A Mini Review on Osteoporosis: From Biology to Pharmacological Management of Bone Loss
Abstract
1. Introduction
2. Pathophysiology of Osteoporosis
3. Detection and Diagnosis of Osteoporosis
4. Epidemiology and Burden of Osteoporosis
5. Risk Factors of Osteoporosis
| Non-Modifiable Risk Factor | Explanation |
|---|---|
| Age | Accumulation of cellular damage, cellular senescence, chronic low-grade ageing and endocrine deficiency [100,136]. |
| Female | Women have lower peak bone mass compared to men and accelerated bone loss during menopause [16]. |
| Genetics | Polymorphisms of genes such as VDR [137], ER [138], OPG [139], COL1A1 [140] and TNFα [141] are associated with osteoporosis and fracture risk. |
| Family history | A family history of fractures in parents, particularly at the hip, is significantly associated with fracture risk [142]. |
| Medical conditions | Rheumatoid arthritis: characterised by systematic inflammation that is pro-osteoclastogenesis [107]. Hypogonadism: sex hormone deficiency that impairs bone formation and stimulates bone resorption [98] Chronic renal failure: characterised by dysregulation of mineral metabolism, leading to hyperphosphataemia, hyperparathyroidism, hypocalcaemia and decreased vitamin D synthesis [143]. Cancer: pathological cancer due to bone metastasis and the damaging effects of anticancer treatments on bone cells [144]. |
| Medications | Glucocorticoids impair osteogenesis and indirectly stimulate effects on osteoclasts [112]. Sex hormone deprivation therapies induce sex hormone deficiency and subsequent bone loss [108]. Proton pump inhibitors induce mineral malabsorption and vitamin B deficiency [109] Anticonvulsants affect vitamin D conversation through cytochrome P450 enzymes, reduce calcitonin synthesis and calcium absorption [145]. Anticoagulants (vitamin K antagonists) prevent the action of bone Gla-protein and mineralisation process [114]. Non-vitamin K antagonist oral anticoagulants do not affect this process, thus not increasing fracture risk. |
| Modifiable risk factors | Explanation |
| Low body weight | Body weight exerts mechanical loading onto the bone [146]. Being underweight is also an indicator of malnutrition, which affects body metabolism [147]. Low body weight is a risk factor for fracture, and this relationship is associated with BMD [148]. |
| Sedentary lifestyle | Physical activities exert mechanical loading onto the bone. They also increase the level of sex hormones and anti-inflammatory cytokines, as well as suppress pro-inflammatory cytokines in the system, which are protective of bone health [149]. |
| Calcium deficiency Vitamin D deficiency | Calcium deficiency will induce hyperparathyroidism, which mobilizes calcium stores from the bone. The deficiency of vitamin D, which helps with calcium absorption, also causes the same effect [129]. |
| Cigarette smoking | Chemicals in cigarettes are toxic to the bone cells, and induce changes in the RANK/RANKL/OPG, calcium absorption, sex hormones and cortisol levels, resulting in net bone loss [42]. |
| Alcohol consumption | Alcohol reduces calcium reserves and damages the pancreas, leading to low vitamin D synthesis and poor calcium absorption [41]. |
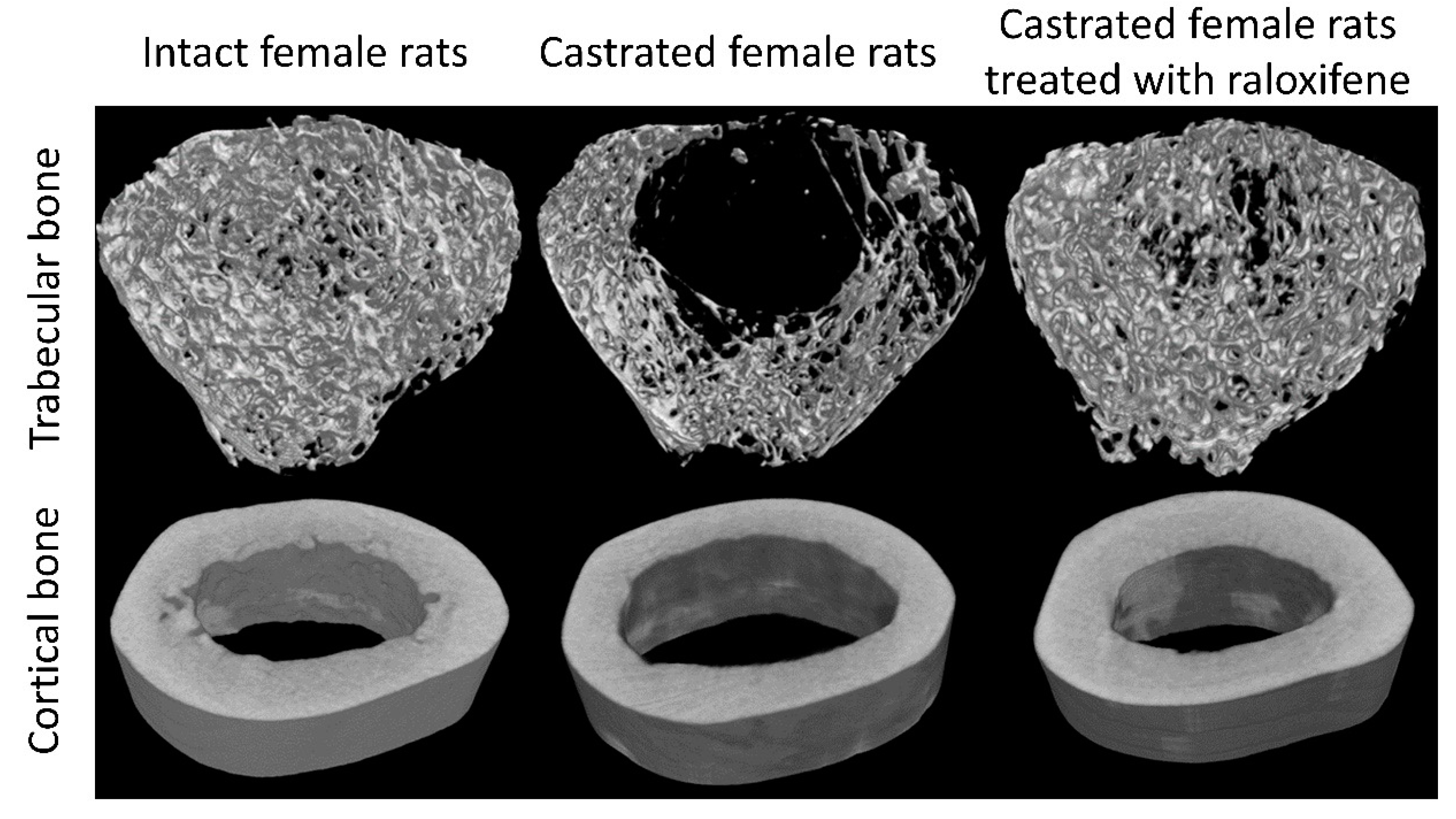
6. Pharmacological Treatments for Osteoporosis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organisation. GHE: Life Expectancy and Healthy Life Expectancy. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-life-expectancy-and-healthy-life-expectancy (accessed on 28 September 2022).
- Niccoli, T.; Partridge, L. Ageing as a Risk Factor for Disease. Curr. Biol. 2012, 22, R741–R752. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.; Goltzman, D.; Langsetmo, L.; Joseph, L.; Jackson, S.; Kreiger, N.; Tenenhouse, A.; Davison, K.S.; Josse, R.G.; Prior, J.C.; et al. Peak bone mass from longitudinal data: Implications for the prevalence, pathophysiology, and diagnosis of osteoporosis. J. Bone Miner. Res. 2010, 25, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Specker, B.L.; Wey, H.E.; Smith, E.P. Rates of bone loss in young adult males. Int. J. Clin. Rheumtol. 2010, 5, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Alswat, K.A. Gender Disparities in Osteoporosis. J. Clin. Med. Res. 2017, 9, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Lorentzon, M.; Cummings, S.R. Osteoporosis: The evolution of a diagnosis. J. Intern. Med. 2015, 277, 650–661. [Google Scholar] [CrossRef]
- Mitchell, P.J.; Chan, D.D.; Lee, J.K.; Tabu, I.; Alpuerto, B.B. The global burden of fragility fractures—what are the differences, and where are the gaps. Best Pract. Res. Clin. Rheumatol. 2022, 101777. [Google Scholar] [CrossRef]
- Hachuła, M.; Pietrzyk, B.; Gruszka, W.; Cedrych, I.; Chudek, J. High rates of undiagnosed and untreated osteoporosis in postmenopausal women receiving medical services in the area of Upper Silesia. Prz. Menopauzalny 2020, 19, 72–79. [Google Scholar] [CrossRef]
- Clarke, B.L.; Khosla, S. Physiology of bone loss. Radiol. Clin. N. Am. 2010, 48, 483–495. [Google Scholar] [CrossRef]
- Ma, Q.; Liang, M.; Wu, Y.; Luo, F.; Ma, Z.; Dong, S.; Xu, J.; Dou, C. Osteoclast-derived apoptotic bodies couple bone resorption and formation in bone remodeling. Bone Res. 2021, 9, 5. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, Y.; Pacios, S.; Li, S.; Graves, D.T. Cellular and Molecular Aspects of Bone Remodeling. Front. Oral Biol. 2016, 18, 9–16. [Google Scholar] [CrossRef]
- Prideaux, M.; Findlay, D.M.; Atkins, G.J. Osteocytes: The master cells in bone remodelling. Curr. Opin. Pharmacol 2016, 28, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG Pathway: A Mechanism Involved in Exercise-Induced Bone Remodeling. Biomed. Res. Int. 2020, 2020, 6910312. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Ekeuku, S.O.; Pang, K.L. Sclerostin in the development of osteoarthritis: A mini review. Malays J. Pathol 2022, 44, 1–18. [Google Scholar] [PubMed]
- Siddiqui, J.A.; Partridge, N.C. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology 2016, 31, 233–245. [Google Scholar] [CrossRef]
- Ji, M.X.; Yu, Q. Primary osteoporosis in postmenopausal women. Chronic Dis. Transl Med. 2015, 1, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y. The Relationship between Follicle-stimulating Hormone and Bone Health: Alternative Explanation for Bone Loss beyond Oestrogen? Int. J. Med. Sci. 2018, 15, 1373–1383. [Google Scholar] [CrossRef]
- Chin, K.Y.; Ima-Nirwana, S. Sex steroids and bone health status in men. Int. J. Endocrinol. 2012, 2012, 208719. [Google Scholar] [CrossRef]
- Mohamad, N.V.; Soelaiman, I.N.; Chin, K.Y. A concise review of testosterone and bone health. Clin. Interv. Aging 2016, 11, 1317–1324. [Google Scholar] [CrossRef]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef]
- Mohamad, N.V.; Ima-Nirwana, S.; Chin, K.Y. Are Oxidative Stress and Inflammation Mediators of Bone Loss Due to Estrogen Deficiency? A Review of Current Evidence. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1478–1487. [Google Scholar] [CrossRef]
- Kim, O.Y.; Chae, J.S.; Paik, J.K.; Seo, H.S.; Jang, Y.; Cavaillon, J.M.; Lee, J.H. Effects of aging and menopause on serum interleukin-6 levels and peripheral blood mononuclear cell cytokine production in healthy nonobese women. Age 2012, 34, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.V.; Ima-Nirwana, S.; Chin, K.Y. Therapeutic potential of annatto tocotrienol with self-emulsifying drug delivery system in a rat model of postmenopausal bone loss. Biomed. Pharmacother. 2021, 137, 111368. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.K.; Dar, H.Y.; Mishra, P.K. Immunoporosis: Immunology of Osteoporosis-Role of T Cells. Front. Immunol. 2018, 9, 657. [Google Scholar] [CrossRef] [PubMed]
- Bozec, A.; Zaiss, M.M. T Regulatory Cells in Bone Remodelling. Curr. Osteoporos. Rep. 2017, 15, 121–125. [Google Scholar] [CrossRef]
- Zaiss, M.M.; Sarter, K.; Hess, A.; Engelke, K.; Böhm, C.; Nimmerjahn, F.; Voll, R.; Schett, G.; David, J.P. Increased bone density and resistance to ovariectomy-induced bone loss in FoxP3-transgenic mice based on impaired osteoclast differentiation. Arthritis Rheum 2010, 62, 2328–2338. [Google Scholar] [CrossRef]
- Cline-Smith, A.; Axelbaum, A.; Shashkova, E.; Chakraborty, M.; Sanford, J.; Panesar, P.; Peterson, M.; Cox, L.; Baldan, A.; Veis, D.; et al. Ovariectomy Activates Chronic Low-Grade Inflammation Mediated by Memory T Cells, Which Promotes Osteoporosis in Mice. J. Bone Miner. Res. 2020, 35, 1174–1187. [Google Scholar] [CrossRef]
- Fessler, J.; Husic, R.; Schwetz, V.; Lerchbaum, E.; Aberer, F.; Fasching, P.; Ficjan, A.; Obermayer-Pietsch, B.; Duftner, C.; Graninger, W.; et al. Senescent T-Cells Promote Bone Loss in Rheumatoid Arthritis. Front. Immunol. 2018, 9, 95. [Google Scholar] [CrossRef]
- Pietschmann, P.; Mechtcheriakova, D.; Meshcheryakova, A.; Föger-Samwald, U.; Ellinger, I. Immunology of Osteoporosis: A Mini-Review. Gerontology 2016, 62, 128–137. [Google Scholar] [CrossRef]
- Das, M.; Cronin, O.; Keohane, D.M.; Cormac, E.M.; Nugent, H.; Nugent, M.; Molloy, C.; O’Toole, P.W.; Shanahan, F.; Molloy, M.G.; et al. Gut microbiota alterations associated with reduced bone mineral density in older adults. Rheumatology 2019, 58, 2295–2304. [Google Scholar] [CrossRef]
- Ding, K.; Hua, F.; Ding, W. Gut Microbiome and Osteoporosis. Aging Dis. 2020, 11, 438–447. [Google Scholar] [CrossRef]
- Rios-Arce, N.D.; Schepper, J.D.; Dagenais, A.; Schaefer, L.; Daly-Seiler, C.S.; Gardinier, J.D.; Britton, R.A.; McCabe, L.R.; Parameswaran, N. Post-antibiotic gut dysbiosis-induced trabecular bone loss is dependent on lymphocytes. Bone 2020, 134, 115269. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Vitetta, L. The Role of Butyrate in Attenuating Pathobiont-Induced Hyperinflammation. Immune Netw. 2020, 20, e15. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gu, X.; Yang, J.; Wei, Y.; Zhao, Y. Gut Microbiota Dysbiosis and Increased Plasma LPS and TMAO Levels in Patients With Preeclampsia. Front. Cell. Infect. Microbiol. 2019, 9, 409. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.D.; Naylor, A.J.; Buckley, C.; Filer, A.; Tak, P.-P. Fibroblasts and Osteoblasts in Inflammation and Bone Damage. In Stromal Immunology; Owens, B.M.J., Lakins, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 37–54. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Lee, Y.J.; Hong, J.Y.; Kim, S.C.; Joo, J.K.; Na, Y.J.; Lee, K.S. The association between oxidative stress and bone mineral density according to menopausal status of Korean women. Obstet. Gynecol. Sci. 2015, 58, 46–52. [Google Scholar] [CrossRef]
- Wu, J.; Su, J.; Wang, Y.; Chen, J.; Shang, Y.; Li, J. Association between total bilirubin and bone mineral density level in adolescents. BMC Musculoskelet. Disord. 2022, 23, 639. [Google Scholar] [CrossRef]
- Chin, K.Y.; Ima-Nirwana, S. The effects of alpha-tocopherol on bone: A double-edged sword? Nutrients 2014, 6, 1424–1441. [Google Scholar] [CrossRef]
- Chin, K.Y.; Ima-Nirwana, S. Vitamin C and Bone Health: Evidence from Cell, Animal and Human Studies. Curr. Drug Targets 2018, 19, 439–450. [Google Scholar] [CrossRef]
- Maurel, D.B.; Boisseau, N.; Benhamou, C.L.; Jaffre, C. Alcohol and bone: Review of dose effects and mechanisms. Osteoporos. Int. 2012, 23, 1–16. [Google Scholar] [CrossRef]
- Al-Bashaireh, A.M.; Haddad, L.G.; Weaver, M.; Chengguo, X.; Kelly, D.L.; Yoon, S. The Effect of Tobacco Smoking on Bone Mass: An Overview of Pathophysiologic Mechanisms. J. Osteoporos. 2018, 2018, 1206235. [Google Scholar] [CrossRef]
- Agidigbi, T.S.; Kim, C. Reactive Oxygen Species in Osteoclast Differentiation and Possible Pharmaceutical Targets of ROS-Mediated Osteoclast Diseases. Int. J. Mol. Sci. 2019, 20, 3576. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis: Report of a World Health Organization Study Group; World Health Organization: Geneva, Switzerland, 1994. [Google Scholar]
- Leslie, W.D.; Lix, L.M.; Tsang, J.F.; Caetano, P.A.; Program, M.B.D. Single-Site vs Multisite Bone Density Measurement for Fracture Prediction. Arch. Intern. Med. 2007, 167, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Lundstam, K.; Godang, K.; Pretorius, M.; Markwardt, P.; Hellström, M.; Bollerslev, J.; Heck, A. The Influence of DXA Hardware, Software, Reference Population and Software Analysis Settings on the Bone Mineral Density and T-Score Relationship. J. Clin. Densitom. 2022, 25, 24–33. [Google Scholar] [CrossRef]
- Yeap, S.S.; Thambiah, S.C.; Samsudin, I.N.; Appannah, G.; Zainuddin, N.; Mohamad-Ismuddin, S.; Shahifar, N.; Md-Said, S.; Zahari-Sham, S.Y.; Suppiah, S.; et al. Different reference ranges affect the prevalence of osteoporosis and osteopenia in an urban adult Malaysian population. Osteoporos. Sarcopenia 2020, 6, 168–172. [Google Scholar] [CrossRef]
- Lewiecki, E.M.; Binkley, N.; Morgan, S.L.; Shuhart, C.R.; Camargos, B.M.; Carey, J.J.; Gordon, C.M.; Jankowski, L.G.; Lee, J.K.; Leslie, W.D. Best Practices for Dual-Energy X-ray Absorptiometry Measurement and Reporting: International Society for Clinical Densitometry Guidance. J. Clin. Densitom. 2016, 19, 127–140. [Google Scholar] [CrossRef]
- Martineau, P.; Leslie, W.D. The utility and limitations of using trabecular bone score with FRAX. Curr. Opin. Rheumatol. 2018, 30, 412–419. [Google Scholar] [CrossRef]
- McCloskey, E.V.; Odén, A.; Harvey, N.C.; Leslie, W.D.; Hans, D.; Johansson, H.; Barkmann, R.; Boutroy, S.; Brown, J.; Chapurlat, R.; et al. A Meta-Analysis of Trabecular Bone Score in Fracture Risk Prediction and Its Relationship to FRAX. J. Bone Miner. Res. 2016, 31, 940–948. [Google Scholar] [CrossRef]
- Rajan, R.; Cherian, K.E.; Kapoor, N.; Paul, T.V. Trabecular Bone Score-An Emerging Tool in the Management of Osteoporosis. Indian J. Endocrinol. Metab. 2020, 24, 237–243. [Google Scholar] [CrossRef]
- Chin, K.Y.; Ima-Nirwana, S. Calcaneal quantitative ultrasound as a determinant of bone health status: What properties of bone does it reflect? Int. J. Med. Sci. 2013, 10, 1778–1783. [Google Scholar] [CrossRef]
- Subramaniam, S.; Chan, C.Y.; Soelaiman, I.N.; Mohamed, N.; Muhammad, N.; Ahmad, F.; Ng, P.Y.; Jamil, N.A.; Aziz, N.A.; Chin, K.Y. The Performance of a Calcaneal Quantitative Ultrasound Device, CM-200, in Stratifying Osteoporosis Risk among Malaysian Population Aged 40 Years and Above. Diagnostics 2020, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Krieg, M.A.; Barkmann, R.; Gonnelli, S.; Stewart, A.; Bauer, D.C.; Del Rio Barquero, L.; Kaufman, J.J.; Lorenc, R.; Miller, P.D.; Olszynski, W.P.; et al. Quantitative ultrasound in the management of osteoporosis: The 2007 ISCD Official Positions. J. Clin. Densitom. 2008, 11, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.L.; Ang, S.B.; Chadha, M.; Chow, E.S.; Chung, Y.S.; Hew, F.L.; Jaisamrarn, U.; Ng, H.; Takeuchi, Y.; Wu, C.H.; et al. An updated hip fracture projection in Asia: The Asian Federation of Osteoporosis Societies study. Osteoporos. Sarcopenia 2018, 4, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Graffy, P.M.; Ziemlewicz, T.J.; Lee, S.J.; Summers, R.M.; Pickhardt, P.J. Opportunistic Osteoporosis Screening at Routine Abdominal and Thoracic CT: Normative L1 Trabecular Attenuation Values in More than 20 000 Adults. Radiology 2019, 291, 360–367. [Google Scholar] [CrossRef]
- Brett, A.D.; Brown, J.K. Quantitative computed tomography and opportunistic bone density screening by dual use of computed tomography scans. J. Orthop. Translat. 2015, 3, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Genant, H.K.; Engelke, K.; Prevrhal, S. Advanced CT bone imaging in osteoporosis. Rheumatology 2008, 47 (Suppl. 4), iv9–iv16. [Google Scholar] [CrossRef]
- Toh, L.S.; Lai, P.S.M.; Wu, D.B.-C.; Bell, B.G.; Dang, C.P.L.; Low, B.Y.; Wong, K.T.; Guglielmi, G.; Anderson, C. A comparison of 6 osteoporosis risk assessment tools among postmenopausal women in Kuala Lumpur, Malaysia. Osteoporos. Sarcopenia 2019, 5, 87–93. [Google Scholar] [CrossRef]
- Crandall, C.J. Risk Assessment Tools for Osteoporosis Screening in Postmenopausal Women: A Systematic Review. Curr. Osteoporos. Rep. 2015, 13, 287–301. [Google Scholar] [CrossRef]
- Chin, K.Y. A review on the performance of osteoporosis self-assessment tool for Asians in determining osteoporosis and fracture risk. Postgrad. Med. 2017, 129, 734–746. [Google Scholar] [CrossRef]
- Subramaniam, S.; Chan, C.Y.; Soelaiman, I.N.; Mohamed, N.; Muhammad, N.; Ahmad, F.; Ng, P.Y.; Jamil, N.A.; Aziz, N.A.; Chin, K.Y. The performance of osteoporosis self-assessment tool for Asians (OSTA) in identifying the risk of osteoporosis among Malaysian population aged 40 years and above. Arch. Osteoporos. 2019, 14, 117. [Google Scholar] [CrossRef]
- Subramaniam, S.; Ima-Nirwana, S.; Chin, K.Y. Performance of Osteoporosis Self-Assessment Tool (OST) in Predicting Osteoporosis-A Review. Int. J. Environ. Res. Public Health 2018, 15, 1445. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, E.V.; Harvey, N.C.; Johansson, H.; Lorentzon, M.; Liu, E.; Vandenput, L.; Leslie, W.D.; Kanis, J.A. Fracture risk assessment by the FRAX model. Climacteric 2022, 25, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Harvey, N.C.; Cooper, C.; Johansson, H.; Odén, A.; McCloskey, E.V. A systematic review of intervention thresholds based on FRAX: A report prepared for the National Osteoporosis Guideline Group and the International Osteoporosis Foundation. Arch. Osteoporos. 2016, 11, 25. [Google Scholar] [CrossRef]
- Kanis, J.A.; Harvey, N.C.; Johansson, H.; Odén, A.; Leslie, W.D.; McCloskey, E.V. FRAX and fracture prediction without bone mineral density. Climacteric 2015, 18 (Suppl. 2), 2–9. [Google Scholar] [CrossRef]
- Greenblatt, M.B.; Tsai, J.N.; Wein, M.N. Bone Turnover Markers in the Diagnosis and Monitoring of Metabolic Bone Disease. Clin. Chem. 2017, 63, 464–474. [Google Scholar] [CrossRef]
- Jain, S.; Camacho, P. Use of bone turnover markers in the management of osteoporosis. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Liu, D.; Wu, F.; Wang, M.; Cen, Y.; Ma, L. Correlation between Bone Turnover Markers and Bone Mineral Density in Patients Undergoing Long-Term Anti-Osteoporosis Treatment: A Systematic Review and Meta-Analysis. Appl. Sci. 2020, 10, 832. [Google Scholar] [CrossRef]
- Eastell, R.; Szulc, P. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol. 2017, 5, 908–923. [Google Scholar] [CrossRef]
- Cheng, X.; Zhao, K.; Zha, X.; Du, X.; Li, Y.; Chen, S.; Wu, Y.; Li, S.; Lu, Y.; Zhang, Y.; et al. Opportunistic Screening Using Low-Dose CT and the Prevalence of Osteoporosis in China: A Nationwide, Multicenter Study. J. Bone Miner. Res. 2021, 36, 427–435. [Google Scholar] [CrossRef]
- Chan, C.Y.; Subramaniam, S.; Mohamed, N.; Muhammad, N.; Ramli, F.F.; Ima-Nirwana, S.; Chin, K.Y. Circulating Biomarkers Related to Osteocyte and Calcium Homeostasis between Postmenopausal Women with and without Osteoporosis. Endocr Metab. Immune Disord. Drug Targets 2021, 21, 2273–2280. [Google Scholar] [CrossRef]
- Ramli, F.F.; Chin, K.Y. A Review of the Potential Application of Osteocyte-Related Biomarkers, Fibroblast Growth Factor-23, Sclerostin, and Dickkopf-1 in Predicting Osteoporosis and Fractures. Diagnostics 2020, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhou, X.; Chen, L.; Huang, S.; Leung, V.; Wu, N.; Pan, H.; Zhen, W.; Lu, W.; Peng, S. The Regulatory Roles of MicroRNAs in Bone Remodeling and Perspectives as Biomarkers in Osteoporosis. Biomed. Res. Int. 2016, 2016, 1652417. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.L.; Esa, M.S.; Li, K.H.C.; Krishnan, S.R.G.; Elgallab, G.M.; Pearce, M.S.; Young, D.A.; Birrell, F.N. Osteoporosis, fracture, osteoarthritis & sarcopenia: A systematic review of circulating microRNA association. Bone 2021, 152, 116068. [Google Scholar] [CrossRef] [PubMed]
- Materozzi, M.; Merlotti, D.; Gennari, L.; Bianciardi, S. The Potential Role of miRNAs as New Biomarkers for Osteoporosis. Int. J. Endocrinol. 2018, 2018, 2342860. [Google Scholar] [CrossRef]
- Kerschan-Schindl, K.; Hackl, M.; Boschitsch, E.; Föger-Samwald, U.; Nägele, O.; Skalicky, S.; Weigl, M.; Grillari, J.; Pietschmann, P. Diagnostic Performance of a Panel of miRNAs (OsteomiR) for Osteoporosis in a Cohort of Postmenopausal Women. Calcif. Tissue Int. 2021, 108, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Pawłowski, W.; Goniewicz, K.; Goniewicz, M.; Lasota, D. Public health impact of osteoporosis in older age. J. Pre-Clin. Clin. Res. 2018, 12, 106–109. [Google Scholar] [CrossRef]
- Xiao, P.L.; Cui, A.Y.; Hsu, C.J.; Peng, R.; Jiang, N.; Xu, X.H.; Ma, Y.G.; Liu, D.; Lu, H.D. Global, regional prevalence, and risk factors of osteoporosis according to the World Health Organization diagnostic criteria: A systematic review and meta-analysis. Osteoporos. Int. 2022, 33, 2137–2153. [Google Scholar] [CrossRef]
- Wright, N.C.; Looker, A.C.; Saag, K.G.; Curtis, J.R.; Delzell, E.S.; Randall, S.; Dawson-Hughes, B. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J. Bone Miner. Res. 2014, 29, 2520–2526. [Google Scholar] [CrossRef]
- Marquez, M.A.; Melton, L.J., 3rd; Muhs, J.M.; Crowson, C.S.; Tosomeen, A.; O’Connor, M.K.; O’Fallon, W.M.; Riggs, B.L. Bone density in an immigrant population from Southeast Asia. Osteoporos. Int. 2001, 12, 595–604. [Google Scholar] [CrossRef]
- Mehta, G.; Taylor, P.; Petley, G.; Dennison, E.; Cooper, C.; Walker-Bone, K. Bone mineral status in immigrant Indo-Asian women. QJM 2004, 97, 95–99. [Google Scholar] [CrossRef][Green Version]
- Lim, P.S.; Ong, F.B.; Adeeb, N.; Seri, S.S.; Noor-Aini, M.Y.; Shamsuddin, K.; Hapizah, N.; Mohamed, A.L.; Mokhtar, A.; Wan, H.W. Bone health in urban midlife Malaysian women: Risk factors and prevention. Osteoporos. Int. 2005, 16, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.Y.; Subramaniam, S.; Mohamed, N.; Ima-Nirwana, S.; Muhammad, N.; Fairus, A.; Ng, P.Y.; Jamil, N.A.; Abd Aziz, N.; Chin, K.Y. Determinants of Bone Health Status in a Multi-Ethnic Population in Klang Valley, Malaysia. Int. J. Environ. Res. Public Health 2020, 17, 384. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). Clinical Guideline [CG146]: Osteoporosis: Assessing the Risk of Fragility Fracture. Available online: https://www.nice.org.uk/guidance/cg146/resources (accessed on 28 September 2022).
- Kanis, J.A.; Norton, N.; Harvey, N.C.; Jacobson, T.; Johansson, H.; Lorentzon, M.; McCloskey, E.V.; Willers, C.; Borgström, F. SCOPE 2021: A new scorecard for osteoporosis in Europe. Arch. Osteoporos. 2021, 16, 82. [Google Scholar] [CrossRef]
- Lau, E.M.; Lee, J.K.; Suriwongpaisal, P.; Saw, S.M.; Das De, S.; Khir, A.; Sambrook, P. The incidence of hip fracture in four Asian countries: The Asian Osteoporosis Study (AOS). Osteoporos. Int. 2001, 12, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Wright, N.C.; Saag, K.G.; Curtis, J.R.; Smith, W.K.; Kilgore, M.L.; Morrisey, M.A.; Yun, H.; Zhang, J.; Delzell, E.S. Recent trends in hip fracture rates by race/ethnicity among older US adults. J. Bone Miner. Res. 2012, 27, 2325–2332. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-K.; Khir, A.S.M. The incidence of hip fracture in Malaysians above 50 years of age: Variation in different ethnic groups. APLAR J. Rheumatol. 2007, 10, 300–305. [Google Scholar] [CrossRef]
- Haentjens, P.; Magaziner, J.; Colón-Emeric, C.S.; Vanderschueren, D.; Milisen, K.; Velkeniers, B.; Boonen, S. Meta-analysis: Excess mortality after hip fracture among older women and men. Ann. Intern. Med. 2010, 152, 380–390. [Google Scholar] [CrossRef]
- Borhan, S.; Papaioannou, A.; Gajic-Veljanoski, O.; Kennedy, C.; Ioannidis, G.; Berger, C.; Goltzman, D.; Josse, R.; Kovacs, C.S.; Hanley, D.A.; et al. Incident Fragility Fractures Have a Long-Term Negative Impact on Health-Related Quality of Life of Older People: The Canadian Multicentre Osteoporosis Study. J. Bone Miner. Res. 2019, 34, e3666. [Google Scholar] [CrossRef]
- Williamson, S.; Landeiro, F.; McConnell, T.; Fulford-Smith, L.; Javaid, M.K.; Judge, A.; Leal, J. Costs of fragility hip fractures globally: A systematic review and meta-regression analysis. Osteoporos. Int. 2017, 28, 2791–2800. [Google Scholar] [CrossRef]
- Clynes, M.A.; Harvey, N.C.; Curtis, E.M.; Fuggle, N.R.; Dennison, E.M.; Cooper, C. The epidemiology of osteoporosis. Br. Med. Bull. 2020, 133, 105–117. [Google Scholar] [CrossRef]
- Woratanarat, P.; Wajanavisit, W.; Lertbusayanukul, C.; Loahacharoensombat, W.; Ongphiphatanakul, B. Cost analysis of osteoporotic hip fractures. J. Med. Assoc. Thai. 2005, 88 (Suppl. 5), S96–S104. [Google Scholar] [PubMed]
- Pouresmaeili, F.; Kamalidehghan, B.; Kamarehei, M.; Goh, Y.M. A comprehensive overview on osteoporosis and its risk factors. Ther. Clin. Risk Manag. 2018, 14, 2029–2049. [Google Scholar] [CrossRef] [PubMed]
- Farr, J.N.; Khosla, S. Cellular senescence in bone. Bone 2019, 121, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Monroe, D.G. Regulation of Bone Metabolism by Sex Steroids. Cold Spring Harb. Perspect. Med. 2018, 8, a031211. [Google Scholar] [CrossRef] [PubMed]
- den Uyl, D.; van Schoor, N.M.; Bravenboer, N.; Lips, P.; Lems, W.F. Low grade inflammation is associated with lower velocity of sound and broadband ultrasound attenuation in older men, but not with bone loss or fracture risk in a longitudinal aging study. Bone 2015, 81, 270–276. [Google Scholar] [CrossRef]
- Josephson, A.M.; Bradaschia-Correa, V.; Lee, S.; Leclerc, K.; Patel, K.S.; Muinos Lopez, E.; Litwa, H.P.; Neibart, S.S.; Kadiyala, M.; Wong, M.Z.; et al. Age-related inflammation triggers skeletal stem/progenitor cell dysfunction. Proc. Natl. Acad. Sci. USA 2019, 116, 6995–7004. [Google Scholar] [CrossRef]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.H.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 609. [Google Scholar] [CrossRef]
- Nam, H.S.; Kweon, S.S.; Choi, J.S.; Zmuda, J.M.; Leung, P.C.; Lui, L.Y.; Hill, D.D.; Patrick, A.L.; Cauley, J.A. Racial/ethnic differences in bone mineral density among older women. J. Bone Miner. Metab. 2013, 31, 190–198. [Google Scholar] [CrossRef]
- Hayhoe, R.P.G.; Chan, R.; Skinner, J.; Leung, J.; Jennings, A.; Khaw, K.-T.; Woo, J.; Welch, A.A. Fracture Incidence and the Relevance of Dietary and Lifestyle Factors Differ in the United Kingdom and Hong Kong: An International Comparison of Longitudinal Cohort Study Data. Calcif. Tissue Int. 2021, 109, 563–576. [Google Scholar] [CrossRef]
- Bijelic, R.; Milicevic, S.; Balaban, J. The Influence of Non-preventable Risk Factors on the Development of Osteoporosis in Postmenopausal Women. Mater. Sociomed. 2019, 31, 62–65. [Google Scholar] [CrossRef]
- Zhai, G.; Andrew, T.; Kato, B.S.; Blake, G.M.; Spector, T.D. Genetic and environmental determinants on bone loss in postmenopausal Caucasian women: A 14-year longitudinal twin study. Osteoporos. Int. 2009, 20, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Llorente, I.; Garcia-Castaneda, N.; Valero, C.; Gonzalez-Alvaro, I.; Castaneda, S. Osteoporosis in Rheumatoid Arthritis: Dangerous Liaisons. Front. Med. (Lausanne) 2020, 7, 601618. [Google Scholar] [CrossRef] [PubMed]
- Sakthiswary, R.; Uma Veshaaliini, R.; Chin, K.-Y.; Das, S.; Sirasanagandla, S.R. Pathomechanisms of bone loss in rheumatoid arthritis. Front. Med. 2022, 9, 962969. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.V.; Soelaiman, I.N.; Chin, K.Y. A Review on the Effects of Androgen Deprivation Therapy (ADT) on Bone Health Status in Men with Prostate Cancer. Endocr. Metab. Immune Disord. Drug Targets 2017, 17, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Thong, B.K.S.; Ima-Nirwana, S.; Chin, K.Y. Proton Pump Inhibitors and Fracture Risk: A Review of Current Evidence and Mechanisms Involved. Int. J. Environ. Res. Public Health 2019, 16, 1571. [Google Scholar] [CrossRef]
- Ekeuku, S.O.; Mohd Ramli, E.S.; Abdullah Sani, N.; Abd Ghafar, N.; Soelaiman, I.N.; Chin, K.-Y. Tocotrienol as a Protecting Agent against Glucocorticoid-Induced Osteoporosis: A Mini Review of Potential Mechanisms. Molecules 2022, 27, 5862. [Google Scholar] [CrossRef]
- Panday, K.; Gona, A.; Humphrey, M.B. Medication-induced osteoporosis: Screening and treatment strategies. Ther. Adv. Musculoskelet. Dis. 2014, 6, 185–202. [Google Scholar] [CrossRef]
- Compston, J. Glucocorticoid-induced osteoporosis: An update. Endocrine 2018, 61, 7–16. [Google Scholar] [CrossRef]
- Yokoyama, S.; Ieda, S.; Nagano, M.; Nakagawa, C.; Iwase, M.; Hosomi, K.; Takada, M. Association between oral anticoagulants and osteoporosis: Real-world data mining using a multi-methodological approach. Int. J. Med. Sci. 2020, 17, 471–479. [Google Scholar] [CrossRef]
- Chin, K.Y.; Pang, K.L.; Wong, S.K.; Chew, D.C.H.; Qodriyah, H.M.S. Relationship Amongst Vitamin K Status, Vitamin K Antagonist Use and Osteoarthritis: A Review. Drugs Aging 2022, 39, 487–504. [Google Scholar] [CrossRef]
- Gu, Z.-C.; Zhou, L.-Y.; Shen, L.; Zhang, C.; Pu, J.; Lin, H.-W.; Liu, X.-Y. Non-vitamin K Antagonist Oral Anticoagulants vs. Warfarin at Risk of Fractures: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2018, 9, 348. [Google Scholar] [CrossRef] [PubMed]
- Sundararaghavan, V.; Mazur, M.M.; Evans, B.; Liu, J.; Ebraheim, N.A. Diabetes and bone health: Latest evidence and clinical implications. Ther. Adv. Musculoskelet. Dis. 2017, 9, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Xu, J.; Zhou, M.; Lian, X.; Xu, J.; Shi, J. Association between type 1 diabetes mellitus and reduced bone mineral density in children: A meta-analysis. Osteoporos. Int. 2021, 32, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Oei, L.; Jiang, L.; Estrada, K.; Chen, H.; Wang, Z.; Yu, Q.; Zillikens, M.C.; Gao, X.; Rivadeneira, F. Association between bone mineral density and type 2 diabetes mellitus: A meta-analysis of observational studies. Eur. J. Epidemiol. 2012, 27, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Li, C.; Dong, Z.; Wang, J. Is diabetes mellitus a risk factor for low bone density: A systematic review and meta-analysis. BMC Endocr. Disord. 2021, 21, 65. [Google Scholar] [CrossRef]
- Chin, K.Y.; Wong, S.K.; Ekeuku, S.O.; Pang, K.L. Relationship Between Metabolic Syndrome and Bone Health—An Evaluation of Epidemiological Studies and Mechanisms Involved. Diabetes Metab. Syndr. Obes. 2020, 13, 3667–3690. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.; Ahmad, F.; Ima-Nirwana, S. The Relationship between Metabolic Syndrome and Osteoporosis: A Review. Nutrients 2016, 8, 347. [Google Scholar] [CrossRef]
- Kumari, C.; Yagoub, G.; Ashfaque, M.; Jawed, S.; Hamid, P. Consequences of Diabetes Mellitus in Bone Health: Traditional Review. Cureus 2021, 13, e13820. [Google Scholar] [CrossRef]
- Yoon, V.; Maalouf, N.M.; Sakhaee, K. The effects of smoking on bone metabolism. Osteoporos. Int. 2012, 23, 2081–2092. [Google Scholar] [CrossRef]
- Nicholson, T.; Scott, A.; Newton Ede, M.; Jones, S.W. Do E-cigarettes and vaping have a lower risk of osteoporosis, nonunion, and infection than tobacco smoking? Bone Jt. Res. 2021, 10, 188–191. [Google Scholar] [CrossRef]
- Cheraghi, Z.; Doosti-Irani, A.; Almasi-Hashiani, A.; Baigi, V.; Mansournia, N.; Etminan, M.; Mansournia, M.A. The effect of alcohol on osteoporosis: A systematic review and meta-analysis. Drug Alcohol. Depend. 2019, 197, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, H.C.; West, D.W.D.; Baehr, L.M.; Tarke, F.D.; Baar, K.; Bodine, S.C.; Christiansen, B.A. Age-dependent bone loss and recovery during hindlimb unloading and subsequent reloading in rats. BMC Musculoskelet. Disord. 2018, 19, 223. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.G.; Furlini, G.; Zati, A.; Letizia Mauro, G. The Effectiveness of Physical Exercise on Bone Density in Osteoporotic Patients. Biomed. Res. Int. 2018, 2018, 4840531. [Google Scholar] [CrossRef]
- Zhu, K.; Prince, R.L. Calcium and bone. Clin. Biochem. 2012, 45, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Overview of Calcium. In Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, C.L., Yaktine, A.L., Eds.; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- National Coordinating Committee on Food and Nutrition. Recommended Nutrient Intakes for Malaysia: A Report of the Technical Working Group on Nutritional Guidelines; Ministry of Health Malaysia: Putrajaya, Malaysia, 2017.
- Chan, C.Y.; Mohamed, N.; Soelaiman, I.-N.; Chin, K.-Y. Attitude of Asians to Calcium and Vitamin D Rich Foods and Supplements: A Systematic Review. Sains Malays. 2018, 47, 1801–1810. [Google Scholar] [CrossRef]
- Chan, C.Y.; Subramaniam, S.; Chin, K.Y.; Ima-Nirwana, S.; Muhammad, N.; Fairus, A.; Ng, P.Y.; Aini, J.N.; Aziz, N.A.; Mohamed, N. Effect of a Screening and Education Programme on Knowledge, Beliefs, and Practices Regarding Osteoporosis among Malaysians. Int. J. Environ. Res. Public Health 2022, 19, 6072. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.Z.; Latiwesh, O.B.; Nouh, F.; Hussain, A.; Kumar, S.; Kaler, J. Response of Parathyroid Hormone to Vitamin D Deficiency in Otherwise Healthy Individuals. Cureus 2020, 12, e9764. [Google Scholar] [CrossRef] [PubMed]
- Ariffin, M.A.S.M.; Fazil, F.N.; Yassin, N.M.; Junaida, N.S.; Gan, P.V.; Rahman, R.A.; Chin, K.-Y.; Aziz, N.H.A. Prevalence of Vitamin D Deficiency and its Associated Risk Factors during Early Pregnancy in a Tropical Country: A Pilot Study. J. Clin. Diagn. Res. 2018, 12, QC18–QC22. [Google Scholar] [CrossRef]
- Chin, K.Y.; Ima-Nirwana, S.; Ibrahim, S.; Mohamed, I.N.; Wan Ngah, W.Z. Vitamin D status in Malaysian men and its associated factors. Nutrients 2014, 6, 5419–5433. [Google Scholar] [CrossRef]
- Qadir, A.; Liang, S.; Wu, Z.; Chen, Z.; Hu, L.; Qian, A. Senile Osteoporosis: The Involvement of Differentiation and Senescence of Bone Marrow Stromal Cells. Int. J. Mol. Sci. 2020, 21, 349. [Google Scholar] [CrossRef]
- Jiang, L.L.; Zhang, C.; Zhang, Y.; Ma, F.; Guan, Y. Associations between polymorphisms in VDR gene and the risk of osteoporosis: A meta-analysis. Arch. Physiol. Biochem. 2020, 128, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jiang, J.; Wang, Q.; Zong, J.; Zhang, L.; Ma, T.; Xu, Y.; Zhang, L. Associations between ERα/β gene polymorphisms and osteoporosis susceptibility and bone mineral density in postmenopausal women: A systematic review and meta-analysis. BMC Endocr. Disord. 2018, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Tang, K.; Quan, Z.; Zhao, Z.; Jiang, D. Association Between Seven Common OPG Genetic Polymorphisms and Osteoporosis Risk: A Meta-Analysis. DNA Cell Biol. 2014, 33, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.H.; Tang, J.; Dai, C.Q.; Yu, Y.; Hong, J.J. COL1A1 gene -1997G/T polymorphism and risk of osteoporosis in postmenopausal women: A meta-analysis. Genet. Mol. Res. 2015, 14, 10991–10998. [Google Scholar] [CrossRef]
- Fu, S.-C.; Wang, P.; Qi, M.-X.; Peng, J.-P.; Lin, X.-Q.; Zhang, C.-Y.; Zhao, G.-X.; He, G.-H. The associations of TNF-α gene polymorphisms with bone mineral density and risk of osteoporosis: A meta-analysis. Int. J. Rheum. Dis. 2019, 22, 1619–1629. [Google Scholar] [CrossRef]
- Kanis, J.A.; Johansson, H.; Oden, A.; Johnell, O.; De Laet, C.; Eisman, J.A.; McCloskey, E.V.; Mellstrom, D.; Melton, L.J., 3rd; Pols, H.A.; et al. A family history of fracture and fracture risk: A meta-analysis. Bone 2004, 35, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Tasnim, N.; Dutta, P.; Nayeem, J.; Masud, P.; Ferdousi, A.; Ghosh, A.S.; Hossain, M.; Rajia, S.; Kubra, K.T.; Sakibuzzaman, M.; et al. Osteoporosis, an Inevitable Circumstance of Chronic Kidney Disease: A Systematic Review. Cureus 2021, 13, e18488. [Google Scholar] [CrossRef]
- Drake, M.T. Osteoporosis and Cancer. Curr. Osteoporos. Rep. 2013, 11, 163–170. [Google Scholar] [CrossRef]
- Pack, A.M. The Association between Antiepileptic Drugs and Bone Disease. Epilepsy Curr. 2003, 3, 91–95. [Google Scholar] [CrossRef]
- Iwaniec, U.T.; Turner, R.T. Influence of body weight on bone mass, architecture and turnover. J. Endocrinol. 2016, 230, R115–R130. [Google Scholar] [CrossRef]
- Coin, A.; Sergi, G.; Benincà, P.; Lupoli, L.; Cinti, G.; Ferrara, L.; Benedetti, G.; Tomasi, G.; Pisent, C.; Enzi, G. Bone mineral density and body composition in underweight and normal elderly subjects. Osteoporos. Int. 2000, 11, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- De Laet, C.; Kanis, J.A.; Odén, A.; Johanson, H.; Johnell, O.; Delmas, P.; Eisman, J.A.; Kroger, H.; Fujiwara, S.; Garnero, P.; et al. Body mass index as a predictor of fracture risk: A meta-analysis. Osteoporos. Int. 2005, 16, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Chen, X.; Zhang, S.; Huang, M.; Shen, X.; Xu, J.; Zou, J. The Effect of Exercise on the Prevention of Osteoporosis and Bone Angiogenesis. Biomed. Res. Int. 2019, 2019, 8171897. [Google Scholar] [CrossRef] [PubMed]
- Riek, A.E.; Towler, D.A. The pharmacological management of osteoporosis. Mo Med. 2011, 108, 118–123. [Google Scholar] [PubMed]
- Khajuria, D.K.; Razdan, R.; Mahapatra, D.R. Drugs for the management of osteoporosis: A review. Rev. Bras. Reumatol. 2011, 51, 365–371, 379–382. [Google Scholar] [PubMed]
- González Macías, J.; Olmos Martínez, J.M. Aminobisphosphonates: Reconsideration 25 years after their approval for the treatment of osteoporosis. Med. Clin. 2022, 159, 336–343. [Google Scholar] [CrossRef]
- Byun, J.H.; Jang, S.; Lee, S.; Park, S.; Yoon, H.K.; Yoon, B.H.; Ha, Y.C. The Efficacy of Bisphosphonates for Prevention of Osteoporotic Fracture: An Update Meta-analysis. J. Bone Metab. 2017, 24, 37–49. [Google Scholar] [CrossRef]
- Kennel, K.A.; Drake, M.T. Adverse effects of bisphosphonates: Implications for osteoporosis management. Mayo Clin. Proc. 2009, 84, 632–637; quiz 638. [Google Scholar] [CrossRef]
- Pang, K.L.; Low, N.Y.; Chin, K.Y. A Review on the Role of Denosumab in Fracture Prevention. Drug Des. Dev. Ther. 2020, 14, 4029–4051. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, J.; Zhou, Y.; Peng, J.; Wang, B. Efficacy and Safety of Denosumab in Osteoporosis or Low Bone Mineral Density Postmenopausal Women. Front. Pharmacol. 2021, 12, 588095. [Google Scholar] [CrossRef]
- Cranney, A.; Adachi, J.D. Benefit-risk assessment of raloxifene in postmenopausal osteoporosis. Drug Saf. 2005, 28, 721–730. [Google Scholar] [CrossRef] [PubMed]
- An, K.C. Selective Estrogen Receptor Modulators. Asian Spine J. 2016, 10, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Zhao, Q.; Lu, P.; Chen, H.; Tan, W.; Guo, W.; Liu, C.; Liu, J. Comparison between teriparatide and bisphosphonates for improving bone mineral density in postmenopausal osteoporosis patients: A meta-analysis. Medicine (Baltimore) 2020, 99, e18964. [Google Scholar] [CrossRef]
- Yuan, F.; Peng, W.; Yang, C.; Zheng, J. Teriparatide versus bisphosphonates for treatment of postmenopausal osteoporosis: A meta-analysis. Int. J. Surg. 2019, 66, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vall, H.; Parmar, M. Teriparatide. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559248/ (accessed on 8 October 2022).
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef]
- Singh, S.; Dutta, S.; Khasbage, S.; Kumar, T.; Sachin, J.; Sharma, J.; Varthya, S.B. A systematic review and meta-analysis of efficacy and safety of Romosozumab in postmenopausal osteoporosis. Osteoporos. Int. 2022, 33, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tian, A.; Jia, H.; Zhu, S.; Lu, B.; Li, Y.; Ma, J.; Ma, X. Romosozumab versus Teriparatide for the Treatment of Postmenopausal Osteoporosis: A Systematic Review and Meta-analysis through a Grade Analysis of Evidence. Orthop. Surg. 2021, 13, 1941–1950. [Google Scholar] [CrossRef]
- Fixen, C.; Tunoa, J. Romosozumab: A Review of Efficacy, Safety, and Cardiovascular Risk. Curr. Osteoporos. Rep. 2021, 19, 15–22. [Google Scholar] [CrossRef]
- Langdahl, B. Treatment of postmenopausal osteoporosis with bone-forming and antiresorptive treatments: Combined and sequential approaches. Bone 2020, 139, 115516. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chin, K.-Y.; Ng, B.N.; Rostam, M.K.I.; Muhammad Fadzil, N.F.D.; Raman, V.; Mohamed Yunus, F.; Syed Hashim, S.A.; Ekeuku, S.O. A Mini Review on Osteoporosis: From Biology to Pharmacological Management of Bone Loss. J. Clin. Med. 2022, 11, 6434. https://doi.org/10.3390/jcm11216434
Chin K-Y, Ng BN, Rostam MKI, Muhammad Fadzil NFD, Raman V, Mohamed Yunus F, Syed Hashim SA, Ekeuku SO. A Mini Review on Osteoporosis: From Biology to Pharmacological Management of Bone Loss. Journal of Clinical Medicine. 2022; 11(21):6434. https://doi.org/10.3390/jcm11216434
Chicago/Turabian StyleChin, Kok-Yong, Ben Nett Ng, Muhd Khairik Imran Rostam, Nur Farah Dhaniyah Muhammad Fadzil, Vaishnavi Raman, Farzana Mohamed Yunus, Syed Alhafiz Syed Hashim, and Sophia Ogechi Ekeuku. 2022. "A Mini Review on Osteoporosis: From Biology to Pharmacological Management of Bone Loss" Journal of Clinical Medicine 11, no. 21: 6434. https://doi.org/10.3390/jcm11216434
APA StyleChin, K.-Y., Ng, B. N., Rostam, M. K. I., Muhammad Fadzil, N. F. D., Raman, V., Mohamed Yunus, F., Syed Hashim, S. A., & Ekeuku, S. O. (2022). A Mini Review on Osteoporosis: From Biology to Pharmacological Management of Bone Loss. Journal of Clinical Medicine, 11(21), 6434. https://doi.org/10.3390/jcm11216434






