important differences between the two entities, which may help clinicians timely screen possible LGMD R2 from seronegative IMNM for genetic testing, thereby shortening the time to diagnosis. No reliable clues indicative of hereditary myopathy was the leading cause of early misdiagnosis of LGMD R2 as seronegative IMNM. In this scenario, we highlighted that abundant p62 staining and muscle edema affecting the adductor magnus on MRI seem to be good markers for IMNM, while the presence of splitting myofibers is a crucial clue to early hereditary myopathy, including LGMD R2. It is important to consider a combination of clinicopathological features in any case. Prospective and multi-center studies with a larger sample of patients are still needed to confirm our preliminary observations and explore the differences between IMNM and others’ hereditary myopathy, especially LGMD.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11216566/s1. Figure S1: Hematoxilin-eosin (H&E) staining and immunological detection of dysferlin in muscle biopsies from LGMD R2; Figure S2: Characterization of inflammatory infiltrates in IMNM and LGMD R2 biopsies; Figure S3: Thigh muscle MRI of patients with LGMD R2; Table S1: Demographic features and pathogenic mutations in patients with LGMD R2.
Author Contributions
Conceptualization, B.B.; Data curation, M.Y., S.J., L.X., Q.Z., Y.L. and H.G.; Funding acquisition, B.B.; Methodology, L.X.; Project administration, B.B.; Visualization, Y.L.; Writing—original draft, M.Y., L.X. and Q.Z.; Writing—review and editing, M.Y., S.J. and H.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant Number: 81873758).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by The Institutional Review Board at Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology (IRB ID: TJ-C20121221). Informed consent was obtained from all subjects involved in the study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The data presented in this study are available in the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Allenbach, Y.; Benveniste, O.; Stenzel, W.; Boyer, O. Immune-mediated necrotizing myopathy: Clinical features and pathogenesis. Nat. Rev. Rheumatol. 2020, 16, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Selva-O’Callaghan, A.; Trallero-Araguás, E.; Milisenda, J.C.; Grau-Junyent, J.M. Differential diagnosis of necrotizing myopathy. Curr. Opin. Rheumatol. 2021, 33, 544–553. [Google Scholar] [CrossRef]
- Merlonghi, G.; Antonini, G.; Garibaldi, M. Immune-mediated necrotizing myopathy (IMNM): A myopathological challenge. Autoimmun. Rev. 2021, 21, 102993. [Google Scholar] [CrossRef] [PubMed]
- Han, R. Muscle membrane repair and inflammatory attack in dysferlinopathy. Skelet. Muscle 2011, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Fanin, M.; Angelini, C. Progress and challenges in diagnosis of dysferlinopathy. Muscle Nerve 2016, 54, 821–835. [Google Scholar] [CrossRef]
- Liang, W.-C.; Jong, Y.-J.; Wang, C.-H.; Wang, C.-H.; Tian, X.; Chen, W.-Z.; Kan, T.-M.; Minami, N.; Nishino, I.; Wong, L.-J.C. Clinical, pathological, imaging, and genetic characterization in a Taiwanese cohort with limb-girdle muscular dystrophy. Orphanet J. Rare Dis. 2020, 15, 160. [Google Scholar] [CrossRef]
- Takahashi, T.; Aoki, M.; Suzuki, N.; Tateyama, M.; Yaginuma, C.; Sato, H.; Hayasaka, M.; Sugawara, H.; Ito, M.; Abe-Kondo, E.; et al. Clinical features and a mutation with late onset of limb girdle muscular dystrophy 2B. J. Neurol. Neurosurg. Psychiatry 2013, 84, 433–440. [Google Scholar] [CrossRef]
- Allenbach, Y.; Mammen, A.L.; Benveniste, O.; Stenzel, W.; On behalf of the Immune-Mediated Necrotizing Myopathies Working Group. 224th ENMC International Workshop: Clinico-sero-pathological classifi-cation of immune-mediated necrotizing myopathies Zandvoort, The Netherlands, 14–16 October 2016. Neuromuscul. Disord 2018, 28, 87–99. [Google Scholar] [CrossRef]
- Barp, A.; Laforet, P.; Bello, L.; Tasca, G.; Vissing, J.; Monforte, M.; Ricci, E.; Choumert, A.; Stojkovic, T.; Malfatti, E.; et al. European muscle MRI study in limb girdle muscular dystrophy type R1/2A (LGMDR1/LGMD2A). J. Neurol. 2020, 267, 45–56. [Google Scholar] [CrossRef]
- Nguyen, K.; Bassez, G.; Krahn, M.; Bernard, R.; Laforêt, P.; Labelle, V.; Urtizberea, J.A.; Figarella-Branger, D.; Romero, N.; Attarian, S.; et al. Phenotypic study in 40 patients with dysferlin gene mutations: High frequency of atypical phenotypes. Arch. Neurol. 2007, 64, 1176–1182. [Google Scholar] [CrossRef]
- Harris, E.; Bladen, C.L.; Mayhew, A.; James, M.; Bettinson, K.; Moore, U.; Smith, F.E.; Rufibach, L.; Cnaan, A.; Bharucha-Goebel, D.X.; et al. The Clinical Outcome Study for dysferlinopathy: An international multicenter study. Neurol Genet. 2016, 2, e89. [Google Scholar] [CrossRef] [PubMed]
- Anquetil, C.; Boyer, O.; Wesner, N.; Benveniste, O.; Allenbach, Y. Myositis-specific autoantibodies, a cornerstone in immune-mediated necrotizing myopathy. Autoimmun. Rev. 2019, 18, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Takahashi, T. Mutational and clinical features of Japanese patients with dysferlinopathy (Miyoshi myopathy and limb girdle muscular dystrophy type 2B). Rinsho Shinkeigaku Clin. Neurol. 2005, 45, 938–942. [Google Scholar]
- Triplett, J.; Kassardjian, C.D.; Liewluck, T.; Tahir, A.; Lennon, V.; Kopecky, S.; Milone, M. Cardiac and Respiratory Complications of Necrotizing Autoimmune Myopathy. Mayo Clin. Proc. 2020, 95, 2144–2149. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xu, L.; Li, Y.; Bu, B. Immunotherapy reversed myopathy but not cardiomyopathy in a necrotizing autoimmune myopathy patient with positive anti-SRP and MDA-5 autoantibodies. BMC Cardiovasc. Disord. 2021, 21, 88. [Google Scholar] [CrossRef]
- Ma, X.; Xu, L.; Ji, S.; Li, Y.; Bu, B. The Clinicopathological Distinction Between Seropositive and Seronegative Immune-Mediated Ne-crotizing Myopathy in China. Front. Neurol 2021, 12, 670784. [Google Scholar] [CrossRef]
- Wenzel, K.; Geier, C.; Qadri, F.; Hubner, N.; Schulz, H.; Erdmann, B.; Gross, V.; Bauer, D.; Dechend, R.; Dietz, R.; et al. Dysfunction of dysferlin-deficient hearts. J. Mol. Med. 2007, 85, 1203–1214. [Google Scholar] [CrossRef]
- Rosales, X.Q.; Moser, S.J.; Tran, T.; McCarthy, B.; Dunn, N.; Habib, P.; Simonetti, O.P.; Mendell, J.R.; Raman, S.V. Cardiovascular magnetic resonance of cardiomyopathy in limb girdle muscular dys-trophy 2B and 2I. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2011, 13, 39. [Google Scholar]
- Nishikawa, A.; Mori-Yoshimura, M.; Segawa, K.; Hayashi, Y.K.; Takahashi, T.; Saito, Y.; Nonaka, I.; Krahn, M.; Levy, N.; Shimizu, J.; et al. Respiratory and cardiac function in japanese patients with dysferli-nopathy. Muscle Nerve 2016, 53, 394–401. [Google Scholar] [CrossRef]
- Marago, I.; Roberts, M.; Roncaroli, F.; DuPlessis, D.; Sewry, C.; Nagaraju, S.; Limbada, F.; Marini-Bettolo, C.; Hudson, J.; Banerjee, S.; et al. Limb girdle muscular dystrophy R12 (LGMD 2L, anoctaminopathy) mimicking idiopathic inflammatory myopathy: Key points to prevent misdiagnosis. Rheumatology 2021, 61, 1645–1650. [Google Scholar] [CrossRef]
- Hsu, W.-C.; Lin, Y.-C.; Chuang, H.-H.; Yeh, K.-Y.; Chan, W.P.; Ro, L.-S. A Muscle Biosignature Differentiating Between Limb-Girdle Muscular Dystrophy and Idiopathic Inflammatory Myopathy on Magnetic Resonance Imaging. Front. Neurol. 2021, 12, 783095. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.L.; Moran, E. The Limb-Girdle Muscular Dystrophies: Is Treatment on the Horizon? Neurotherapeutics 2018, 15, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Mohassel, P.; Landon-Cardinal, O.; Foley, A.R.; Donkervoort, S.; Pak, K.S.; Wahl, C.; Shebert, R.T.; Harper, A.; Fequiere, P.; Meriggioli, M.; et al. Anti-HMGCR myopathy may resemble limb-girdle muscular dystrophy. Neurol. Neuroimmunol. Neuroinflamm. 2018, 6, e523. [Google Scholar] [CrossRef]
- Tard, C.; Tiffreau, V.; Jaillette, E.; Jouen, F.; Nelson, I.; Bonne, G.; Yaou, R.B.; Romero, N.; Vallée, L.; Vermersch, P.; et al. Anti-HMGCR Antibody-Related Necrotizing Autoimmune Myopathy Mimicking Mus-cular Dystrophy. Neuropediatrics 2017, 48, 473–476. [Google Scholar] [PubMed]
- Mohassel, P.; Foley, A.R.; Donkervoort, S.; Fequiere, P.R.; Pak, K.; Bönnemann, C.G.; Mammen, A.L. Anti-3-hydroxy-3-methylglutaryl-coenzyme a reductase necrotizing myopathy masquerading as a muscular dystrophy in a child. Muscle Nerve 2017, 56, 1177–1181. [Google Scholar] [CrossRef]
- Day, J.A.; Limaye, V. Immune-mediated necrotising myopathy: A critical review of current concepts. Semin. Arthritis Rheum. 2019, 49, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Call, J.A.; Wilson, R.J.; Laker, R.C.; Zhang, M.; Kundu, M.; Yan, Z. Ulk1-mediated autophagy plays an essential role in mitochondrial remodeling and functional regeneration of skeletal muscle. Am. J. Physiol. Physiol. 2017, 312, C724–C732. [Google Scholar] [CrossRef]
- Lee, D.E.; Bareja, A.; Bartlett, D.B.; White, J.P. Autophagy as a Therapeutic Target to Enhance Aged Muscle Regeneration. Cells 2019, 8, 183. [Google Scholar] [CrossRef]
- Cohen, T.V.; Cohen, J.E.; Partridge, T.A. Myogenesis in dysferlin-deficient myoblasts is inhibited by an intrinsic inflam-matory response. Neuromuscul. Disord. NMD 2012, 22, 648–658. [Google Scholar] [CrossRef][Green Version]
- Ganassi, M.; Muntoni, F.; Zammit, P.S. Defining and identifying satellite cell-opathies within muscular dystrophies and myopathies. Exp. Cell Res. 2022, 411, 112906. [Google Scholar] [CrossRef]
- Girolamo, F.; Lia, A.; Annese, T.; Giannini, M.; Amati, A.; D′Abbicco, D.; Tampoia, M.; Virgintino, D.; Ribatti, D.; Serlenga, L.; et al. Autophagy markers LC3 and p62 accumulate in immune-mediated necrotizing myopathy. Muscle Nerve 2019, 60, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Krahn, M.; Illa, I.; Levy, N.; Bushby, K. 172nd ENMC International Workshop: Dysferlinopathies 29–31 January 2010, Naarden, The Netherlands. Neuromuscul Disord 2011, 21, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Lerario, A.; Cogiamanian, F.; Marchesi, C.; Belicchi, M.; Bresolin, N.; Porretti, L.; Torrente, Y. Effects of rituximab in two patients with dysferlin-deficient muscular dystrophy. BMC Musculoskelet. Disord. 2010, 11, 157. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.C.; Reilich, P.; Thiele, S.; Schessl, J.; Schreiber, H.; Reiners, K.; Kress, W.; Müller-Reible, C.; Vorgerd, M.; Urban, P.; et al. Treatment of dysferlinopathy with deflazacort: A double-blind, placebo-controlled clinical trial. Orphanet J. Rare Dis. 2013, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zheng, Y.; Jin, S.; Gang, Q.; Wang, Q.; Yu, P.; Lv, H.; Zhang, W.; Yuan, Y.; Wang, Z. Mutational spectrum of Chinese LGMD patients by targeted next-generation sequencing. PLoS ONE 2017, 12, e0175343. [Google Scholar] [CrossRef]
- Becker, N.; Moore, S.A.; Jones, K.A. The inflammatory pathology of dysferlinopathy is distinct from calpainopathy, Becker muscular dystrophy, and inflammatory myopathies. Acta Neuropathol. Commun. 2022, 10, 17. [Google Scholar] [CrossRef]
- Hu, Y.-Y.; Lian, Y.-J.; Xu, H.-L.; Zheng, Y.-K.; Li, C.-F.; Zhang, J.-W.; Yan, S.-P. Novel, de novo dysferlin gene mutations in a patient with Miyoshi myopathy. Neurosci. Lett. 2018, 664, 107–109. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).