Early Changes in Androgen Levels in Individuals with Spinal Cord Injury: A Longitudinal SwiSCI Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting
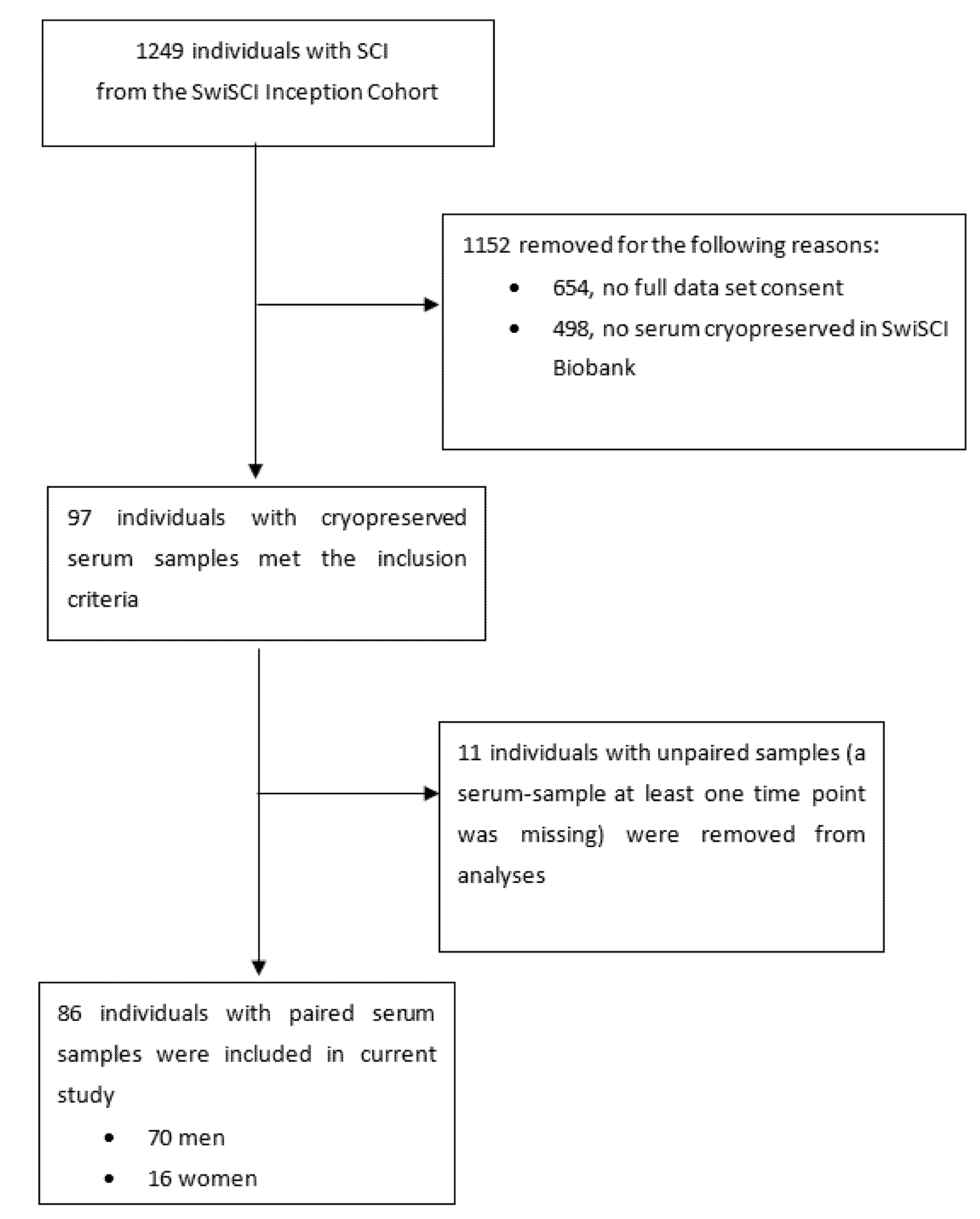
2.2. Study Population, Inclusion and Exclusion Criteria
2.3. Study Measures
Androgen Hormones
2.4. Clinical and SCI Characteristics
2.5. Power Calculation
2.6. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Association between Clinical Characteristics and Androgens at Baseline
3.3. Longitudinal Changes in Androgen Levels
4. Discussion
4.1. Androgen Changes during Initial Inpatient Rehabilitation
4.2. Clinical Implications of Our Findings and Directions for Future Research
4.3. Strengths and Limitations of the Current Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsitouras, P.D.; Zhong, Y.G.; Spungen, A.M.; Bauman, W.A. Serum testosterone and growth hormone/insulin-like growth factor-I in adults with spinal cord injury. Horm. Metab. Res. 1995, 27, 287–292. [Google Scholar] [CrossRef]
- Naderi, A.R.; Safarinejad, M.R. Endocrine profiles and semen quality in spinal cord injured men. Clin. Endocrinol. 2003, 58, 177–184. [Google Scholar] [CrossRef]
- Miranda, E.P.; Gomes, C.M.; de Bessa, J., Jr.; Najjar Abdo, C.H.; Suzuki Bellucci, C.H.; de Castro Filho, J.E.; de Carvalho, F.L.; de Souza, D.R.; Battistella, L.R.; Scazufca, M.; et al. Evaluation of Sexual Dysfunction in Men With Spinal Cord Injury Using the Male Sexual Quotient. Arch. Phys. Med. Rehabil. 2016, 97, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Beauchet, O. Testosterone and cognitive function: Current clinical evidence of a relationship. Eur. J. Endocrinol. 2006, 155, 773–781. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.W.; Glisic, M.; Kumarendran, B.; Subramanian, A.; Manolopoulos, K.N.; Tahrani, A.A.; Keerthy, D.; Muka, T.; Toulis, K.A.; Hanif, W.; et al. Serum testosterone, sex hormone-binding globulin and sex-specific risk of incident type 2 diabetes in a retrospective primary care cohort. Clin. Endocrinol. 2019, 90, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Traish, A.M.; Kang, H.P.; Saad, F.; Guay, A.T. Dehydroepiandrosterone (DHEA)—A precursor steroid or an active hormone in human physiology. J. Sex. Med. 2011, 8, 2960–2982. [Google Scholar] [CrossRef]
- Wu, T.T.; Chen, Y.; Zhou, Y.; Adi, D.; Zheng, Y.Y.; Liu, F.; Ma, Y.T.; Xie, X. Prognostic Value of Dehydroepiandrosterone Sulfate for Patients with Cardiovascular Disease: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2017, 6, e004896. [Google Scholar] [CrossRef]
- Brahimaj, A.; Muka, T.; Kavousi, M.; Laven, J.S.; Dehghan, A.; Franco, O.H. Serum dehydroepiandrosterone levels are associated with lower risk of type 2 diabetes: The Rotterdam Study. Diabetologia 2017, 60, 98–106. [Google Scholar] [CrossRef]
- Ding, E.L.; Song, Y.; Malik, V.S.; Liu, S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA 2006, 295, 1288–1299. [Google Scholar] [CrossRef]
- Xing, D.; Nozell, S.; Chen, Y.F.; Hage, F.; Oparil, S. Estrogen and mechanisms of vascular protection. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 289–295. [Google Scholar] [CrossRef]
- Liu, S.; Sun, Q. Sex differences, endogenous sex-hormone hormones, sex-hormone binding globulin, and exogenous disruptors in diabetes and related metabolic outcomes. J. Diabetes 2018, 10, 428–441. [Google Scholar] [CrossRef]
- Ding, E.L.; Song, Y.; Manson, J.E.; Hunter, D.J.; Lee, C.C.; Rifai, N.; Buring, J.E.; Gaziano, J.M.; Liu, S. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N. Engl. J. Med. 2009, 361, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Muka, T.; Nano, J.; Jaspers, L.; Meun, C.; Bramer, W.M.; Hofman, A.; Dehghan, A.; Kavousi, M.; Laven, J.S.; Franco, O.H. Associations of Steroid Sex Hormones and Sex Hormone-Binding Globulin With the Risk of Type 2 Diabetes in Women: A Population-Based Cohort Study and Meta-analysis. Diabetes 2017, 66, 577–586. [Google Scholar] [CrossRef]
- Elkabes, S.; Nicot, A.B. Sex steroids and neuroprotection in spinal cord injury: A review of preclinical investigations. Exp. Neurol. 2014, 259, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.N.; MacLean, S.M.; Stromberg, A.J.; Whelan, J.P.; Bailey, W.M.; Gensel, J.C.; Wilson, M.E. Considerations for Studying Sex as a Biological Variable in Spinal Cord Injury. Front. Neurol. 2020, 11, 802. [Google Scholar] [CrossRef] [PubMed]
- Arbo, B.D.; Ribeiro, F.S.; Ribeiro, M.F. Astrocyte Neuroprotection and Dehydroepiandrosterone. Vitam. Horm. 2018, 108, 175–203. [Google Scholar] [CrossRef] [PubMed]
- Bauman, W.A.; La Fountaine, M.F.; Spungen, A.M. Age-related prevalence of low testosterone in men with spinal cord injury. J. Spinal Cord Med. 2014, 37, 32–39. [Google Scholar] [CrossRef]
- Durga, A.; Sepahpanah, F.; Regozzi, M.; Hastings, J.; Crane, D.A. Prevalence of testosterone deficiency after spinal cord injury. PM&R 2011, 3, 929–932. [Google Scholar]
- Clark, M.J.; Schopp, L.H.; Mazurek, M.O.; Zaniletti, I.; Lammy, A.B.; Martin, T.A.; Thomas, F.P.; Acuff, M.E. Testosterone levels among men with spinal cord injury: Relationship between time since injury and laboratory values. Am. J. Phys. Med. Rehabil. 2008, 87, 758–767. [Google Scholar] [CrossRef]
- Schopp, L.H.; Clark, M.; Mazurek, M.O.; Hagglund, K.J.; Acuff, M.E.; Sherman, A.K.; Childers, M.K. Testosterone levels among men with spinal cord injury admitted to inpatient rehabilitation. Am. J. Phys. Med. Rehabil. 2006, 85, 678–684. [Google Scholar] [CrossRef]
- Naftchi, N.E.; Viau, A.T.; Sell, G.H.; Lowman, E.W. Pituitary-testicular axis dysfunction in spinal cord injury. Arch. Phys. Med. Rehabil. 1980, 61, 402–405. [Google Scholar] [PubMed]
- Dirlikov, B.; Lavoie, S.; Shem, K. Correlation between thyroid function, testosterone levels, and depressive symptoms in females with spinal cord injury. Spinal Cord Ser. Cases 2019, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Mondaini, N.; Macchiarella, A.; Del Popolo, G. Female sexual dysfunction and hormonal status in spinal cord injured (SCI) patients. J. Androl. 2007, 28, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Raguindin, P.F.; Muka, T.; Glisic, M. Sex and gender gap in spinal cord injury research: Focus on cardiometabolic diseases. A mini review. Maturitas 2021, 147, 14–18. [Google Scholar] [CrossRef]
- Barbonetti, A.; Vassallo, M.R.; Pacca, F.; Cavallo, F.; Costanzo, M.; Felzani, G.; Francavilla, S.; Francavilla, F. Correlates of low testosterone in men with chronic spinal cord injury. Andrology 2014, 2, 721–728. [Google Scholar] [CrossRef]
- Safarinejad, M.R. Level of injury and hormone profiles in spinal cord-injured men. Urology 2001, 58, 671–676. [Google Scholar] [CrossRef]
- Fekete, C.; Gurtner, B.; Kunz, S.; Gemperli, A.; Gmünder, H.P.; Hund-Georgiadis, M.; Jordan, X.; Schubert, M.; Stoyanov, J.; Stucki, G. Inception cohort of the Swiss Spinal Cord Injury Cohort Study (SwiSCI): Design, participant characteristics, response rates and non-response. J. Rehabil. Med. 2021, 53, jrm00159. [Google Scholar] [CrossRef]
- MyAssays Ltd. Four Parameter Logistic Curve. Available online: http://www.myassays.com/four-parameter-logistic-curve.assay (accessed on 5 April 2022).
- Marino, R.J.; Barros, T.; Biering-Sorensen, F.; Burns, S.P.; Donovan, W.H.; Graves, D.E.; Haak, M.; Hudson, L.M.; Priebe, M.M. International standards for neurological classification of spinal cord injury. J. Spinal Cord. Med. 2003, 26 (Suppl. S1), S50–S56. [Google Scholar] [CrossRef]
- Alexander, M.S.; New, P.W.; Biering-Sorensen, F.; Courtois, F.; Popolo, G.D.; Elliott, S.; Kiekens, C.; Vogel, L.; Previnaire, J.G. International spinal cord injury male sexual function and female sexual and reproductive function basic data sets-version 2.0. Spinal Cord Ser. Cases 2017, 3, 17050. [Google Scholar] [CrossRef]
- Celik, B.; Sahin, A.; Caglar, N.; Erhan, B.; Gunduz, B.; Gultekin, O.; Karabulut, M. Sex hormone levels and functional outcomes: A controlled study of patients with spinal cord injury compared with healthy subjects. Am. J. Phys. Med. Rehabil. 2007, 86, 784–790. [Google Scholar] [CrossRef]
- Behnaz, M.; Majd, Z.; Radfar, M.; Ajami, H.; Qorbani, M.; Kokab, A. Prevalence of androgen deficiency in chronic spinal cord injury patients suffering from erectile dysfunction. Spinal Cord 2017, 55, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Gabriela Boehl, P.F.R.; Valido, E.; Bertolo, A.; Itodo, O.A.; Minder, B.; Lampart, P.; Scheel-Sailer, A.; Leichtle, A.; Glisic, M.; Stoyanov, J. Endocrinological and inflammatory markers in individuals with spinal cord injury: A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2022, 23, 1035–1050. [Google Scholar] [CrossRef]
- Sullivan, S.D.; Nash, M.S.; Tefera, E.; Tinsley, E.; Blackman, M.R.; Groah, S. Prevalence and Etiology of Hypogonadism in Young Men With Chronic Spinal Cord Injury: A Cross-Sectional Analysis From Two University-Based Rehabilitation Centers. PM&R 2017, 9, 751–760. [Google Scholar] [CrossRef]
- Barbonetti, A.; Vassallo, M.; Felzani, R.G.; Francavilla, S.; Francavilla, F. Association between 25(OH)-vitamin D and testosterone levels: Evidence from men with chronic spinal cord injury. J. Spinal. Cord. Med. 2016, 39, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Goulis, D.G.; Huhtaniemi, I.; Zitzmann, M.; Toppari, J.; Forti, G.; Vanderschueren, D.; Wu, F.C. European Academy of Andrology (EAA) guidelines on investigation, treatment and monitoring of functional hypogonadism in males: Endorsing organization: European Society of Endocrinology. Andrology 2020, 8, 970–987. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.G.; Zhao, F.; Lee, R.K. Androgen deficiency and metabolic syndrome in men. Transl. Androl. Urol. 2014, 3, 50–58. [Google Scholar] [CrossRef]
- Cumming, D.C.; Quigley, M.E.; Yen, S.S. Acute suppression of circulating testosterone levels by cortisol in men. J. Clin. Endocrinol. Metab. 1983, 57, 671–673. [Google Scholar] [CrossRef]
- Cruse, J.M.; Lewis, R.E., Jr.; Bishop, G.R.; Kliesch, W.F.; Gaitan, E.; Britt, R. Decreased immune reactivity and neuroendocrine alterations related to chronic stress in spinal cord injury and stroke patients. Pathobiology 1993, 61, 183–192. [Google Scholar] [CrossRef]
- Kumagai, H.; Zempo-Miyaki, A.; Yoshikawa, T.; Tsujimoto, T.; Tanaka, K.; Maeda, S. Increased physical activity has a greater effect than reduced energy intake on lifestyle modification-induced increases in testosterone. J. Clin. Biochem. Nutr. 2016, 58, 84–89. [Google Scholar] [CrossRef]
- Brignardello-Petersen, R.; Izcovich, A.; Rochwerg, B.; Florez, I.D.; Hazlewood, G.; Alhazanni, W.; Yepes-Nunez, J.; Santesso, N.; Guyatt, G.H.; Schunemann, H.J.; et al. GRADE approach to drawing conclusions from a network meta-analysis using a partially contextualised framework. BMJ 2020, 371, m3907. [Google Scholar] [CrossRef]
- Holman, M.E.; Chang, G.; Ghatas, M.P.; Saha, P.K.; Zhang, X.; Khan, M.R.; Sima, A.P.; Adler, R.A.; Gorgey, A.S. Bone and non-contractile soft tissue changes following open kinetic chain resistance training and testosterone treatment in spinal cord injury: An exploratory study. Osteoporos. Int. 2021, 32, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Holman, M.E.; Gorgey, A.S. Testosterone and Resistance Training Improve Muscle Quality in Spinal Cord Injury. Med. Sci. Sport. Exerc. 2019, 51, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Gorgey, A.S.; Khalil, R.E.; Gill, R.; Gater, D.R.; Lavis, T.D.; Cardozo, C.P.; Adler, R.A. Low-Dose Testosterone and Evoked Resistance Exercise after Spinal Cord Injury on Cardio-Metabolic Risk Factors: An Open-Label Randomized Clinical Trial. J. Neurotrauma 2019, 36, 2631–2645. [Google Scholar] [CrossRef]
- Bauman, W.A.; Cirnigliaro, C.M.; La Fountaine, M.F.; Jensen, A.M.; Wecht, J.M.; Kirshblum, S.C.; Spungen, A.M. A small-scale clinical trial to determine the safety and efficacy of testosterone replacement therapy in hypogonadal men with spinal cord injury. Horm. Metab. Res. 2011, 43, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.M.; Vandenborne, K.; Huang, H.F.; Ottenweller, J.E.; Dudley, G.A. Effects of testosterone replacement therapy on skeletal muscle after spinal cord injury. Spinal Cord 2003, 41, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Qiu, L.; Jiang, F.; Gaman, M.A.; Abudoraehem, O.S.; Okunade, K.S.; Zhang, M. The effect of dehydroepiandrosterone (DHEA) supplementation on estradiol levels in women: A dose-response and meta-analysis of randomized clinical trials. Steroids 2021, 173, 108889. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Zhong, Y.; Xue, Q.; Wu, M.; Deng, X.; Santos, H.O.; Tan, S.C.; Kord-Varkaneh, H.; Jiao, P. Impact of dehydroepianrosterone (DHEA) supplementation on serum levels of insulin-like growth factor 1 (IGF-1): A dose-response meta-analysis of randomized controlled trials. Exp. Gerontol. 2020, 136, 110949. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jin, Z.; Sun, C.; Santos, H.; Kord Varkaneh, H. Effects of dehydroepiandrosterone (DHEA) supplementation on cortisol, leptin, adiponectin, and liver enzyme levels: A systematic review and meta-analysis of randomised clinical trials. Int. J. Clin. Pract. 2021, 75, e14698. [Google Scholar] [CrossRef]
- Hu, Y.; Wan, P.; An, X.; Jiang, G. Impact of dehydroepiandrosterone (DHEA) supplementation on testosterone concentrations and BMI in elderly women: A meta-analysis of randomized controlled trials. Complement. Ther. Med. 2021, 56, 102620. [Google Scholar] [CrossRef]
- Daniell, H.W. DHEAS deficiency during consumption of sustained-action prescribed opioids: Evidence for opioid-induced inhibition of adrenal androgen production. J. Pain 2006, 7, 901–907. [Google Scholar] [CrossRef]
- Medina, W.A., II; Conermann, T. Opioid-induced Endocrinopathy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Dokras, A. Cardiovascular disease risk in women with PCOS. Steroids 2013, 78, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Haring, R.; Teumer, A.; Volker, U.; Dorr, M.; Nauck, M.; Biffar, R.; Volzke, H.; Baumeister, S.E.; Wallaschofski, H. Mendelian randomization suggests non-causal associations of testosterone with cardiometabolic risk factors and mortality. Andrology 2013, 1, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Au Yeung, S.L.; Zhao, J.V.; Burgess, S.; Schooling, C.M. Association of genetically predicted testosterone with thromboembolism, heart failure, and myocardial infarction: Mendelian randomisation study in UK Biobank. BMJ 2019, 364, l476. [Google Scholar] [CrossRef] [PubMed]
- Tracz, M.J.; Sideras, K.; Bolona, E.R.; Haddad, R.M.; Kennedy, C.C.; Uraga, M.V.; Caples, S.M.; Erwin, P.J.; Montori, V.M. Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J. Clin. Endocrinol. Metab. 2006, 91, 2011–2016. [Google Scholar] [CrossRef]
- Krebs, J.; Gocking, K.; Pannek, J. Testicular resistive index determined by Doppler ultrasonography in men with spinal cord injury—A case series. Andrologia 2015, 47, 811–815. [Google Scholar] [CrossRef]

| Characteristic | All Observations (n = 86) | Males (n = 70, 81 %) | Females (n = 16, 19 %) | p Value 1 |
|---|---|---|---|---|
| Age, years, median (IQR) | 51 (36–64) | 52.5 (35–63) | 47 (38–73.5) | 0.68 |
| Injury characteristics | ||||
| Tetraplegia, n (%) | 29 (33.7) | 24 (34.3) | 5 (31.3) | 0.19 |
| Paraplegia, n (%) | 43 (50) | 37 (52.9) | 6 (37.5) | |
| Missing, n (%) | 14 (16.3) | 9 (12.9) | 5 (31.3) | |
| Time since injury, days | 15.5 (10–27) | 16 (10–27) | 12.5 (8.5–24.5) | 0.48 |
| Length of rehabilitation, months | 5.6 (4.2–7.5) | 5.7 (4.2–7.5) | 5.1 (4.3–5.9) | 0.29 |
| Cause of injury | ||||
| Traumatic, n (%) | 61 (70.9) | 52 (74.3) | 9 (56.2) | 0.15 |
| Non-traumatic, n (%) | 25 (29.1) | 18 (25.7) | 7 (43.8) | |
| Completeness of injury | ||||
| Motor Complete | 33 (38.4) | 29 (41.4) | 4 (25.0) | 0.16 |
| Motor Incomplete | 39 (45.4) | 32 (45.7) | 7 (43.7) | |
| Missing | 14 (16.3) | 9 (12.9) | 5 (31.3) | |
| Cause of traumatic injury | ||||
| Vehicular | 22 (36.1) | 22 (42.3) | 0 (0) | 0.03 |
| Violence | 1 (1.6) | 0 (0) | 1 (11.1) | |
| Sports | 17 (27.9) | 12 (23.1) | 5 (55.6) | |
| Falls | 15 (24.6) | 13 (25.0) | 2 (22.2) | |
| Medical complication | 5 (8.2) | 4 (7.7) | 1 (11.1) | |
| Unknown | 1 (1.6) | 1 (1.9) | 0 (0) | |
| Cause of non-traumatic injury | ||||
| Genetic disorders | 8 (32.0) | 5 (27.8) | 3 (42.9) | 0.80 |
| Metabolic | 9 (36.0) | 6 (33.3) | 3 (42.9) | |
| Toxic | 6 (24.0) | 5 (27.8) | 1 (14.3) | |
| Infections | 1 (4.0) | 1 (5.6) | 0 | |
| Miscellaneous | 1 (4.0) | 1 (5.6) | 0 | |
| ISNCSCI Scale | ||||
| A, n (%) | 19 (26.4) | 17 (27.9) | 2 (18.2) | 0.69 |
| B, n (%) | 14 (19.4) | 12 (19.7) | 2 (18.2) | |
| C, n (%) | 11 (15.3) | 10 (16.4) | 1 (9.1) | |
| D, n (%) | 28 (38.9) | 22 (36.1) | 6 (54.6) | |
| Anthropometric measurements | ||||
| BMI, kg/m2 | 23.7 (21.3–26.3) | 23.7 (21.5–26.0) | 23.4 (20.4–26.3) | 0.76 |
| Waist circumference, cm | 86.5 (79.3–96) | 87 (79.5–97) | 83 (78–90) | 0.63 |
| Androgens | ||||
| Total testosterone, nmol/L, median (IQR) | 10.4 (5.3–17.3) | 12.5 (7.9–17.7) | 1.9 (1.4–2.5) | <0.01 |
| Free testosterone, pmol/L, median (IQR) | 24.1 (11.5–33.0) | 27.5 (16.9–36.6) | 2.9 (2.3–3.7) | <0.01 |
| SHBG, pg/mL, median (IQR) 3 | 2606 (2053–2944) | 2558 (2053–2777) | 2890 (2020.5–3947) | 0.11 |
| DHEA, nmol/L, median (IQR) | 19.7 (13.5–38.0) | 20.2 (12.8–40.2) | 18.9 (15.7–35.0) | 0.50 |
| DHEA-S, umol/L, median (IQR) | 3.3 (1.6–6.5) | 3.6 (1.6–6.9) | 3.1 (1.5–4.6) | 0.35 |
| Medication use | ||||
| Opioids, n (%) | 26 (30.2) | 23 (32.9) | 3 (18.8) | 0.27 |
| Corticosteroids, n (%) | 8 (9.3) | 7 (10.0) | 1 (6.3) | 0.64 |
| Thyroid hormones, n (%) | 4 (4.7) | 2 (2.9) | 2 (12.5) | 0.10 |
| Comorbidities | ||||
| Cardiovascular diseases 2, n (%) | 4 (4.7) | 2 (2.9) | 2 (12.5) | 0.10 |
| Type 2 diabetes/prediabetes, n (%) | 6 (7.0) | 4 (5.7) | 2 (12.5) | 0.34 |
| Hypertension, n (%) | 8 (9.3) | 7 (10.0) | 1 (6.3) | 0.64 |
| Chronic kidney disease, n (%) | 3 (3.5) | 1 (1.4) | 2 (12.5) | 0.03 |
| Men (N = 70) | |||||
|---|---|---|---|---|---|
| Parameter | TT (β, 95%CI) | FT (β, 95%CI) | SHBG (β, 95%CI) | DHEA (β, 95%CI) | DHEA-S (β, 95%CI) |
| Etiology of injury | |||||
| Non-traumatic | 2.30 (−2.02, 6.61) | 21.46 (−67.1, 110.00) | −167.28 (−544.62, 210.06) | 6.78 (−4.4, 18.00) | −0.09 (−1.97, 1.80) |
| Injury level | |||||
| Paraplegics | 2.07 (−1.67, 5.79) | 15.30 (−64.58, 95.17) | 88.56 (−291.17, 468.29) | −2.32 (−11.39, 6.76) | −1.77 (−3.60, 0.05) |
| Injury completeness | |||||
| Incomplete | 2.25 (−1.61, 6.12) | 21.32 (−55.33, 97.98) | 179.33 (−200.05, 558.70) | −3.18 (−11.97, 5.61) | −1.78 (−3.43, −0.12) * |
| Medication use | |||||
| Opioids use | 1.97 (−1.87, 5.80) | −56.40 (−126.62, 13.82) | 557.76 (281.48, 834.03) *** | −9.02 (−17.01, −1.03) * | −2.18 (−3.58, −0.78) ** |
| Corticosteroids use | 4.51 (0.08, 8.94) | 45.99 ( −52.07, 144.05) | 31.60 (−568.62, 631.82) | 3.66 (−12.15, 19.47) | 0.02 (−1.95, 2.00) |
| Women (N = 16) | |||||
| Parameter | TT (β, 95%CI) | FT (β, 95%CI) | SHBG (β, 95%CI) | DHEA (β, 95%CI) | DHEA-S (β, 95%CI) |
| Etiology of injury | |||||
| Non-traumatic | 0.36 (−1.91, 2.63) | 4.65 (−21.98, 31.29) | 55.14 (−1131.25, 1241.54) | −18.05 (−35.51, −0.58) * | −1.32 (−3.91, 1.27) |
| Injury level | |||||
| Paraplegics | 0.97 (−1.98, 3.92) | 10.47 (−21.48, 42.42) | 132.93 (−1428.11, 1693.97) | 0.32 (−18.78, 19.42) | −1.61 (−4.05, 0.83) |
| Injury completeness | |||||
| Incomplete | 0.12 (−2.54, 2.77) | 9.02 (−18.74, 36.77) | −416.18 (−1815.78, 983.43) | −12.79 (−29.09, 3.51) | −0.19 (−2.49, 2.12) |
| Medication use | |||||
| Opioids use | 0.21 (−1.29, 1.71) | −4.54 (−20.29, 11.22) | 1158.41 (310.59, 2006.23) * | −5.70 (−22.46, 11.07) | −1.93 (−3.95, 0.08) |
| Corticosteroids use | 0.38 (−0.71, 1.47) | −5.96 (−18.93, 7.00) | 388.13 (−248.53, 1024.80) | 7.31 (−3.97, 18.59) | −0.24 (−1.70, 1.22) |
| Admission to Rehabilitation | End of Rehabilitation | p-Value | Unadjusted Model | p-Value | Adjusted Model 1 | p-Value | |
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | (Paired t Test) | (β, 95%CI) | (LMM) | (β, 95%CI) | (LMM) | |
| Men (N = 70) | |||||||
| TT, nmol/L | 13.4 (7.2) | 17.0 (6.2) | <0.01 | 3.66 (1.87, 5.46) | <0.01 | 3.96 (1.37, 6.56) | 0.003 |
| FT, pmol/L | 29.4 (15.8) | 30.8 (18.5) | 0.32 | 13.85 (−26.89, 54.59) | 0.51 | 2.99 (−53.56, 59.53) | 0.92 |
| SHBG, pg/mL | 2360.12 (744.88) | 2294.10 (706.31) | 0.12 | −66.01 (−140.40, 8.37) | 0.08 | −37.61 (−145.04, 69.81) | 0.49 |
| DHEA, nmol/L | 26.2 (17.6) | 26.9 (21.9) | 0.60 | 0.65 (−3.20, 4.49) | 0.74 | −1.59 (−7.39, 4.21) | 0.59 |
| DHEA-S, umol/L | 4.5 (3.3) | 5.9 (4.4) | <0.01 | 1.44 (0.76, 2.12) | <0.01 | 1.77 (0.73, 2.81) | <0.01 |
| Women (N = 16) | |||||||
| TT, nmol/L | 2.1 (1.7) | 2.2 (1.9) | 0.80 | 0.06 (−0.47, 0.60) | 0.82 | 0.23 (−0.34, 0.79) | 0.43 |
| FT, pmol/L | 3.4 (2.2) | 3.1(3.2) | 0.54 | −2.09 (−11.78, 7.60) | 0.67 | 2.71 (−10.22, 15.65) | 0.68 |
| SHBG, pg/mL | 2770.13 (1079.79) | 2767.06 (903.71) | 0.60 | −3.06 (−322.10, 315.97) | 0.99 | 514.08 (−87.06, 1115.23) | 0.09 |
| DHEA, nmol/L | 28.2 (18.3) | 21.1 (14.0) | 0.05 | −7.11 (−13.98, −0.24) | 0.04 | −11.91 (−24.39, −4.11) | <0.01 |
| DHEA-S, umol/L | 3.4 (2.5) | 4.4 (3.6) | 0.29 | 1.01 (−0.31, 2.32) | 0.13 | 2.71 (−0.02, 5.43) | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itodo, O.A.; Raguindin, P.F.; Wöllner, J.; Eriks-Hoogland, I.; Jordan, X.; Hund-Georgiadis, M.; Muka, T.; Pannek, J.; Stoyanov, J.; Glisic, M. Early Changes in Androgen Levels in Individuals with Spinal Cord Injury: A Longitudinal SwiSCI Study. J. Clin. Med. 2022, 11, 6559. https://doi.org/10.3390/jcm11216559
Itodo OA, Raguindin PF, Wöllner J, Eriks-Hoogland I, Jordan X, Hund-Georgiadis M, Muka T, Pannek J, Stoyanov J, Glisic M. Early Changes in Androgen Levels in Individuals with Spinal Cord Injury: A Longitudinal SwiSCI Study. Journal of Clinical Medicine. 2022; 11(21):6559. https://doi.org/10.3390/jcm11216559
Chicago/Turabian StyleItodo, Oche Adam, Peter Francis Raguindin, Jens Wöllner, Inge Eriks-Hoogland, Xavier Jordan, Margret Hund-Georgiadis, Taulant Muka, Jürgen Pannek, Jivko Stoyanov, and Marija Glisic. 2022. "Early Changes in Androgen Levels in Individuals with Spinal Cord Injury: A Longitudinal SwiSCI Study" Journal of Clinical Medicine 11, no. 21: 6559. https://doi.org/10.3390/jcm11216559
APA StyleItodo, O. A., Raguindin, P. F., Wöllner, J., Eriks-Hoogland, I., Jordan, X., Hund-Georgiadis, M., Muka, T., Pannek, J., Stoyanov, J., & Glisic, M. (2022). Early Changes in Androgen Levels in Individuals with Spinal Cord Injury: A Longitudinal SwiSCI Study. Journal of Clinical Medicine, 11(21), 6559. https://doi.org/10.3390/jcm11216559











