Proactive versus Rank-Down Topical Corticosteroid Therapy for Maintenance of Remission in Pediatric Atopic Dermatitis: A Randomized, Open-Label, Active-Controlled, Parallel-Group Study (Anticipate Study)
Abstract
1. Introduction
2. Materials and Methods
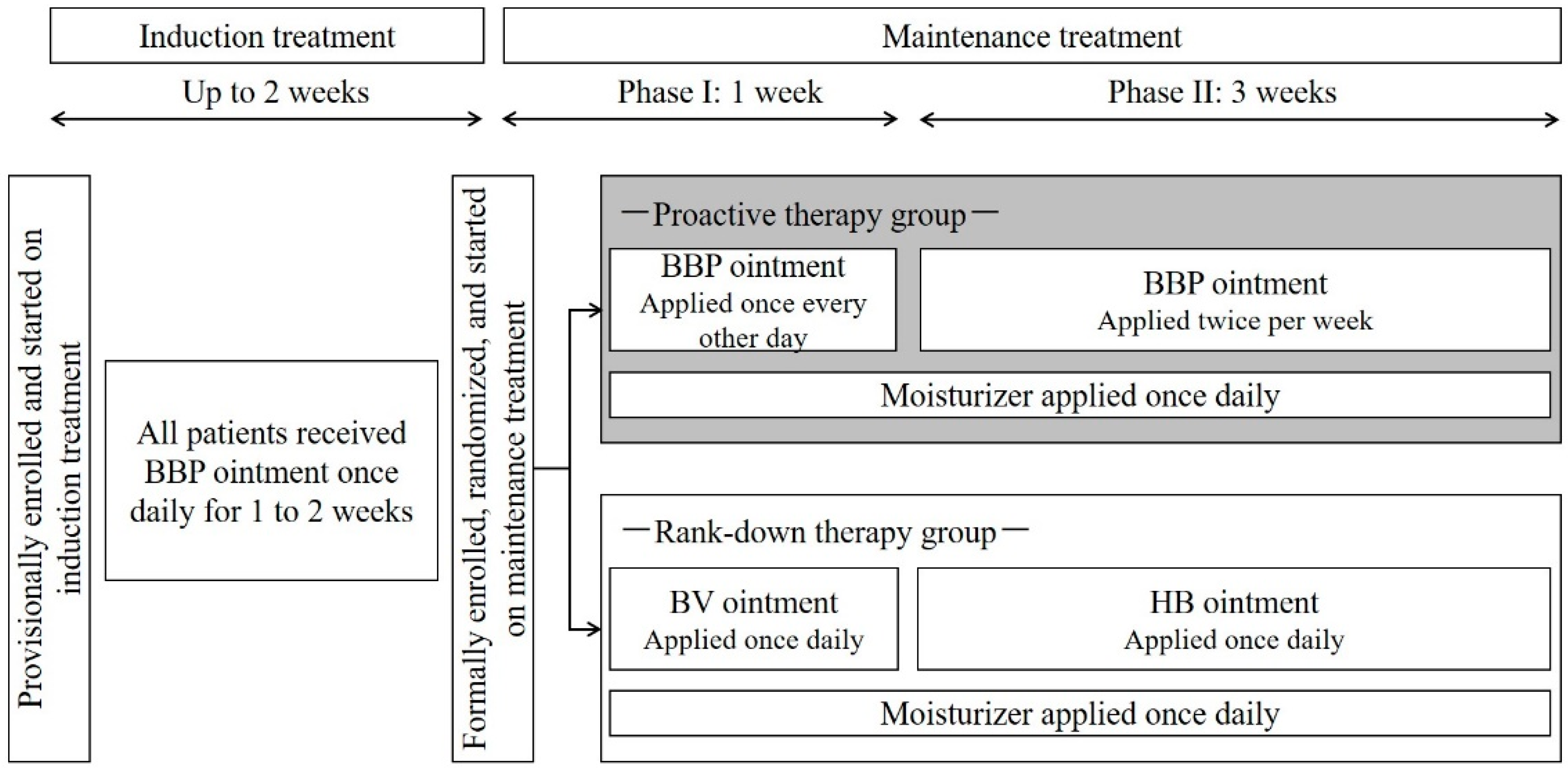
2.1. Study Design
2.2. Patients
2.3. Study Endpoints
2.4. Statistical Analyses
3. Results
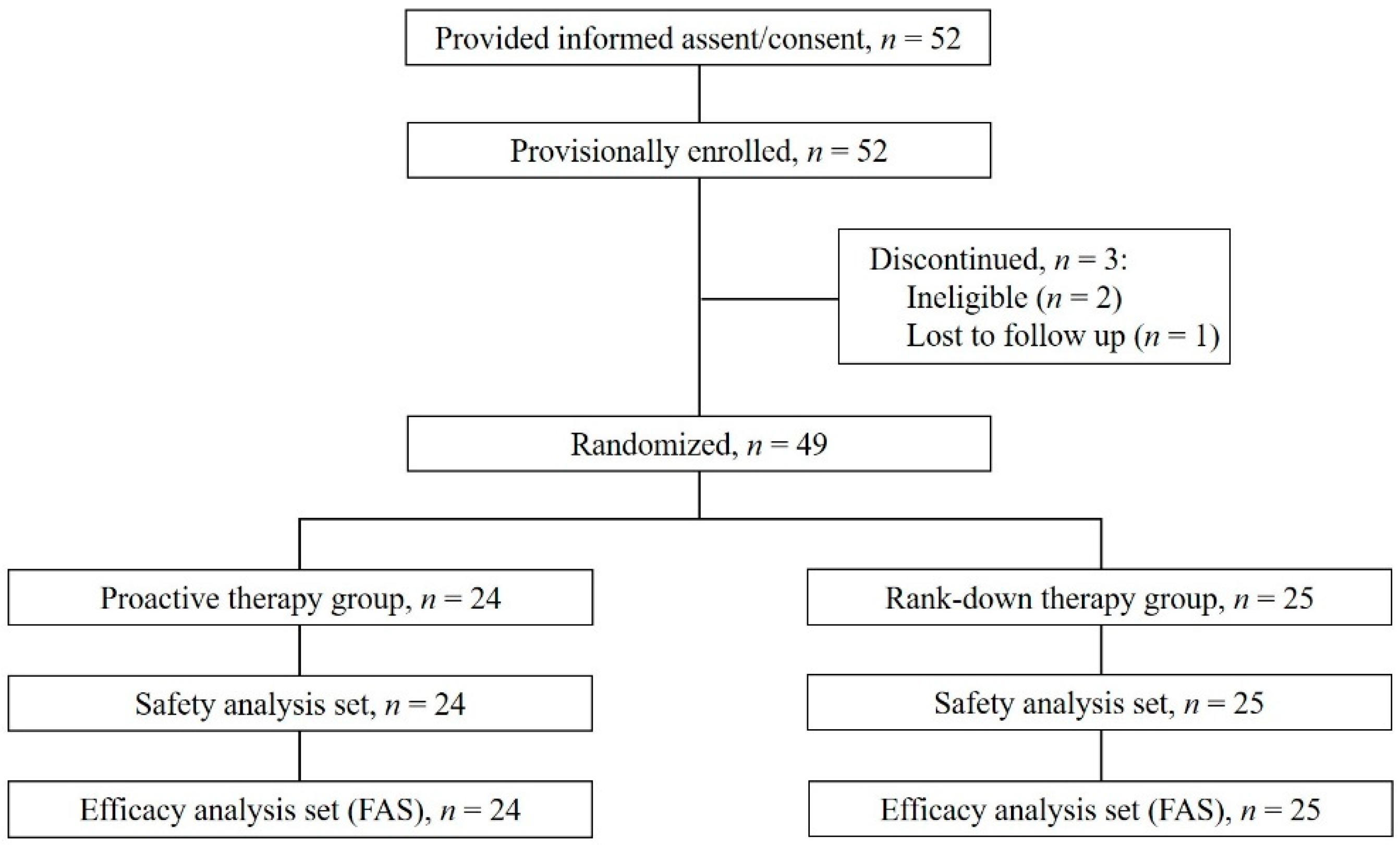
3.1. Patient Characteristics
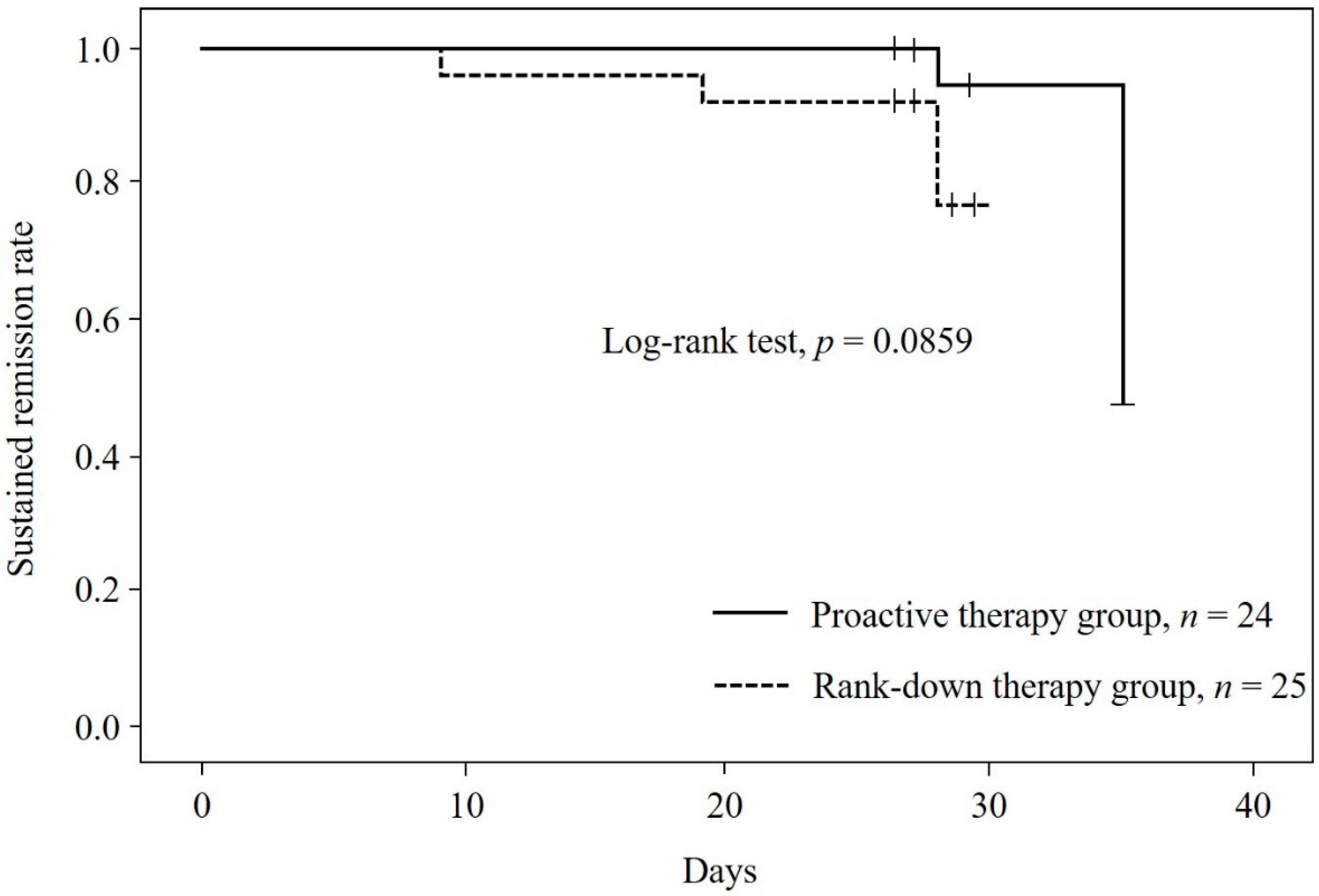
3.2. Relapse Rate
3.3. Secondary Outcome Measures
3.4. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katoh, N.; Ohya, Y.; Ikeda, M.; Ebihara, T.; Katayama, I.; Saeki, H.; Shimojo, N.; Tanaka, A.; Nakahara, T.; Nagao, M.; et al. Japanese guidelines for atopic dermatitis 2020. Allergol. Int. 2020, 69, 356–369. [Google Scholar] [CrossRef]
- Jackson, K.D.; Howie, L.D.; Akinbami, L.J. Trends in allergic conditions among children: United States, 1997–2011. NCHS Data Brief. 2013, 121, 1–8. [Google Scholar]
- Saeki, H.; Iizuka, H.; Mori, Y.; Akasaka, T.; Takagi, H.; Kitajima, Y.; Tezuka, T.; Tanaka, T.; Hide, M.; Yamamoto, S.; et al. Prevalence of atopic dermatitis in Japanese elementary schoolchildren. Br. J. Dermatol. 2005, 152, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Eichenfield, L.F.; Tom, W.L.; Berger, T.G.; Krol, A.; Paller, A.S.; Schwarzenberger, K.; Bergman, J.N.; Chamlin, S.L.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of care for the management of atopic dermatitis: Section 2. Management and treatment of atopic dermatitis with topical therapies. J. Am. Acad. Dermatol. 2014, 71, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Barbarot, S.; Bieber, T.; Christen-Zaech, S.; Deleuran, M.; Fink-Wagner, A.; Gieler, U.; Girolomoni, G.; Lau, S.; Muraro, A.; et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: Part I. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 657–682. [Google Scholar] [CrossRef]
- Ellison, J.A.; Patel, L.; Ray, D.W.; David, T.J.; Clayton, P.E. Hypothalamic-pituitary-adrenal function and glucocorticoid sensitivity in atopic dermatitis. Pediatrics 2000, 105, 794–799. [Google Scholar] [CrossRef]
- Schlessinger, J.; Miller, B.; Gilbert, R.D.; Plott, R.T. An open-label adrenal suppression study of 0.1% fluocinonide cream in pediatric patients with atopic dermatitis. Arch. Dermatol. 2006, 142, 1568–1572. [Google Scholar] [CrossRef]
- Friedlander, S.F.; Hebert, A.A.; Allen, D.B. Safety of fluticasone propionate cream 0.05% for the treatment of severe and extensive atopic dermatitis in children as young as 3 months. J. Am. Acad. Dermatol. 2002, 46, 387–393. [Google Scholar] [CrossRef][Green Version]
- Hebert, A.A.; Friedlander, S.F.; Allen, D.B. Topical fluticasone propionate lotion does not cause HPA axis suppression. J. Pediatrics 2006, 149, 378–382. [Google Scholar] [CrossRef]
- Coondoo, A.; Phiske, M.; Verma, S.; Lahiri, K. Side-effects of topical steroids: A long overdue revisit. Indian Dermatol. Online J. 2014, 5, 416–425. [Google Scholar] [CrossRef]
- Hengge, U.R.; Ruzicka, T.; Schwartz, R.A.; Cork, M.J. Adverse effects of topical glucocorticosteroids. J. Am. Acad. Dermatol. 2006, 54, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Arase, S.; Takahashi, S. Side effects of topical corticosteroids and their prevention. Drugs 1988, 36 (Suppl. S5), 15–23. [Google Scholar] [CrossRef] [PubMed]
- Sastre, J.; Baldrich, E.S.; Armario Hita, J.C.; Herráez, L.; Jáuregui, I.; Martín-Santiago, A.; de Frutos, J.O.; Silvestre, J.F.; Valero, A. Consensus on the clinical approach to moderate-to-severe atopic dermatitis in Spain: A Delphi survey. Dermatol. Res. Pract. 2020, 2020, 1524293. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kim, H.J.; Lew, B.L.; Lee, K.H.; Hong, S.P.; Jang, Y.H.; Park, K.Y.; Seo, S.J.; Bae, J.M.; Choi, E.H.; et al. Consensus guidelines for the treatment of atopic dermatitis in Korea (Part I): General management and topical treatment. Ann. Dermatol. 2015, 27, 563–577. [Google Scholar] [CrossRef]
- Kulthanan, K.; Tuchinda, P.; Nitiyarom, R.; Chunharas, A.; Chantaphakul, H.; Aunhachoke, K.; Chularojanamontri, L.; Rajatanavin, N.; Jirapongsananuruk, O.; Vichyanond, P.; et al. Clinical practice guidelines for the diagnosis and management of atopic dermatitis. Asian Pac. J. Allergy Immunol. 2021, 39, 145–155. [Google Scholar]
- Klasa, B.; Cichocka-Jarosz, E. Atopic dermatitis—Current state of research on biological treatment. J. Mother Child. 2020, 24, 53–66. [Google Scholar]
- Peserico, A.; Städtler, G.; Sebastian, M.; Fernandez, R.S.; Vick, K.; Bieber, T. Reduction of relapses of atopic dermatitis with methylprednisolone aceponate cream twice weekly in addition to maintenance treatment with emollient: A multicentre, randomized, double-blind, controlled study. Br. J. Dermatol. 2008, 158, 801–807. [Google Scholar] [CrossRef]
- Berth-Jones, J.; Damstra, R.J.; Golsch, S.; Livden, J.K.; Van Hooteghem, O.; Allegra, F.; Parker, C.A. Twice weekly fluticasone propionate added to emollient maintenance treatment to reduce risk of relapse in atopic dermatitis: Randomised, double blind, parallel group study. BMJ 2003, 326, 1367. [Google Scholar] [CrossRef]
- Liu, L.; Ong, G. A randomized, open-label study to evaluate an intermittent dosing regimen of fluticasone propionate 0.05% cream in combination with regular emollient skin care in reducing the risk of relapse in pediatric patients with stabilized atopic dermatitis. J. Dermatol. Treat. 2018, 29, 501–509. [Google Scholar] [CrossRef]
- Hanifin, J.; Gupta, A.K.; Rajagopalan, R. Intermittent dosing of fluticasone propionate cream for reducing the risk of relapse in atopic dermatitis patients. Br. J. Dermatol. 2002, 147, 528–537. [Google Scholar] [CrossRef]
- Glazenburg, E.J.; Wolkerstorfer, A.; Gerretsen, A.L.; Mulder, P.G.; Oranje, A.P. Efficacy and safety of fluticasone propionate 0.005% ointment in the long-term maintenance treatment of children with atopic dermatitis: Differences between boys and girls? Pediatric Allergy Immunol. 2009, 20, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Van Der Meer, J.B.; Glazenburg, E.J.; Mulder, P.G.; Eggink, H.F.; Coenraads, P.J. The management of moderate to severe atopic dermatitis in adults with topical fluticasone propionate. The Netherlands Adult Atopic Dermatitis Study Group. Br. J. Dermatol. 1999, 140, 1114–1121. [Google Scholar] [CrossRef]
- Rubio-Gomis, E.; Martinez-Mir, I.; Morales-Olivas, F.J.; Martorell-Aragones, A.; Palop-Larrea, V.; Bernalte-Sesé, A.; Cerda-Mir, J.C.; Polo-Martín, P.; Febrer, I.; Aranda-Grau, L.; et al. Fluticasone in mild to moderate atopic dermatitis relapse: A randomized controlled trial. Allergol. Immunopathol. 2018, 46, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Fukuie, T.; Hirakawa, S.; Narita, M.; Nomura, I.; Matsumoto, K.; Tokura, Y.; Ohya, Y. Potential preventive effects of proactive therapy on sensitization in moderate to severe childhood atopic dermatitis: A randomized, investigator-blinded, controlled study. J. Dermatol. 2016, 43, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Murota, H.; Katayama, I. Exacerbating factors of itch in atopic dermatitis. Allergol. Int. 2017, 66, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Umehara, Y.; Kiatsurayanon, C.; Trujillo-Paez, J.V.; Chieosilapatham, P.; Peng, G.; Yue, H.; Nguyen, H.L.T.; Song, P.; Okumura, K.; Ogawa, H.; et al. Intractable itch in atopic dermatitis: Causes and treatments. Biomedicines 2021, 9, 229. [Google Scholar] [CrossRef]
- Mochizuki, H.; Tanaka, S.; Morita, T.; Wasaka, T.; Sadato, N.; Kakigi, R. The cerebral representation of scratching-induced pleasantness. J. Neurophysiol. 2014, 111, 488–498. [Google Scholar] [CrossRef]
- Harrison, I.P.; Spada, F. Breaking the itch-scratch cycle: Topical options for the management of chronic cutaneous itch in atopic dermatitis. Medicines 2019, 6, 76. [Google Scholar] [CrossRef]
- Kakinuma, T.; Nakamura, K.; Wakugawa, M.; Mitsui, H.; Tada, Y.; Saeki, H.; Torii, H.; Asahina, A.; Onai, N.; Matsushima, K.; et al. Thymus and activation-regulated chemokine in atopic dermatitis: Serum thymus and activation-regulated chemokine level is closely related with disease activity. J. Allergy Clin. Immunol. 2001, 107, 535–541. [Google Scholar] [CrossRef]
- Thijs, J.; Krastev, T.; Weidinger, S.; Buckens, C.F.; de Bruin-Weller, M.; Bruijnzeel-Koomen, C.; Carsten, F.; DirkJan, H. Biomarkers for atopic dermatitis: A systematic review and meta-analysis. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 453–460. [Google Scholar] [CrossRef]
- Caproni, M.; Torchia, D.; Antiga, E.; Terranova, M.; Volpi, W.; del Bianco, E.; D’Agata, A.; Fabbri, P. The comparative effects of tacrolimus and hydrocortisone in adult atopic dermatitis: An immunohistochemical study. Br. J. Dermatol. 2007, 156, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Vassina, E.; Yousefi, S.; Kozlowski, E.; Braathen, L.R.; Simon, H.U. Reduced dermal infiltration of cytokine-expressing inflammatory cells in atopic dermatitis after short-term topical tacrolimus treatment. J. Allergy Clin. Immunol. 2004, 114, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Chernyshov, P.V.; Sampogna, F.; Pustišek, N.; Marinovic, B.; Manolache, L.; Suru, A.; Salavastru, C.M.; Tiplica, G.S.; Stoleriu, G.; Kakourou, T.; et al. Validation of the dermatology-specific proxy instrument the Infants and Toddlers Dermatology Quality of Life. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 49) | Proactive Therapy Group (n = 24) | Rank-Down Therapy Group (n = 25) | |
|---|---|---|---|
| Sex, n (%), male | 25 (51.0%) | 11 (45.8%) | 14 (56.0%) |
| Age, mean ± SD, y | 9.3 ± 2.2 | 9.1± 2.2 | 9.5 ± 2.2 |
| Height, mean ± SD, cm | 134.0 ± 13.4 | 133.4 ± 14.6 | 134.5 ± 12.5 |
| Weight, mean ± SD, kg | 32.4 ± 12.5 | 31.0 ± 12.4 | 33.7 ± 12.8 |
| Duration of AD, n (%) † | |||
| <5 years | 26 (54.2%) | 12 (50.0%) | 14 (58.3%) |
| ≥5 years, <10 years | 13 (27.1%) | 7 (29.2%) | 6 (25.0%) |
| ≥10 years | 9 (18.8%) | 5 (20.8%) | 4 (16.7%) |
| Family history, n (%) | |||
| No | 5 (10.2%) | 2 (8.3%) | 3 (12.0%) |
| Yes | 44 (89.8%) | 22 (91.7%) | 22 (88.0%) |
| Previous medical history, n (%) | |||
| No | 36 (73.5%) | 18 (75.0%) | 18 (72.0%) |
| Yes | 13 (26.5%) | 6 (25.0%) | 7 (28.0%) |
| Concomitant illnesses, n (%) | |||
| No | 16 (32.7%) | 8 (33.3%) | 8 (32.0%) |
| Yes | 33 (67.3%) | 16 (66.7%) | 17 (68.0%) |
| Prior anti-AD therapy, n (%) | |||
| No | 0 | 0 | 0 |
| Yes | 49 (100.0%) | 24 (100.0%) | 25 (100.0%) |
| Itching, n (%) | |||
| No | 5 (10.2%) | 2 (8.3%) | 3 (12.0%) |
| Yes | 44 (89.8%) | 22 (91.7%) | 22 (88.0%) |
| Acute skin lesions, n (%) | |||
| None | 12 (24.5%) | 6 (25.0%) | 6 (24.0%) |
| Erythema | 35 (71.4%) | 17 (70.8%) | 18 (72.0%) |
| Weeping erythema | 8 (16.3%) | 2 (8.3%) | 6 (24.0%) |
| Papule | 19 (38.8%) | 8 (33.3%) | 11 (44.0%) |
| Serous papule | 5 (10.2%) | 1 (4.2%) | 4 (16.0%) |
| Scale | 14 (28.6%) | 5 (20.8%) | 9 (36.0%) |
| Scab | 9 (18.4%) | 4 (16.7%) | 5 (20.0%) |
| Chronic skin lesions, n (%) | |||
| None | 7 (14.3%) | 3 (12.5%) | 4 (16.0%) |
| Weeping Erythema/lichenification | 35 (71.4%) | 17 (70.8%) | 18 (72.0%) |
| Prurigo | 7 (14.3%) | 2 (8.3%) | 5 (20.0%) |
| Scale | 20 (40.8%) | 7 (29.2%) | 13 (52.0%) |
| Scab | 13 (26.5%) | 5 (20.8%) | 8 (32.0%) |
| Proactive Therapy Group (n = 24) | p †: vs. Start of Maintenance Treatment | Rank-Down Therapy Group (n = 25) | p †: vs. Start of Maintenance Treatment | p ‡: Between-Group Comparison | |
|---|---|---|---|---|---|
| Duration of remission, mean ± SD, Days | 28.3 ± 2.3 | 26.9 ± 4.3 | - | ||
| IGA score, mean ± SD | |||||
| At start of induction treatment | 3.0 ± 0.2 | 3.1 ± 0.3 | - | ||
| At start of maintenance treatment | 1.5 ± 0.5 | - | 1.8 ± 0.4 | - | p = 0.0618 |
| At 4 weeks | 1.4 ± 0.8 | p = 0.5637 | 1.6 ± 0.9 | p = 0.3711 | p = 0.5441 |
| Modified SCORAD score, mean ± SD | |||||
| Severity score | |||||
| At start of induction treatment | 48.7 ± 19.6 | 46.6 ± 18.3 | - | ||
| At start of maintenance treatment | 16.2 ± 6.2 | - | 21.5 ± 10.4 | - | p = 0.1025 |
| At 4 weeks | 17.3 ± 16.0 | p = 0.4579 | 25.4 ± 18.2 | p = 0.3100 | p = 0.1136 |
| Disease extent score | |||||
| At start of induction treatment | 43.2 ± 13.7 | 37.2 ± 12.4 | - | ||
| At start of maintenance treatment | 25.5 ± 10.2 | - | 26.0 ± 11.2 | - | p = 0.5174 |
| At 4 weeks | 26.3 ± 11.6 | p = 0.7514 | 25.9 ± 14.2 | p = 0.8079 | p = 0.9275 |
| NRS symptom score, mean ± SD | |||||
| Itching score | |||||
| At start of induction treatment | 5.4 ± 2.4 | 5.1 ± 2.1 | - | ||
| At start of maintenance treatment | 2.2 ± 2.0 | - | 2.5 ± 1.9 | - | p = 0.5491 |
| At 4 weeks | 2.8 ± 2.2 | p = 0.1877 | 3.6 ± 2.6 | p = 0.0438 | p = 0.2706 |
| Sleep disturbance score | |||||
| At start of induction treatment | 1.7 ± 2.4 | 2.6 ± 3.1 | - | ||
| At start of maintenance treatment | 1.2 ± 1.8 | - | 1.1 ± 1.6 | - | p = 0.9299 |
| At 4 weeks | 1.1 ± 2.1 | p = 0.8571 | 1.5 ± 1.9 | p = 0.3101 | p = 0.2619 |
| Total | Proactive Therapy Group | Rank-Down Therapy Group | |
|---|---|---|---|
| Safety analysis set, n | 49 | 24 | 25 |
| Patients with any adverse event, n (%) | 5 (10.2%) | 2 (8.3%) | 3 (12.0%) |
| Adverse events, n (%) | |||
| Infections and infestations | 2 (4.1%) | 0 | 2 (8.0%) |
| Upper respiratory tract infection | 1 (2.0%) | 0 | 1 (4.0%) |
| Infections and infestations, other (Kaposi’s varicelliform eruption) | 1 (2.0%) | 0 | 1 (4.0%) |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | 2 (4.1%) | 2 (8.3%) | 0 |
| Skin papilloma | 2 (4.1%) | 2 (8.3%) | 0 |
| Skin and subcutaneous tissue disorders | 1 (2.0%) | 0 | 1 (4.0%) |
| Cutaneous ulceration | 1 (2.0%) | 0 | 1 (4.0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamiya, K.; Saeki, H.; Tokura, Y.; Yoshihara, S.; Sugai, J.; Ohtsuki, M. Proactive versus Rank-Down Topical Corticosteroid Therapy for Maintenance of Remission in Pediatric Atopic Dermatitis: A Randomized, Open-Label, Active-Controlled, Parallel-Group Study (Anticipate Study). J. Clin. Med. 2022, 11, 6477. https://doi.org/10.3390/jcm11216477
Kamiya K, Saeki H, Tokura Y, Yoshihara S, Sugai J, Ohtsuki M. Proactive versus Rank-Down Topical Corticosteroid Therapy for Maintenance of Remission in Pediatric Atopic Dermatitis: A Randomized, Open-Label, Active-Controlled, Parallel-Group Study (Anticipate Study). Journal of Clinical Medicine. 2022; 11(21):6477. https://doi.org/10.3390/jcm11216477
Chicago/Turabian StyleKamiya, Koji, Hidehisa Saeki, Yoshiki Tokura, Shigemi Yoshihara, Junichi Sugai, and Mamitaro Ohtsuki. 2022. "Proactive versus Rank-Down Topical Corticosteroid Therapy for Maintenance of Remission in Pediatric Atopic Dermatitis: A Randomized, Open-Label, Active-Controlled, Parallel-Group Study (Anticipate Study)" Journal of Clinical Medicine 11, no. 21: 6477. https://doi.org/10.3390/jcm11216477
APA StyleKamiya, K., Saeki, H., Tokura, Y., Yoshihara, S., Sugai, J., & Ohtsuki, M. (2022). Proactive versus Rank-Down Topical Corticosteroid Therapy for Maintenance of Remission in Pediatric Atopic Dermatitis: A Randomized, Open-Label, Active-Controlled, Parallel-Group Study (Anticipate Study). Journal of Clinical Medicine, 11(21), 6477. https://doi.org/10.3390/jcm11216477






